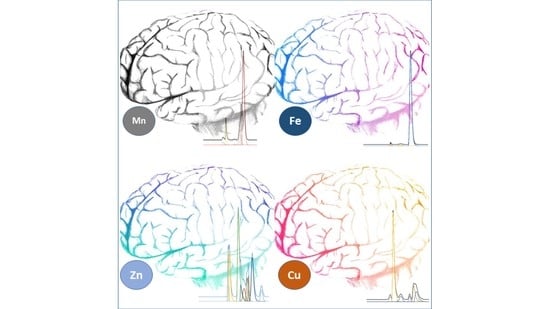
Simultaneous Quantification and Speciation of Trace Metals in Paired Serum and CSF Samples by Size Exclusion Chromatography–Inductively Coupled Plasma–Dynamic Reaction Cell–Mass Spectrometry (SEC-DRC-ICP-MS)
Abstract
:1. Introduction
2. Results and Discussion
2.1. Total Quantification and SEC-Separation of Metal Species in Serum and CSF Samples
2.2. Correlation of Trace Elements in Serum and CSF Samples
2.3. SEC-Separation of Metal Species in Brain Extracts from Mn-Exposed Rats
3. Conclusions and limitations of the Study
4. Materials and Methods
4.1. Patient Samples
4.2. Chemicals
4.3. Standard and Sample Preparation
4.4. SEC Parameters
4.5. Columns Mass Calibration
4.6. ICP-MS Parameters
4.7. ICP-OES
4.8. Calculations and Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Barnett, J.P.; Scanlan, D.J.; Blindauer, C.A. Protein fractionation and detection for metalloproteomics: Challenges and approaches. Anal. Bioanal. Chem. 2012, 402, 3311–3322. [Google Scholar] [CrossRef]
- Waldron, K.J.; Rutherford, J.C.; Ford, D.; Robinson, N.J. Metalloproteins and metal sensing. Nature 2009, 460, 823–830. [Google Scholar] [CrossRef] [PubMed]
- Jensen, O.N. Interpreting the protein language using proteomics. Nat. Rev. Mol. Cell Biol. 2006, 7, 391–403. [Google Scholar] [CrossRef] [PubMed]
- Lothian, A.; Hare, D.J.; Grimm, R.; Ryan, T.M.; Masters, C.L.; Roberts, B.R. Metalloproteomics: Principles, challenges and applications to neurodegeneration. Front. Aging Neurosci. 2013, 5, 35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bertini, I.; Rosato, A. From Genes to Metalloproteins: A Bioinformatic Approach. Eur. J. Inorg. Chem. 2007, 2007, 2546–2555. [Google Scholar] [CrossRef]
- Shi, W.; Chance, M.R. Metallomics and metalloproteomics. Cell. Mol. Life Sci. CMLS 2008, 65, 3040–3048. [Google Scholar] [CrossRef]
- Wandt, V.K.; Winkelbeiner, N.; Bornhorst, J.; Witt, B.; Raschke, S.; Simon, L.; Ebert, F.; Kipp, A.P.; Schwerdtle, T. A matter of concern-Trace element dyshomeostasis and genomic stability in neurons. Redox Biol. 2021, 41, 101877. [Google Scholar] [CrossRef]
- Daneman, R.; Prat, A. The blood-brain barrier. Cold Spring Harb. Perspect. Biol. 2015, 7, a020412. [Google Scholar] [CrossRef] [Green Version]
- Basun, H.; Forssell, L.G.; Wetterberg, L.; Winblad, B. Metals and trace elements in plasma and cerebrospinal fluid in normal aging and Alzheimer’s disease. J. Neural. Transm. Park Dis. Dement. Sect. 1991, 3, 231–258. [Google Scholar]
- Zheng, W.; Aschner, M.; Ghersi-Egea, J.F. Brain barrier systems: A new frontier in metal neurotoxicological research. Toxicol. Appl. Pharmacol. 2003, 192, 1–11. [Google Scholar] [CrossRef] [Green Version]
- D’Autreaux, B.; Toledano, M.B. ROS as signalling molecules: Mechanisms that generate specificity in ROS homeostasis. Nat. Rev. Mol. Cell Biol. 2007, 8, 813–824. [Google Scholar] [CrossRef] [PubMed]
- Stockwell, B.R.; Friedmann Angeli, J.P.; Bayir, H.; Bush, A.I.; Conrad, M.; Dixon, S.J.; Fulda, S.; Gascon, S.; Hatzios, S.K.; Kagan, V.E.; et al. Ferroptosis: A Regulated Cell Death Nexus Linking Metabolism, Redox Biology, and Disease. Cell 2017, 171, 273–285. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saporito-Magrina, C.M.; Musacco-Sebio, R.N.; Andrieux, G.; Kook, L.; Orrego, M.T.; Tuttolomondo, M.V.; Desimone, M.F.; Boerries, M.; Borner, C.; Repetto, M.G. Copper-induced cell death and the protective role of glutathione: The implication of impaired protein folding rather than oxidative stress. Metallomics 2018, 10, 1743–1754. [Google Scholar] [CrossRef]
