Combinational Therapy of Cardiac Atrial Appendage Stem Cells and Pyridoxamine: The Road to Cardiac Repair?
Abstract
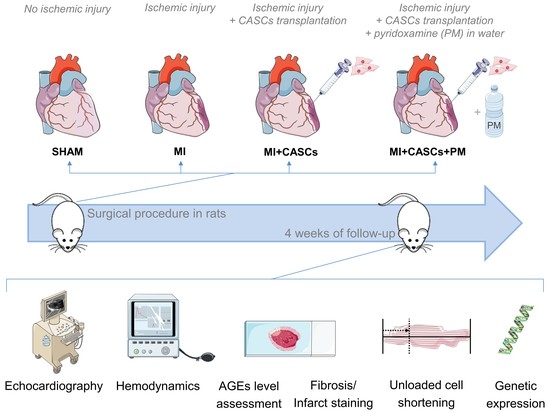
:1. Introduction
2. Results
2.1. AGEs’ Levels Are Reduced with PM Treatment
2.2. CASCs Transplantation Prevents Loss of LV Function after MI
2.3. CASCs Transplantation Tended to Reduce Infarct Size
2.4. CASCs Transplantation Prevents the Increased Interstitial Collagen Deposition Seen with MI
2.5. CASCs Transplantation Prevents Resident Cardiomyocyte Functional Remodeling
2.6. PM Treatment Tended to Reduced Tissue Pro-Inflammatory Cytokine Levels
3. Discussion
3.1. Combining CASCs and PM to Enhance Cardiac Repair
3.2. CASCs Alone Are an Effective Therapy for MI
4. Materials and Methods
4.1. Animal Experiments
4.2. Rat CASCs Isolation and Expansion
4.3. Experimental Protocol
4.4. Echocardiographic Measurements
4.5. Hemodynamic Measurements
4.6. Cardiomyocyte Isolation and Unloaded Cell Shortening
4.7. AGEs’ Content in Heart Tissue
4.8. Interstitial Fibrosis and Infarct Size Measurement
4.9. Real-Time PCR
4.10. Statistics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- The Top 10 Causes of Death. Available online: http://www.who.int/en/news-room/fact-sheets/detail/the-top-10-causes-of-death (accessed on 1 June 2021).
- Xin, M.; Olson, E.N.; Bassel-Duby, R. Mending broken hearts: Cardiac development as a basis for adult heart regeneration and repair. Nat. Rev. Mol. Cell Biol. 2013, 14, 529–541. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosamond, W.; Flegal, K.; Furie, K.; Go, A.; Greenlund, K.; Haase, N.; Hailpern, S.M.; Ho, M.; Howard, V.; Kissela, B.; et al. Heart disease and stroke statistics—2008 update: A report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation 2008, 117, e25–e146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Packer, M.; Bristow, M.R.; Cohn, J.N.; Colucci, W.S.; Fowler, M.B.; Gilbert, E.M.; Shusterman, N.H. The effect of carvedilol on morbidity and mortality in patients with chronic heart failure. U.S. Carvedilol Heart Failure Study Group. N. Engl. J. Med. 1996, 334, 1349–1355. [Google Scholar] [CrossRef] [PubMed]
- Soonpaa, M.H.; Rubart, M.; Field, L.J. Challenges measuring cardiomyocyte renewal. Biochim. Biophys. Acta 2013, 1833, 799–803. [Google Scholar] [CrossRef] [Green Version]
- Cambria, E.; Pasqualini, F.S.; Wolint, P.; Gunter, J.; Steiger, J.; Bopp, A.; Hoerstrup, S.P.; Emmert, M.Y. Translational cardiac stem cell therapy: Advancing from first-generation to next-generation cell types. NPJ Regen. Med. 2017, 2, 17. [Google Scholar] [CrossRef]
- Zhu, K.; Wu, Q.; Ni, C.; Zhang, P.; Zhong, Z.; Wu, Y.; Wang, Y.; Xu, Y.; Kong, M.; Cheng, H.; et al. Lack of Remuscularization Following Transplantation of Human Embryonic Stem Cell-Derived Cardiovascular Progenitor Cells in Infarcted Nonhuman Primates. Circ. Res. 2018, 122, 958–969. [Google Scholar] [CrossRef]
