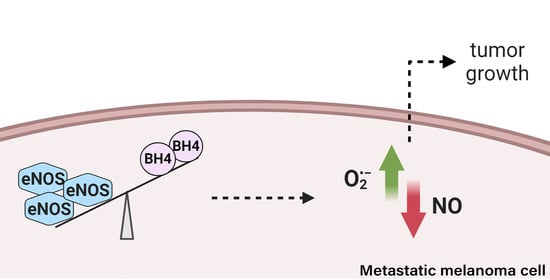
Metastatic Melanoma Progression Is Associated with Endothelial Nitric Oxide Synthase Uncoupling Induced by Loss of eNOS:BH4 Stoichiometry
Abstract
:1. Introduction
2. Results
2.1. Increased Expression of de Novo Tetrahydrobiopterin Biosynthesis Enzymes during Melanoma Progression
2.2. Tetrahydrobiopterin Concentration Is Elevated in Melanoma Cells
2.3. Increased Expression of Endothelial Nitric Oxide Synthase in Metastatic Melanoma Cells
2.4. Downregulation of eNOS Protein Restores Its Function
2.5. Decreased Expression and Coupling of eNOS in the Presence of a Superoxide Scavenger
2.6. L-Sepiapterin Supplementation Decreases Superoxide Anion and Increases Nitric Oxide Levels
2.7. Restoration of eNOS Function Reduced Cell Survival of Metastatic Melanoma Cells
2.8. Downregulation of eNOS Attenuated Metastatic Melanoma Growth In Vivo
2.9. L-Sepiapterin Supplementation Inhibits the Growth of Metastatic Melanoma Cells
2.10. Increased NOS3 Expression in Human Metastatic Compared with Primary Melanomas
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Stable Silencing of eNOS by shRNA
4.3. Nitric Oxide Quantification
4.4. Superoxide Anion Quantification
4.5. Biopterin Quantification
4.6. RNAm Expression Analysis
4.7. Western Blot
4.8. MTT Assay
4.9. Colony Formation Assay
4.10. Anoikis Resistance Assay
4.11. Tumor Growth Assay
4.12. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Lo, J.A.; Fisher, D.E. The Melanoma Revolution: From UV Carcinogenesis to a New Era in Therapeutics. Science 2014, 346, 945–949. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rastrelli, M.; Tropea, S.; Rossi, C.R.; Alaibac, M. Melanoma: Epidemiology, Risk Factors, Pathogenesis, Diagnosis and Classification. In Vivo 2014, 28, 1005–1011. [Google Scholar] [PubMed]
- Curti, B.D.; Faries, M.B. Recent Advances in the Treatment of Melanoma. N. Engl. J. Med. 2021, 89, 73–74. [Google Scholar] [CrossRef]
- Strashilov, S.; Yordanov, A. Aetiology and Pathogenesis of Cutaneous Melanoma: Current Concepts and Advances. Int. J. Mol. Sci. 2021, 22, 6395. [Google Scholar] [CrossRef]
- Campos, A.C.E.; Molognoni, F.; Melo, F.H.M.; Galdieri, L.C.; Carneiro, C.R.W.; D’Almeida, V.; Correa, M.; Jasiulionis, M.G. Oxidative Stress Modulates DNA Methylation during Melanocyte Anchorage Blockade Associated with Malignant Transformation. Neoplasia 2007, 9, 1111–1121. [Google Scholar] [CrossRef] [Green Version]
- Melo, F.H.M.; Molognoni, F.; Morais, A.S.; Toricelli, M.; Mouro, M.G.; Higa, E.M.S.; Lopes, J.D.; Jasiulionis, M.G. Endothelial Nitric Oxide Synthase Uncoupling as a Key Mediator of Melanocyte Malignant Transformation Associated with Sustained Stress Conditions. Free Radic. Biol. Med. 2011, 50, 1263–1273. [Google Scholar] [CrossRef] [Green Version]
- Molognoni, F.; De Melo, F.H.M.; Da Silva, C.T.; Jasiulionis, M.G. Ras and Rac1, Frequently Mutated in Melanomas, Are Activated by Superoxide Anion, Modulate Dnmt1 Level and Are Causally Related to Melanocyte Malignant Transformation. PLoS ONE 2013, 8, e81937. [Google Scholar] [CrossRef] [Green Version]