- Lei, P.; Ayton, S.; Bush, A.I. The essential elements of Alzheimer’s disease. J. Biol. Chem. 2021, 296, 100105. [Google Scholar] [CrossRef] [PubMed]
- Mezzaroba, L.; Alfieri, D.F.; Colado Simao, A.N.; Vissoci Reiche, E.M. The role of zinc, copper, manganese and iron in neurodegenerative diseases. Neurotoxicology 2019, 74, 230–241. [Google Scholar] [CrossRef] [PubMed]
- Vinceti, M.; Solovyev, N.; Mandrioli, J.; Crespi, C.M.; Bonvicini, F.; Arcolin, E.; Georgoulopoulou, E.; Michalke, B. Cerebrospinal fluid of newly diagnosed amyotrophic lateral sclerosis patients exhibits abnormal levels of selenium species including elevated selenite. Neurotoxicology 2013, 38, 25–32. [Google Scholar] [CrossRef] [Green Version]
- Sies, H.; Jones, D.P. Reactive oxygen species (ROS) as pleiotropic physiological signalling agents. Nat. Rev. Mol. Cell Biol. 2020, 21, 363–383. [Google Scholar] [CrossRef]
- Liu, B.; Moloney, A.; Meehan, S.; Morris, K.; Thomas, S.E.; Serpell, L.C.; Hider, R.; Marciniak, S.J.; Lomas, D.A.; Crowther, D.C. Iron promotes the toxicity of amyloid beta peptide by impeding its ordered aggregation. J. Biol. Chem. 2011, 286, 4248–4256. [Google Scholar] [CrossRef] [Green Version]
- Dong, J.; Atwood, C.S.; Anderson, V.E.; Siedlak, S.L.; Smith, M.A.; Perry, G.; Carey, P.R. Metal binding and oxidation of amyloid-beta within isolated senile plaque cores: Raman microscopic evidence. Biochemistry 2003, 42, 2768–2773. [Google Scholar] [CrossRef]
- Opazo, C.; Huang, X.; Cherny, R.A.; Moir, R.D.; Roher, A.E.; White, A.R.; Cappai, R.; Masters, C.L.; Tanzi, R.E.; Inestrosa, N.C.; et al. Metalloenzyme-like activity of Alzheimer’s disease beta-amyloid. Cu-dependent catalytic conversion of dopamine, cholesterol, and biological reducing agents to neurotoxic H(2)O(2). J. Biol. Chem. 2002, 277, 40302–40308. [Google Scholar] [CrossRef] [Green Version]
- Huang, X.; Atwood, C.S.; Hartshorn, M.A.; Multhaup, G.; Goldstein, L.E.; Scarpa, R.C.; Cuajungco, M.P.; Gray, D.N.; Lim, J.; Moir, R.D.; et al. The A beta peptide of Alzheimer’s disease directly produces hydrogen peroxide through metal ion reduction. Biochemistry 1999, 38, 7609–7616. [Google Scholar] [CrossRef]
- Aguirre, J.D.; Culotta, V.C. Battles with iron: Manganese in oxidative stress protection. J. Biol. Chem. 2012, 287, 13541–13548. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Erikson, K.M.; Aschner, M. Manganese neurotoxicity and glutamate-GABA interaction. Neurochem. Int. 2003, 43, 475–480. [Google Scholar] [CrossRef]
- Butterworth, J. Changes in nine enzyme markers for neurons, glia, and endothelial cells in agonal state and Huntington’s disease caudate nucleus. J. Neurochem. 1986, 47, 583–587. [Google Scholar] [CrossRef]
- Martins, A.C., Jr.; Morcillo, P.; Ijomone, O.M.; Venkataramani, V.; Harrison, F.E.; Lee, E.; Bowman, A.B.; Aschner, M. New Insights on the Role of Manganese in Alzheimer’s Disease and Parkinson’s Disease. Int. J. Environ. Res. Public Health 2019, 16, 3546. [Google Scholar] [CrossRef] [PubMed]