- Ayyat, K.S.; Argawi, A.; Mende, M.; Steinhoff, G.; Borger, M.A.; Deebis, A.M.; McCurry, K.R.; Garbade, J. Combined Coronary Artery Bypass Surgery With Bone Marrow Stem Cell Transplantation: Are We There Yet? Ann. Thorac. Surg. 2019, 108, 1913–1921. [Google Scholar] [CrossRef]
- Wolfien, M.; Klatt, D.; Salybekov, A.A.; Ii, M.; Komatsu-Horii, M.; Gaebel, R.; Philippou-Massier, J.; Schrinner, E.; Akimaru, H.; Akimaru, E.; et al. Hematopoietic stem-cell senescence and myocardial repair—Coronary artery disease genotype/phenotype analysis of post-MI myocardial regeneration response induced by CABG/CD133+ bone marrow hematopoietic stem cell treatment in RCT PERFECT Phase 3. EBioMedicine 2020, 57, 102862. [Google Scholar] [CrossRef]
- Hendrikx, M.; Hensen, K.; Clijsters, C.; Jongen, H.; Koninckx, R.; Bijnens, E.; Ingels, M.; Jacobs, A.; Geukens, R.; Dendale, P.; et al. Recovery of regional but not global contractile function by the direct intramyocardial autologous bone marrow transplantation: Results from a randomized controlled clinical trial. Circulation 2006, 114, I101–I107. [Google Scholar] [CrossRef] [Green Version]
- Jeevanantham, V.; Butler, M.; Saad, A.; Abdel-Latif, A.; Zuba-Surma, E.K.; Dawn, B. Adult bone marrow cell therapy improves survival and induces long-term improvement in cardiac parameters: A systematic review and meta-analysis. Circulation 2012, 126, 551–568. [Google Scholar] [CrossRef] [Green Version]
- Bearzi, C.; Rota, M.; Hosoda, T.; Tillmanns, J.; Nascimbene, A.; De Angelis, A.; Yasuzawa-Amano, S.; Trofimova, I.; Siggins, R.W.; Lecapitaine, N.; et al. Human cardiac stem cells. Proc. Natl. Acad. Sci. USA 2007, 104, 14068–14073. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Genead, R.; Danielsson, C.; Andersson, A.B.; Corbascio, M.; Franco-Cereceda, A.; Sylven, C.; Grinnemo, K.H. Islet-1 cells are cardiac progenitors present during the entire lifespan: From the embryonic stage to adulthood. Stem Cells Dev. 2010, 19, 1601–1615. [Google Scholar] [CrossRef] [PubMed]
- Smith, R.R.; Barile, L.; Cho, H.C.; Leppo, M.K.; Hare, J.M.; Messina, E.; Giacomello, A.; Abraham, M.R.; Marban, E. Regenerative potential of cardiosphere-derived cells expanded from percutaneous endomyocardial biopsy specimens. Circulation 2007, 115, 896–908. [Google Scholar] [CrossRef] [Green Version]
- Bolli, R.; Chugh, A.R.; D’Amario, D.; Loughran, J.H.; Stoddard, M.F.; Ikram, S.; Beache, G.M.; Wagner, S.G.; Leri, A.; Hosoda, T.; et al. Cardiac stem cells in patients with ischaemic cardiomyopathy (SCIPIO): Initial results of a randomised phase 1 trial. Lancet 2011, 378, 1847–1857. [Google Scholar] [CrossRef] [Green Version]
- Makkar, R.R.; Smith, R.R.; Cheng, K.; Malliaras, K.; Thomson, L.E.; Berman, D.; Czer, L.S.; Marban, L.; Mendizabal, A.; Johnston, P.V.; et al. Intracoronary cardiosphere-derived cells for heart regeneration after myocardial infarction (CADUCEUS): A prospective, randomised phase 1 trial. Lancet 2012, 379, 895–904. [Google Scholar] [CrossRef] [Green Version]