- Bisevac, J.P.; Djukic, M.; Stanojevic, I.; Stevanovic, I.; Mijuskovic, Z.; Djuric, A.; Gobeljic, B.; Banovic, T.; Vojvodic, D. Association between Oxidative Stress and Melanoma Progression. J. Med. Biochem. 2018, 37, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, D.A.; Xisto, R.; Gonçalves, J.D.; da Silva, D.B.; Moura Soares, J.P.; Icimoto, M.Y.; Sant’Anna, C.; Gimenez, M.; de Angelis, K.; Llesuy, S.; et al. Imbalance between Nitric Oxide and Superoxide Anion Induced by Uncoupled Nitric Oxide Synthase Contributes to Human Melanoma Development. Int. J. Biochem. Cell Biol. 2019, 115, 105592. [Google Scholar] [CrossRef]
- Alves-Fernandes, D.K.; Jasiulionis, M.G. The Role of SIRT1 on DNA Damage Response and Epigenetic Alterations in Cancer. Int. J. Mol. Sci. 2019, 20, 3153. [Google Scholar] [CrossRef] [Green Version]
- Ng, C.Y.; Yen, H.; Hsiao, H.-Y.; Su, S.-C. Phytochemicals in Skin Cancer Prevention and Treatment: An Updated Review. Int. J. Mol. Sci. 2018, 19, 941. [Google Scholar] [CrossRef] [Green Version]
- Meitzler, J.L.; Makhlouf, H.R.; Antony, S.; Wu, Y.; Butcher, D.; Jiang, G.; Juhasz, A.; Lu, J.; Dahan, I.; Jansen-Dürr, P.; et al. Decoding NADPH Oxidase 4 Expression in Human Tumors. Redox Biol. 2017, 13, 182–195. [Google Scholar] [CrossRef]
- Chong, S.J.F.; Lai, J.X.H.; Qu, J.; Hirpara, J.; Kang, J.; Swaminathan, K.; Loh, T.; Kumar, A.; Vali, S.; Abbasi, T.; et al. A Feedforward Relationship between Active Rac1 and Phosphorylated Bcl-2 Is Critical for Sustaining Bcl-2 Phosphorylation and Promoting Cancer Progression. Cancer Lett. 2019, 457, 151–167. [Google Scholar] [CrossRef] [PubMed]
- Aminzadeh-gohari, S.; Weber, D.D.; Catalano, L.; Feichtinger, R.G.; Kofler, B.; Lang, R. Targeting Mitochondria in Melanoma. Biomolecules 2020, 10, 1395. [Google Scholar] [CrossRef]
- Peng, H.; Zhuang, Y.; Chen, Y.; Rizzo, A.N.; Chen, W. The Characteristics and Regulatory Mechanisms of Superoxide Generation from ENOS Reductase Domain. PLoS ONE 2015, 10, e0140365. [Google Scholar] [CrossRef] [PubMed]
- Yuyun, M.F.; Ng, L.L.; Ng, G.A. Endothelial Dysfunction, Endothelial Nitric Oxide Bioavailability, Tetrahydrobiopterin, and 5-Methyltetrahydrofolate in Cardiovascular Disease. Where Are We with Therapy? Microvasc. Res. 2018, 119, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Takata, T.; Araki, S.; Tsuchiya, Y.; Watanabe, Y. Oxidative Stress Orchestrates Mapk and Nitric-Oxide Synthase Signal. Int. J. Mol. Sci. 2020, 21, 8750. [Google Scholar] [CrossRef]
- Alp, N.J.; Channon, K.M. Regulation of Endothelial Nitric Oxide Synthase by Tetrahydrobiopterin in Vascular Disease. Arterioscler. Thromb. Vasc. Biol. 2004, 24, 413–420. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.K.; Han, J. Tetrahydrobiopterin in Energy Metabolism and Metabolic Diseases. Pharmacol. Res. 2020, 157, 104827. [Google Scholar] [CrossRef]