- Michalke, B.; Fernsebner, K. New insights into manganese toxicity and speciation. J. Trace Elem. Med. Biol. 2014, 28, 106–116. [Google Scholar] [CrossRef] [PubMed]
- Kaur, G.; Kumar, V.; Arora, A.; Tomar, A.; Sur, R.; Dutta, D. Affected energy metabolism under manganese stress governs cellular toxicity. Sci. Rep. 2017, 7, 11645. [Google Scholar] [CrossRef] [Green Version]
- Oteiza, P.I. Zinc and the modulation of redox homeostasis. Free. Radic. Biol. Med. 2012, 53, 1748–1759. [Google Scholar] [CrossRef] [Green Version]
- Hubner, C.; Haase, H. Interactions of zinc- and redox-signaling pathways. Redox Biol. 2021, 41, 101916. [Google Scholar] [CrossRef]
- Bitanihirwe, B.K.; Cunningham, M.G. Zinc: The brain’s dark horse. Synapse 2009, 63, 1029–1049. [Google Scholar] [CrossRef]
- Takeda, A. Zinc homeostasis and functions of zinc in the brain. Biometals 2001, 14, 343–351. [Google Scholar] [CrossRef] [PubMed]
- Szewczyk, B. Zinc homeostasis and neurodegenerative disorders. Front. Aging Neurosci. 2013, 5, 33. [Google Scholar] [CrossRef] [Green Version]
- Roos, P.M.; Lierhagen, S.; Flaten, T.P.; Syversen, T.; Vesterberg, O.; Nordberg, M. Manganese in cerebrospinal fluid and blood plasma of patients with amyotrophic lateral sclerosis. Exp. Biol. Med. (Maywood) 2012, 237, 803–810. [Google Scholar] [CrossRef] [PubMed]
- Korvela, M.; Lind, A.L.; Wetterhall, M.; Gordh, T.; Andersson, M.; Pettersson, J. Quantification of 10 elements in human cerebrospinal fluid from chronic pain patients with and without spinal cord stimulation. J. Trace Elem. Med. Bio. 2016, 37, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Laur, N.; Kinscherf, R.; Pomytkin, K.; Kaiser, L.; Knes, O.; Deigner, H.P. ICP-MS trace element analysis in serum and whole blood. PLoS ONE 2020, 15, e0233357. [Google Scholar] [CrossRef] [PubMed]
- Gerhardsson, L.; Lundh, T.; Minthon, L.; Londos, E. Metal concentrations in plasma and cerebrospinal fluid in patients with Alzheimer’s disease. Dement. Geriatr. Cogn. Disord. 2008, 25, 508–515. [Google Scholar] [CrossRef] [PubMed]
- Gerhardsson, L.; Lundh, T.; Londos, E.; Minthon, L. Cerebrospinal fluid/plasma quotients of essential and non-essential metals in patients with Alzheimer’s disease. J. Neural Transm. (Vienna) 2011, 118, 957–962. [Google Scholar] [CrossRef]
- Roos, P.M. Ultraclean paired sampling for metal analysis in neurodegenerative disorders. J. Trace Elem. Med. Biol. 2019, 52, 48–52. [Google Scholar] [CrossRef]
- Michalke, B.; Halbach, S.; Berthele, A.; Mistritiotis, P.; Ochsenkuhn-Petropoulou, M. Size characterization of manganese species from human serum and cerebrospinal fluid using size exclusion chromatography coupled to inductively coupled plasma mass spectrometry. J. Anal. Atom. Spectrom. 2007, 22, 267–272. [Google Scholar] [CrossRef]
- Nischwitz, V.; Berthele, A.; Michalke, B. Speciation analysis of selected metals and determination of their total contents in paired serum and cerebrospinal fluid samples: An approach to investigate the permeability of the human blood-cerebrospinal fluid-barrier. Anal. Chim. Acta 2008, 627, 258–269. [Google Scholar] [CrossRef]
- Michalke, B.; Lucio, M.; Berthele, A.; Kanawati, B. Manganese speciation in paired serum and CSF samples using SEC-DRC-ICP-MS and CE-ICP-DRC-MS. Anal. Bioanal. Chem. 2013, 405, 2301–2309. [Google Scholar] [CrossRef]