- van Berlo, J.H.; Kanisicak, O.; Maillet, M.; Vagnozzi, R.J.; Karch, J.; Lin, S.C.; Middleton, R.C.; Marban, E.; Molkentin, J.D. c-kit+ cells minimally contribute cardiomyocytes to the heart. Nature 2014, 509, 337–341. [Google Scholar] [CrossRef]
- Koninckx, R.; Daniels, A.; Windmolders, S.; Mees, U.; Macianskiene, R.; Mubagwa, K.; Steels, P.; Jamaer, L.; Dubois, J.; Robic, B.; et al. The cardiac atrial appendage stem cell: A new and promising candidate for myocardial repair. Cardiovasc. Res. 2013, 97, 413–423. [Google Scholar] [CrossRef] [Green Version]
- Fanton, Y.; Robic, B.; Rummens, J.L.; Daniels, A.; Windmolders, S.; Willems, L.; Jamaer, L.; Dubois, J.; Bijnens, E.; Heuts, N.; et al. Cardiac atrial appendage stem cells engraft and differentiate into cardiomyocytes in vivo: A new tool for cardiac repair after MI. Int. J. Cardiol. 2015, 201, 10–19. [Google Scholar] [CrossRef]
- Kralev, S.; Zimmerer, E.; Brueckmann, M.; Lang, S.; Kalsch, T.; Rippert, A.; Lin, J.; Borggrefe, M.; Hammes, H.P.; Suselbeck, T. Elevation of the glycoxidation product N(epsilon)-(carboxymethyl)lysine in patients presenting with acute myocardial infarction. Clin. Chem. Lab. Med. 2009, 47, 446–451. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Barden, A.; Mori, T.; Beilin, L. Advanced glycation end-products: A review. Diabetologia 2001, 44, 129–146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hartog, J.W.; Voors, A.A.; Bakker, S.J.; Smit, A.J.; van Veldhuisen, D.J. Advanced glycation end-products (AGEs) and heart failure: Pathophysiology and clinical implications. Eur. J. Heart Fail. 2007, 9, 1146–1155. [Google Scholar] [CrossRef]
- Ramasamy, R.; Yan, S.F.; Schmidt, A.M. Stopping the primal RAGE reaction in myocardial infarction: Capturing adaptive responses to heal the heart? Circulation 2008, 117, 3165–3167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.; Qu, Y.; Wang, R.; Ma, Y.; Xia, C.; Gao, C.; Liu, J.; Lian, K.; Xu, A.; Lu, X.; et al. The alternative crosstalk between RAGE and nitrative thioredoxin inactivation during diabetic myocardial ischemia-reperfusion injury. Am. J. Physiol. Endocrinol. Metab. 2012, 303, E841–E852. [Google Scholar] [CrossRef] [Green Version]
- Fishman, S.L.; Sonmez, H.; Basman, C.; Singh, V.; Poretsky, L. The role of advanced glycation end-products in the development of coronary artery disease in patients with and without diabetes mellitus: A review. Mol. Med. 2018, 24, 59. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, F.; Shi, H.; Gao, Y.; Dong, Z.; Ma, L.; Sun, X.; Li, X.; Chang, S.; Wang, Z.; et al. Neutrophil-derived advanced glycation end products-Nepsilon-(carboxymethyl) lysine promotes RIP3-mediated myocardial necroptosis via RAGE and exacerbates myocardial ischemia/reperfusion injury. FASEB J. 2019, 33, 14410–14422. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Son, M.; Kang, W.C.; Oh, S.; Bayarsaikhan, D.; Ahn, H.; Lee, J.; Park, H.; Lee, S.; Choi, J.; Lee, H.S.; et al. Advanced glycation end-product (AGE)-albumin from activated macrophage is critical in human mesenchymal stem cells survival and post-ischemic reperfusion injury. Sci. Rep. 2017, 7, 11593. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Evens, L.; Belien, H.; Deluyker, D.; Bronckaers, A.; Gervois, P.; Hendrikx, M.; Bito, V. The Impact of Advanced Glycation End-Products (AGEs) on Proliferation and Apoptosis of Primary Stem Cells: A Systematic Review. Stem Cells Int. 2020, 2020, 8886612. [Google Scholar] [CrossRef]
- Evens, L.; Heeren, E.; Rummens, J.L.; Bronckaers, A.; Hendrikx, M.; Deluyker, D.; Bito, V. Advanced Glycation End Products Impair Cardiac Atrial Appendage Stem Cells Properties. J. Clin. Med. 2021, 10, 2964. [Google Scholar] [CrossRef]
- Ramis, R.; Ortega-Castro, J.; Caballero, C.; Casasnovas, R.; Cerrillo, A.; Vilanova, B.; Adrover, M.; Frau, J. How Does Pyridoxamine Inhibit the Formation of Advanced Glycation End Products? The Role of Its Primary Antioxidant Activity. Antioxidants 2019, 8, 344. [Google Scholar] [CrossRef] [Green Version]
- Voziyan, P.A.; Hudson, B.G. Pyridoxamine as a multifunctional pharmaceutical: Targeting pathogenic glycation and oxidative damage. Cell. Mol. Life Sci. 2005, 62, 1671–1681. [Google Scholar] [CrossRef]
- Paul, L.; Ueland, P.M.; Selhub, J. Mechanistic perspective on the relationship between pyridoxal 5’-phosphate and inflammation. Nutr. Rev. 2013, 71, 239–244. [Google Scholar] [CrossRef] [PubMed]
- Lemcke, H.; Voronina, N.; Steinhoff, G.; David, R. Recent Progress in Stem Cell Modification for Cardiac Regeneration. Stem Cells Int. 2018, 2018, 1909346. [Google Scholar] [CrossRef] [PubMed]
- Qiu, H.; Li, W.P.; Shen, X.H.; Guo, X.Y.; Hua, B.; Li, H.W. Dynamic fluctuations of advanced glycation end products and its C-terminal truncated receptor level in patients with acute ST-segment elevation myocardial infarction and undergoing diabetes or not: A retrospective study. Medicine 2018, 97, e11278. [Google Scholar] [CrossRef]
- Greven, W.L.; Smit, J.M.; Rommes, J.H.; Spronk, P.E. Accumulation of advanced glycation end (AGEs) products in intensive care patients: An observational, prospective study. BMC Clin. Pathol. 2010, 10, 4. [Google Scholar] [CrossRef] [Green Version]
- Celec, P.; Hodosy, J.; Jani, P.; Janega, P.; Kudela, M.; Kalousova, M.; Holzerova, J.; Parrak, V.; Halcak, L.; Zima, T.; et al. Advanced glycation end products in myocardial reperfusion injury. Heart Vessel. 2012, 27, 208–215. [Google Scholar] [CrossRef] [PubMed]
- Dwyer, J.P.; Greco, B.A.; Umanath, K.; Packham, D.; Fox, J.W.; Peterson, R.; Broome, B.R.; Greene, L.E.; Sika, M.; Lewis, J.B. Pyridoxamine dihydrochloride in diabetic nephropathy (PIONEER-CSG-17): Lessons learned from a pilot study. Nephron 2015, 129, 22–28. [Google Scholar] [CrossRef] [Green Version]