- Bendall, J.K.; Alp, N.J.; Warrick, N.; Cai, S.; Adlam, D.; Rockett, K.; Yokoyama, M.; Kawashima, S.; Channon, K.M. Stoichiometric Relationships between Endothelial Tetrahydrobiopterin, Endothelial NO Synthese (ENOS) Activity, and ENOS Coupling in Vivo: Insights from Transgenic Mice with Endothelial-Targeted GTP Cyclohydrolase 1 and ENOS Overexpression. Circ. Res. 2005, 97, 864–871. [Google Scholar] [CrossRef]
- Crabtree, M.J.; Tatham, A.L.; Al-Wakeel, Y.; Warrick, N.; Hale, A.B.; Cai, S.; Channon, K.M.; Alp, N.J. Quantitative Regulation of Intracellular Endothelial Nitric-Oxide Synthase (ENOS) Coupling by Both Tetrahydrobiopterin-ENOS Stoichiometry and Biopterin Redox Status Insights from Cells with TET-Regulated GTP Cyclohydrolasei Expression. J. Biol. Chem. 2009, 284, 1136–1144. [Google Scholar] [CrossRef] [Green Version]
- Tu, Y.T.; Tao, J.; Liu, Y.Q.; Li, Y.; Huang, C.Z.; Zhang, X.B.; Lin, Y. Expression of Endothelial Nitric Oxide Synthase and Vascular Endothelial Growth Factor in Human Malignant Melanoma and Their Relation to Angiogenesis. Clin. Exp. Dermatol. 2006, 31, 413–418. [Google Scholar] [CrossRef]
- Li, C.; Hu, Z.; Liu, Z.; Wang, L.E.; Gershenwald, J.E.; Lee, J.E.; Prieto, V.G.; Duvic, M.; Grimm, E.A.; Wei, Q. Polymorphisms of the Neuronal and Inducible Nitric Oxide Synthase Genes and the Risk of Cutaneous Melanoma: A Case-Control Study. Cancer 2007, 109, 1570–1578. [Google Scholar] [CrossRef]
- Thomas, D.D.; Wink, D.A. NOS2 as an Emergent Player in Progression of Cancer. Antioxidants Redox Signal. 2017, 26, 963–965. [Google Scholar] [CrossRef] [PubMed]
- Ekmekcioglu, S.; Ellerhorst, J.; Smid, C.M.; Prieto, V.G.; Munsell, M.; Buzaid, A.C.; Grimm, E.A. Inducible Nitric Oxide Synthase and Nitrotyrosine in Human Metastatic Melanoma Tumors Correlate with Poor Survival. Clin. Cancer Res. 2000, 6, 4768–4775. [Google Scholar] [PubMed]
- Ekmekcioglu, S.; Ellerhorst, J.A.; Prieto, V.G.; Johnson, M.M.; Broemeling, L.D.; Grimm, E.A. Tumor INOS Predicts Poor Survival for Stage III Melanoma Patients. Int. J. Cancer 2006, 119, 861–866. [Google Scholar] [CrossRef]
- Ahmed, B.; Van Den Oord, J.J. Expression of the Neuronal Isoform of Nitric Oxide Synthase (NNOS) and Its Inhibitor, Protein Inhibitor of NNOS, in Pigment Cell Lesions of the Skin. Br. J. Dermatol. 1999, 141, 12–19. [Google Scholar] [CrossRef]
- Oba-Shinjo, S.M.; Correa, M.; Ricca, T.I.; Molognoni, F.; Pinhal, M.A.; Neves, I.A.; Marie, S.K.; Sampaio, L.O.; Nader, H.B.; Chammas, R.; et al. Melanocyte Transformation Associated with Substrate Adhesion Impediment. Neoplasia 2006, 8, 231–241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, Z.; Kang, B.; Li, C.; Chen, T.; Zhang, Z. GEPIA2: An Enhanced Web Server for Large-Scale Expression Profiling and Interactive Analysis. Nucleic Acids Res. 2019, 47, W556. [Google Scholar] [CrossRef] [Green Version]