- Neth, K.; Lucio, M.; Walker, A.; Zorn, J.; Schmitt-Kopplin, P.; Michalke, B. Changes in Brain Metallome/Metabolome Pattern due to a Single i.v. Injection of Manganese in Rats. PLoS ONE 2015, 10, e0138270. [Google Scholar] [CrossRef] [PubMed]
- Michalke, B.; Aslanoglou, L.; Ochsenkuhn-Petropoulou, M.; Bergstrom, B.; Berthele, A.; Vinceti, M.; Lucio, M.; Liden, G. An approach for manganese biomonitoring using a manganese carrier switch in serum from transferrin to citrate at slightly elevated manganese concentration. J. Trace Elem. Med. Biol. 2015, 32, 145–154. [Google Scholar] [CrossRef]
- Quintana, M.; Klouda, A.D.; Gondikas, A.; Ochsenkuhn-Petropoulou, M.; Michalke, B. Analysis of size characterized manganese species from liver extracts using capillary zone electrophoresis coupled to inductively coupled plasma mass spectrometry (CZE-ICP-MS). Anal. Chim. Acta 2006, 573–574, 172–180. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Sevillano, M.A.; Garcia-Barrera, T.; Navarro, F.; Gailer, J.; Gomez-Ariza, J.L. Use of elemental and molecular-mass spectrometry to assess the toxicological effects of inorganic mercury in the mouse Mus musculus. Anal. Bioanal. Chem. 2014, 406, 5853–5865. [Google Scholar] [CrossRef] [PubMed]
- Jahromi, E.Z.; White, W.; Wu, Q.A.; Yamdagni, R.; Gailer, J. Remarkable effect of mobile phase buffer on the SEC-ICP-AES derived Cu, Fe and Zn-metalloproteome pattern of rabbit blood plasma. Metallomics 2010, 2, 460–468. [Google Scholar] [CrossRef]
- De Cremer, K.; Van Hulle, M.; Chery, C.; Cornelis, R.; Strijckmans, K.; Dams, R.; Lameire, N.; Vanholder, R. Fractionation of vanadium complexes in serum, packed cells and tissues of Wistar rats by means of gel filtration and anion-exchange chromatography. J. Biol. Inorg. Chem. 2002, 7, 884–890. [Google Scholar] [CrossRef]
- Bytzek, A.K.; Boeck, K.; Hermann, G.; Hann, S.; Keppler, B.K.; Hartinger, C.G.; Koellensperger, G. LC- and CZE-ICP-MS approaches for the in vivo analysis of the anticancer drug candidate sodium trans-[tetrachloridobis(1H-indazole)ruthenate(III)] (KP1339) in mouse plasma. Metallomics 2011, 3, 1049–1055. [Google Scholar] [CrossRef]
- Arias-Borrego, A.; Callejon-Leblic, B.; Rodriguez-Moro, G.; Velasco, I.; Gomez-Ariza, J.L.; Garcia-Barrera, T. A novel HPLC column switching method coupled to ICP-MS/QTOF for the first determination of selenoprotein P (SELENOP) in human breast milk. Food Chem. 2020, 321, 126692. [Google Scholar] [CrossRef]
- Boulyga, S.F.; Loreti, V.; Bettmer, J.; Heumann, K.G. Application of SEC-ICP-MS for comparative analyses of metal-containing species in cancerous and healthy human thyroid samples. Anal. Bioanal. Chem. 2004, 380, 198–203. [Google Scholar] [CrossRef]
- Gellein, K.; Roos, P.M.; Evje, L.; Vesterberg, O.; Flaten, T.P.; Nordberg, M.; Syversen, T. Separation of proteins including metallothionein in cerebrospinal fluid by size exclusion HPLC and determination of trace elements by HR-ICP-MS. Brain Res. 2007, 1174, 136–142. [Google Scholar] [CrossRef]
- Ellis, J.; Grimm, R.; Clark, J.F.; Pyne-Gaithman, G.; Wilbur, S.; Caruso, J.A. Studying protein phosphorylation in low MW CSF fractions with capLC-ICPMS and nanoLC-CHIP-ITMS for identification of phosphoproteins. J. Proteome Res. 2008, 7, 4736–4742. [Google Scholar] [CrossRef] [Green Version]