- Voziyan, P.A.; Hudson, B.G. Pyridoxamine: The many virtues of a maillard reaction inhibitor. Ann. N. Y. Acad. Sci. 2005, 1043, 807–816. [Google Scholar] [CrossRef]
- Williams, M.E. Clinical studies of advanced glycation end product inhibitors and diabetic kidney disease. Curr. Diabetes Rep. 2004, 4, 441–446. [Google Scholar] [CrossRef]
- Deluyker, D.; Ferferieva, V.; Driesen, R.B.; Verboven, M.; Lambrichts, I.; Bito, V. Pyridoxamine improves survival and limits cardiac dysfunction after MI. Sci. Rep. 2017, 7, 16010. [Google Scholar] [CrossRef] [Green Version]
- Borg, D.J.; Forbes, J.M. Targeting advanced glycation with pharmaceutical agents: Where are we now? Glycoconj. J. 2016, 33, 653–670. [Google Scholar] [CrossRef]
- Vagnozzi, R.J.; Maillet, M.; Sargent, M.A.; Khalil, H.; Johansen, A.K.Z.; Schwanekamp, J.A.; York, A.J.; Huang, V.; Nahrendorf, M.; Sadayappan, S.; et al. An acute immune response underlies the benefit of cardiac stem cell therapy. Nature 2020, 577, 405–409. [Google Scholar] [CrossRef]
- Oh, S.; Son, M.; Choi, J.; Lee, S.; Byun, K. sRAGE prolonged stem cell survival and suppressed RAGE-related inflammatory cell and T lymphocyte accumulations in an Alzheimer’s disease model. Biochem. Biophys. Res. Commun. 2018, 495, 807–813. [Google Scholar] [CrossRef] [PubMed]
- Park, M.J.; Lee, S.H.; Moon, S.J.; Lee, J.A.; Lee, E.J.; Kim, E.K.; Park, J.S.; Lee, J.; Min, J.K.; Kim, S.J.; et al. Overexpression of soluble RAGE in mesenchymal stem cells enhances their immunoregulatory potential for cellular therapy in autoimmune arthritis. Sci. Rep. 2016, 6, 35933. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Bayarsaikhan, D.; Arivazhagan, R.; Park, H.; Lim, B.; Gwak, P.; Jeong, G.B.; Lee, J.; Byun, K.; Lee, B. CRISPR/Cas9 Edited sRAGE-MSCs Protect Neuronal Death in Parkinsons Disease Model. Int. J. Stem Cells 2019, 12, 114–124. [Google Scholar] [CrossRef] [PubMed]
- Lang, C.I.; Wolfien, M.; Langenbach, A.; Muller, P.; Wolkenhauer, O.; Yavari, A.; Ince, H.; Steinhoff, G.; Krause, B.J.; David, R.; et al. Cardiac Cell Therapies for the Treatment of Acute Myocardial Infarction: A Meta-Analysis from Mouse Studies. Cell. Physiol. Biochem. 2017, 42, 254–268. [Google Scholar] [CrossRef] [PubMed]
- Biernacka, A.; Dobaczewski, M.; Frangogiannis, N.G. TGF-beta signaling in fibrosis. Growth Factors 2011, 29, 196–202. [Google Scholar] [CrossRef] [Green Version]
- Frangogiannis, N.G. The role of transforming growth factor (TGF)-beta in the infarcted myocardium. J. Thorac. Dis. 2017, 9, S52–S63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Voloshenyuk, T.G.; Landesman, E.S.; Khoutorova, E.; Hart, A.D.; Gardner, J.D. Induction of cardiac fibroblast lysyl oxidase by TGF-beta1 requires PI3K/Akt, Smad3, and MAPK signaling. Cytokine 2011, 55, 90–97. [Google Scholar] [CrossRef] [PubMed]