- Talantov, D.; Mazumder, A.; Yu, J.X.; Briggs, T.; Jiang, Y.; Backus, J.; Atkins, D.; Wang, Y. Novel Genes Associated with Malignant Melanoma but Not Benign Melanocytic Lesions. Clin. Cancer Res. 2005, 11, 7234–7242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gesierich, A.; Niroomand, F.; Tiefenbacher, C.P. Role of Human GTP Cyclohydrolase I and Its Regulatory Protein in Tetrahydrobiopterin Metabolism. Basic Res. Cardiol. 2003, 98, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Tatham, A.L.; Crabtree, M.J.; Warrick, N.; Cai, S.; Alp, N.J.; Channon, K.M. GTP Cyclohydrolase I Expression, Protein, and Activity Determine Intracellular Tetrahydrobiopterin Levels, Independent of GTP Cyclohydrolase Feedback Regulatory Protein Expression. J. Biol. Chem. 2009, 284, 13660–13668. [Google Scholar] [CrossRef] [Green Version]
- Zhen, J.; Lu, H.; Wang, X.Q.; Vaziri, N.D.; Zhou, X.J. Upregulation of Endothelial and Inducible Nitric Oxide Synthase Expression by Reactive Oxygen Species. Am. J. Hypertens. 2008, 21, 28–34. [Google Scholar] [CrossRef] [Green Version]
- Ji, X.W.; Lyu, H.J.; Zhou, G.H.; Wu, B.; Zhu, Y.Y.; Wu, T.H.; Zhang, F.; Jin, S.N.; Cho, K.W.; Wen, J.F. Physcion, a Tetra-Substituted 9,10-Anthraquinone, Prevents Homocysteine-Induced Endothelial Dysfunction by Activating Ca2+- and Akt-ENOS-NO Signaling Pathways. Phytomedicine 2021, 81, 153410. [Google Scholar] [CrossRef] [PubMed]
- Ozaki, M.; Kawashima, S.; Yamashita, T.; Hirase, T.; Namiki, M.; Inoue, N.; Hirata, K.; Yasui, H.; Sakurai, H.; Yoshida, Y.; et al. Overexpression of Endothelial Nitric Oxide Synthase Accelerates Atherosclerotic Lesion Formation in ApoE-Deficient Mice. J. Clin. Invest. 2002, 110, 331–340. [Google Scholar] [CrossRef] [PubMed]
- Riker, A.I.; Enkemann, S.A.; Fodstad, O.; Liu, S.; Ren, S.; Morris, C.; Xi, Y.; Howell, P.; Metge, B.; Samant, R.S.; et al. The Gene Expression Profiles of Primary and Metastatic Melanoma Yields a Transition Point of Tumor Progression and Metastasis. BMC Med. Genom. 2008, 1, 13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thöny, B.; Auerbach, G.; Blau, N. Tetrahydrobiopterin Biosynthesis, Regeneration and Functions. Biochem. J. 2000, 347, 1–16. [Google Scholar] [CrossRef]
- Katusic, Z.S.; Stelter, A.; Milstien, S. Cytokines Stimulate GTP Cyclohydrolase I Gene Expression in Cultured Human Umbilical Vein Endothelial Cells. Arterioscler. Thromb. Vasc. Biol. 1998, 18, 27–32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shimizu, S.; Hiroi, T.; Ishii, M.; Hagiwara, T.; Wajima, T.; Miyazaki, A.; Kiuchi, Y. Hydrogen Peroxide Stimulates Tetrahydrobiopterin Synthesis through Activation of the Jak2 Tyrosine Kinase Pathway in Vascular Endothelial Cells. Int. J. Biochem. Cell Biol. 2008, 40, 755–765. [Google Scholar] [CrossRef]
- Chen, L.; Zeng, X.; Kleibeuker, E.; Buffa, F.; Barberis, A.; Leek, R.D.; Roxanis, I.; Zhang, W.; Worth, A.; Beech, J.S.; et al. Paracrine Effect of GTP Cyclohydrolase and Angiopoietin-1 Interaction in Stromal Fibroblasts on Tumor Tie2 Activation and Breast Cancer Growth. Oncotarget 2016, 7, 9353–9367. [Google Scholar] [CrossRef] [Green Version]
- Tran, A.N.; Walker, K.; Harrison, D.G.; Chen, W.; Mobley, J.; Hocevar, L.; Hackney, J.R.; Sedaka, R.S.; Pollock, J.S.; Goldberg, M.S.; et al. Reactive Species Balance via GTP Cyclohydrolase i Regulates Glioblastoma Growth and Tumor Initiating Cell Maintenance. Neuro. Oncol. 2018, 20, 1055–1067. [Google Scholar] [CrossRef]