- Berger, F.; Kubik-Huch, R.A.; Niemann, T.; Schmid, H.R.; Poetzsch, M.; Froehlich, J.M.; Beer, J.H.; Thali, M.J.; Kraemer, T. Gadolinium Distribution in Cerebrospinal Fluid after Administration of a Gadolinium-based MR Contrast Agent in Humans. Radiology 2018, 288, 703–709. [Google Scholar] [CrossRef] [Green Version]
- Michalke, B.; Berthele, A.; Mistriotis, P.; Ochsenkiihn-Petropoulou, M.; Halbach, S. Manganese species from human serum, cerebrospional fluid analysed by size exclusion chromatography-, capillary electropheresis coupled inductively coupled plasma massspectrometry. J. Trace Elem. Med. Bio. 2007, 21, 4–9. [Google Scholar] [CrossRef] [PubMed]
- Fernsebner, K.; Zorn, J.; Kanawati, B.; Walker, A.; Michalke, B. Manganese leads to an increase in markers of oxidative stress as well as to a shift in the ratio of Fe(II)/(III) in rat brain tissue. Metallomics 2014, 6, 921–931. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Venkataramani, V.; Doeppner, T.R.; Willkommen, D.; Cahill, C.M.; Xin, Y.; Ye, G.; Liu, Y.; Southon, A.; Aron, A.; Au-Yeung, H.Y.; et al. Manganese causes neurotoxic iron accumulation via translational repression of amyloid precursor protein and H-Ferritin. J. Neurochem. 2018, 147, 831–848. [Google Scholar] [CrossRef] [PubMed]
- Ramos, P.; Santos, A.; Pinto, N.R.; Mendes, R.; Magalhaes, T.; Almeida, A. Iron levels in the human brain: A post-mortem study of anatomical region differences and age-related changes. J. Trace Elem. Med. Biol. 2014, 28, 13–17. [Google Scholar] [CrossRef]
- Ramos, P.; Santos, A.; Pinto, N.R.; Mendes, R.; Magalhaes, T.; Almeida, A. Anatomical region differences and age-related changes in copper, zinc, and manganese levels in the human brain. Biol. Trace Elem. Res. 2014, 161, 190–201. [Google Scholar] [CrossRef]
- Kambe, T.; Tsuji, T.; Hashimoto, A.; Itsumura, N. The Physiological, Biochemical, and Molecular Roles of Zinc Transporters in Zinc Homeostasis and Metabolism. Physiol. Rev. 2015, 95, 749–784. [Google Scholar] [CrossRef]
- Kardos, J.; Heja, L.; Simon, A.; Jablonkai, I.; Kovacs, R.; Jemnitz, K. Copper signalling: Causes and consequences (vol 16, 71, 2018). Cell Commun. Signal. 2018, 16, 1–22. [Google Scholar] [CrossRef] [Green Version]
- Neth, K.; Lucio, M.; Walker, A.; Kanawati, B.; Zorn, J.; Schmitt-Kopplin, P.; Michalke, B. Diverse Serum Manganese Species Affect Brain Metabolites Depending on Exposure Conditions. Chem. Res. Toxicol. 2015, 28, 1434–1442. [Google Scholar] [CrossRef] [Green Version]
- Willkommen, D.; Lucio, M.; Moritz, F.; Forcisi, S.; Kanawati, B.; Smirnov, K.S.; Schroeter, M.; Sigaroudi, A.; Schmitt-Kopplin, P.; Michalke, B. Metabolomic investigations in cerebrospinal fluid of Parkinson’s disease. PLoS ONE 2018, 13, e0208752. [Google Scholar] [CrossRef] [Green Version]
- Willkommen, D.; Lucio, M.; Schmitt-Kopplin, P.; Gazzaz, M.; Schroeter, M.; Sigaroudi, A.; Michalke, B. Species fractionation in a case-control study concerning Parkinson’s disease: Cu-amino acids discriminate CSF of PD from controls. J. Trace Elem. Med. Biol. 2018, 49, 164–170. [Google Scholar] [CrossRef]
- Merck-Ferritin-Data-Sheet. Ferritin Data Sheet. Available online: https://www.sigmaaldrich.com/DE/de/product/sigma/f4503?gclid=EAIaIQobChMIy77KuIDM8QIVB6p3Ch3XAQNEEAAYASAAEgLXEfD_BwE (accessed on 5 July 2021).