- Jin, L.; Zhang, J.; Deng, Z.; Liu, J.; Han, W.; Chen, G.; Si, Y.; Ye, P. Mesenchymal stem cells ameliorate myocardial fibrosis in diabetic cardiomyopathy via the secretion of prostaglandin E2. Stem Cell Res. Ther. 2020, 11, 122. [Google Scholar] [CrossRef]
- Richardson, W.J.; Clarke, S.A.; Quinn, T.A.; Holmes, J.W. Physiological Implications of Myocardial Scar Structure. Compr. Physiol. 2015, 5, 1877–1909. [Google Scholar] [CrossRef] [Green Version]
- Kanda, P.; Davis, D.R. Cellular mechanisms underlying cardiac engraftment of stem cells. Expert Opin. Biol. Ther. 2017, 17, 1127–1143. [Google Scholar] [CrossRef]
- Baraniak, P.R.; McDevitt, T.C. Stem cell paracrine actions and tissue regeneration. Regen. Med. 2010, 5, 121–143. [Google Scholar] [CrossRef] [Green Version]
- Wu, S.Z.; Li, Y.L.; Huang, W.; Cai, W.F.; Liang, J.; Paul, C.; Jiang, L.; Wu, Z.C.; Xu, M.; Zhu, P.; et al. Paracrine effect of CXCR4-overexpressing mesenchymal stem cells on ischemic heart injury. Cell Biochem. Funct. 2017, 35, 113–123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dulak, J.; Szade, K.; Szade, A.; Nowak, W.; Jozkowicz, A. Adult stem cells: Hopes and hypes of regenerative medicine. Acta Biochim. Pol. 2015, 62, 329–337. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zacchigna, S.; Paldino, A.; Falcao-Pires, I.; Daskalopoulos, E.P.; Dal Ferro, M.; Vodret, S.; Lesizza, P.; Cannata, A.; Miranda-Silva, D.; Lourenco, A.P.; et al. Towards standardization of echocardiography for the evaluation of left ventricular function in adult rodents: A position paper of the ESC Working Group on Myocardial Function. Cardiovasc. Res. 2021, 117, 43–59. [Google Scholar] [CrossRef] [PubMed]
- Haesen, S.; Col, U.; Schurgers, W.; Evens, L.; Verboven, M.; Driesen, R.B.; Bronckaers, A.; Lambrichts, I.; Deluyker, D.; Bito, V. Glycolaldehyde-modified proteins cause adverse functional and structural aortic remodeling leading to cardiac pressure overload. Sci. Rep. 2020, 10, 12220. [Google Scholar] [CrossRef] [PubMed]
- Deluyker, D.; Evens, L.; Belien, H.; Bito, V. Acute exposure to glycated proteins reduces cardiomyocyte contractile capacity. Exp. Physiol. 2019, 104, 997–1003. [Google Scholar] [CrossRef]
- Deluyker, D.; Evens, L.; Haesen, S.; Driesen, R.B.; Kuster, D.; Verboven, M.; Belien, H.; van der Velden, J.; Lambrichts, I.; Bito, V. Glycolaldehyde-Derived High-Molecular-Weight Advanced Glycation End-Products Induce Cardiac Dysfunction through Structural and Functional Remodeling of Cardiomyocytes. Cell. Physiol. Biochem. 2020, 54, 809–824. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [Green Version]
- Deluyker, D.; Ferferieva, V.; Noben, J.P.; Swennen, Q.; Bronckaers, A.; Lambrichts, I.; Rigo, J.M.; Bito, V. Cross-linking versus RAGE: How do high molecular weight advanced glycation products induce cardiac dysfunction? Int. J. Cardiol. 2016, 210, 100–108. [Google Scholar] [CrossRef]