- Bio-Rad-Ferritin-Data-Sheet. Ferritin Data Sheet. Available online: https://www.bio-rad.com/de-de/product/ferritin?ID=MUBL8UE8Z (accessed on 5 July 2021).
- Solovyev, N.; Berthele, A.; Michalke, B. Selenium speciation in paired serum and cerebrospinal fluid samples. Anal. Bioanal. Chem. 2013, 405, 1875–1884. [Google Scholar] [CrossRef]
- Reiber, H.; Thompson, E.J.; Grimsley, G.; Bernardi, G.; Adam, P.; Monteiro de Almeida, S.; Fredman, P.; Keir, G.; Lammers, M.; Liblau, R.; et al. Quality assurance for cerebrospinal fluid protein analysis: International consensus by an Internet-based group discussion. Clin. Chem. Lab. Med. 2003, 41, 331–337. [Google Scholar] [CrossRef] [PubMed]
- Andreini, C.; Banci, L.; Bertini, I.; Rosato, A. Counting the zinc-proteins encoded in the human genome. J. Proteome Res. 2006, 5, 196–201. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Stewart, A.J.; Sadler, P.J.; Pinheiro, T.J.; Blindauer, C.A. Albumin as a zinc carrier: Properties of its high-affinity zinc-binding site. Biochem. Soc. Trans. 2008, 36, 1317–1321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kirsipuu, T.; Zadoroznaja, A.; Smirnova, J.; Friedemann, M.; Plitz, T.; Tougu, V.; Palumaa, P. Copper(II)-binding equilibria in human blood. Sci. Rep. 2020, 10, 5686. [Google Scholar] [CrossRef] [PubMed]
- Mikes, O. High Performance Liquid Chromatography of Bio-Polymers and Bio-Oligomeres; Elsevier: Amsterdam, The Netherlands, 1979; Volume 41B. [Google Scholar]
- Sotak, C.H.; Sharer, K.; Koretsky, A.P. Manganese cell labeling of murine hepatocytes using manganese(III)-transferrin. Contrast Media Mol. Imaging 2008, 3, 95–105. [Google Scholar] [CrossRef] [Green Version]
- Aschner, M.; Guilarte, T.R.; Schneider, J.S.; Zheng, W. Manganese: Recent advances in understanding its transport and neurotoxicity. Toxicol. Appl. Pharm. 2007, 221, 131–147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gunter, T.E.; Gerstner, B.; Gunter, K.K.; Malecki, J.; Gelein, R.; Valentine, W.M.; Aschner, M.; Yule, D.I. Manganese transport via the transferrin mechanism. Neurotoxicology 2013, 34, 118–127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crossgrove, J.S.; Allen, D.D.; Bukaveckas, B.L.; Rhineheimer, S.S.; Yokel, R.A. Manganese distribution across the blood-brain barrier. I. Evidence for carrier-mediated influx of managanese citrate as well as manganese and manganese transferrin. Neurotoxicology 2003, 24, 3–13. [Google Scholar] [CrossRef]
- Ackley, D.C.; Yokel, R.A. Aluminum citrate is transported from brain into blood via the monocarboxylic acid transporter located at the blood-brain barrier. Toxicology 1997, 120, 89–97. [Google Scholar] [CrossRef]
- Yokel, R.A. Manganese flux across the blood-brain barrier. Neuromolecular Med. 2009, 11, 297–310. [Google Scholar] [CrossRef]
- Chua, A.C.; Morgan, E.H. Effects of iron deficiency and iron overload on manganese uptake and deposition in the brain and other organs of the rat. Biol. Trace Elem. Res. 1996, 55, 39–54. [Google Scholar] [CrossRef] [PubMed]