- Derveaux, S.; Vandesompele, J.; Hellemans, J. How to do successful gene expression analysis using real-time PCR. Methods 2010, 50, 227–230. [Google Scholar] [CrossRef] [PubMed]
- Bustin, S.A.; Benes, V.; Garson, J.A.; Hellemans, J.; Huggett, J.; Kubista, M.; Mueller, R.; Nolan, T.; Pfaffl, M.W.; Shipley, G.L.; et al. The MIQE guidelines: Minimum information for publication of quantitative real-time PCR experiments. Clin. Chem. 2009, 55, 611–622. [Google Scholar] [CrossRef] [PubMed] [Green Version]





| Parameters | 4 Weeks Post-Operative | |||
|---|---|---|---|---|
| SHAM | MI | MI + CASCs | MI + CASCs + PM | |
| EF (%) | 80 ± 4 | 59 ± 4 ## | 79 ± 3 *** | 72 ± 3 |
| HR (bpm) | 333 ± 14 | 318 ± 12 | 332 ± 12 | 327 ± 13 |
| SV (µL) | 170 ± 16 | 162 ± 17 | 172 ± 17 | 192 ± 17 |
| CO (mL/min) | 57 ± 4 | 51 ± 5 | 59 ± 6 | 65 ± 6 |
| EDV (µL) | 215 ± 26 | 298 ± 51 | 217 ± 17 | 267 ± 22 |
| ESV (µL) | 45 ± 13 | 136 ± 36 | 44 ± 5 * | 77 ± 11 |
| AWT (mm) | 1.74 ± 0.13 | 1.55 ± 0.16 | 1.65 ± 0.12 | 1.80 ± 0.16 |
| PWT (mm) | 1.54 ± 0.16 | 1.63 ± 0.15 | 1.57 ± 0.09 | 1.62 ± 0.17 |
| Parameters | 4 Weeks Post-Operative | |||
|---|---|---|---|---|
| SHAM | MI | MI + CASCs | MI + CASCs + PM | |
| Max LV pressure (mmHg) | 99 ± 3 | 90 ± 2 | 96 ± 3 | 103 ± 4 * |
| dP/dtmax (mmHg/s) | 6773 ± 529 | 6038 ± 242 | 6923 ± 340 | 6551 ± 257 |
| dP/dtmin (mmHg/s) | −7269 ± 683 | −6550 ± 705 | −6816 ± 354 | −6917 ± 273 |
| Tau (s) | 0.0130 ± 0.001 | 0.0499 ± 0.017 | 0.0148 ± 0.002 | 0.0117 ± 0.001 ** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Evens, L.; Beliën, H.; D’Haese, S.; Haesen, S.; Verboven, M.; Rummens, J.-L.; Bronckaers, A.; Hendrikx, M.; Deluyker, D.; Bito, V. Combinational Therapy of Cardiac Atrial Appendage Stem Cells and Pyridoxamine: The Road to Cardiac Repair? Int. J. Mol. Sci. 2021, 22, 9266. https://doi.org/10.3390/ijms22179266
Evens L, Beliën H, D’Haese S, Haesen S, Verboven M, Rummens J-L, Bronckaers A, Hendrikx M, Deluyker D, Bito V. Combinational Therapy of Cardiac Atrial Appendage Stem Cells and Pyridoxamine: The Road to Cardiac Repair? International Journal of Molecular Sciences. 2021; 22(17):9266. https://doi.org/10.3390/ijms22179266
Chicago/Turabian StyleEvens, Lize, Hanne Beliën, Sarah D’Haese, Sibren Haesen, Maxim Verboven, Jean-Luc Rummens, Annelies Bronckaers, Marc Hendrikx, Dorien Deluyker, and Virginie Bito. 2021. "Combinational Therapy of Cardiac Atrial Appendage Stem Cells and Pyridoxamine: The Road to Cardiac Repair?" International Journal of Molecular Sciences 22, no. 17: 9266. https://doi.org/10.3390/ijms22179266
APA StyleEvens, L., Beliën, H., D’Haese, S., Haesen, S., Verboven, M., Rummens, J. -L., Bronckaers, A., Hendrikx, M., Deluyker, D., & Bito, V. (2021). Combinational Therapy of Cardiac Atrial Appendage Stem Cells and Pyridoxamine: The Road to Cardiac Repair? International Journal of Molecular Sciences, 22(17), 9266. https://doi.org/10.3390/ijms22179266