- Balachandran, R.C.; Mukhopadhyay, S.; McBride, D.; Veevers, J.; Harrison, F.E.; Aschner, M.; Haynes, E.N.; Bowman, A.B. Brain manganese and the balance between essential roles and neurotoxicity. J. Biol. Chem. 2020, 295, 6312–6329. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hagège, T.N.S.H.A. Marc Hébrant. Separative techniques for metalloproteomics require balance between separation and perturbation. Trends Anal. Chem. 2015, 64, 64–74. [Google Scholar] [CrossRef]
- Michalke, B.; Berthele, A. Contribution to selenium speciation in cerebrospinal fluid samples. J. Anal. Atom. Spectrom. 2011, 26, 165–170. [Google Scholar] [CrossRef]
- Michalke, B. Quality control and reference materials in speciation analysis. Fresen. J. Anal. Chem. 1999, 363, 439–445. [Google Scholar] [CrossRef]
- Diederich, J.; Michalke, B. Enhanced extract preparation of native manganese and iron species from brain and liver tissue. Anal. Bioanal. Chem. 2011, 399, 1799–1806. [Google Scholar] [CrossRef]
- Chen, S.Y.; Feng, Z.; Yi, X. A general introduction to adjustment for multiple comparisons. J. Thorac. Dis. 2017, 9, 1725–1729. [Google Scholar] [CrossRef] [PubMed] [Green Version]




| Mn (µg/L) | Fe (µg/L) | Cu (µg/L) | Zn (µg/L) | |
|---|---|---|---|---|
| CSF (mean ± SD) | 0.5 ± 0.2 | 19.4 ± 6.7 | 17.0 ± 2.0 | 18 ± 6.9 |
| Serum (mean ± SD) | 1.35 ± 0.3 | 1220 ± 293 | 1380 ± 560 | 704 ± 159 |
| Q (CSF/Serum) (this paper) | 0.37 | 0.016 | 0.012 | 0.026 |
| Q ctrl (CSF/Serum) (ref. [37]) | 0.69 (0.26–1.87) | 0.14 (0.06–0.38) | 0.02 (0.01–0.03) | 0.02 (0.01–0.20) |
| Q AD (CSF/Serum) (ref. [37]) | 0.45 (0.08–1.50) | 0.13 (0.04–0.52) | 0.01 (0.01–0.11) | 0.02 (0.00–0.19) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Michalke, B.; Berthele, A.; Venkataramani, V. Simultaneous Quantification and Speciation of Trace Metals in Paired Serum and CSF Samples by Size Exclusion Chromatography–Inductively Coupled Plasma–Dynamic Reaction Cell–Mass Spectrometry (SEC-DRC-ICP-MS). Int. J. Mol. Sci. 2021, 22, 8892. https://doi.org/10.3390/ijms22168892
Michalke B, Berthele A, Venkataramani V. Simultaneous Quantification and Speciation of Trace Metals in Paired Serum and CSF Samples by Size Exclusion Chromatography–Inductively Coupled Plasma–Dynamic Reaction Cell–Mass Spectrometry (SEC-DRC-ICP-MS). International Journal of Molecular Sciences. 2021; 22(16):8892. https://doi.org/10.3390/ijms22168892
Chicago/Turabian StyleMichalke, Bernhard, Achim Berthele, and Vivek Venkataramani. 2021. "Simultaneous Quantification and Speciation of Trace Metals in Paired Serum and CSF Samples by Size Exclusion Chromatography–Inductively Coupled Plasma–Dynamic Reaction Cell–Mass Spectrometry (SEC-DRC-ICP-MS)" International Journal of Molecular Sciences 22, no. 16: 8892. https://doi.org/10.3390/ijms22168892
APA StyleMichalke, B., Berthele, A., & Venkataramani, V. (2021). Simultaneous Quantification and Speciation of Trace Metals in Paired Serum and CSF Samples by Size Exclusion Chromatography–Inductively Coupled Plasma–Dynamic Reaction Cell–Mass Spectrometry (SEC-DRC-ICP-MS). International Journal of Molecular Sciences, 22(16), 8892. https://doi.org/10.3390/ijms22168892