- Chavan, B.; Gillbro, J.M.; Rokos, H.; Schallreuter, K.U. GTP Cyclohydrolase Feedback Regulatory Protein Controls Cofactor 6-Tetrahydrobiopterin Synthesis in the Cytosol and in the Nucleus of Epidermal Keratinocytes and Melanocytes. J. Investig. Dermatol. 2006, 126, 2481–2489. [Google Scholar] [CrossRef] [Green Version]
- Kalivendi, S.; Hatakeyama, K.; Whitsett, J.; Konorev, E.; Kalyanaraman, B.; Vásquez-Vivar, J. Changes in Tetrahydrobiopterin Levels in Endothelial Cells and Adult Cardiomyocytes Induced by LPS and Hydrogen Peroxide—A Role for GFRP? Free Radic. Biol. Med. 2005, 38, 481–491. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Zheng, K.; Ma, C.; Li, J.; Zhuo, L.; Huang, W.; Chen, T.; Jiang, Y. PTPS Facilitates Compartmentalized LTBP1 S-Nitrosylation and Promotes Tumor Growth under Hypoxia. Mol. Cell 2020, 77, 95.e5–107.e5. [Google Scholar] [CrossRef] [PubMed]
- De Souza, C.F.; Xander, P.; Monteiro, A.C.; dos Santos Silva, A.G.; da Silva, D.C.P.; Mai, S.; Bernardo, V.; Lopes, J.D.; Jasiulionis, M.G. Mining Gene Expression Signature for the Detection of Pre-Malignant Melanocytes and Early Melanomas with Risk for Metastasis. PLoS ONE 2012, 7, e44800. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lange, I.; Geerts, D.; Feith, D.J.; Mocz, G.; Koster, J.; Bachmann, A.S. Novel Interaction of Ornithine Decarboxylase with Sepiapterin Reductase Regulates Neuroblastoma Cell Proliferation. J. Mol. Biol. 2014, 426, 332–346. [Google Scholar] [CrossRef] [Green Version]
- Wu, Y.; Chen, P.; Sun, L.; Yuan, S.; Cheng, Z.; Lu, L.; Du, H.; Zhan, M. Sepiapterin Reductase: Characteristics and Role in Diseases. J. Cell. Mol. Med. 2020, 24, 9495–9506. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, Y.; Wang, K.; Tang, J.; Chen, Y.; Jin, G.; Liu, X. The Knockdown of the Sepiapterin Reductase Gene Suppresses the Proliferation of Breast Cancer by Inducing ROS-Mediated Apoptosis. Int. J. Clin. Exp. Pathol. 2020, 13, 2228–2239. [Google Scholar]
- Harada, T.; Kagamiyama, H.; Hatakeyama, K. Feedback Regulation Mechanisms for the Control of GTP Cyclohydrolase I Activity. Science 1993, 260, 1507–1510. [Google Scholar] [CrossRef]
- Vásquez-Vivar, J. Tetrahydrobiopterin, Superoxide and Vascular Dysfunction. Free Radic. Biol. Med. 2009, 47, 1108. [Google Scholar] [CrossRef] [Green Version]
- Crabtree, M.J.; Smith, C.L.; Lam, G.; Goligorsky, M.S.; Gross, S.S. Ratio of 5,6,7,8-Tetrahydrobiopterin to 7,8-Dihydrobiopterin in Endothelial Cells Determines Glucose-Elicited Changes in NO vs. Superoxide Production by ENOS. Am. J. Physiol. Heart Circ. Physiol. 2008, 294. [Google Scholar] [CrossRef] [Green Version]
- Lim, K.H.; Ancrile, B.B.; Kashatus, D.F.; Counter, C.M. Tumour Maintenance Is Mediated by ENOS. Nature 2008, 452, 646–649. [Google Scholar] [CrossRef] [Green Version]
- Lampson, B.L.; Kendall, S.D.S.; Ancrile, B.B.; Morrison, M.M.; Shealy, M.J.; Barrientos, K.S.; Crowe, M.S.; Kashatus, D.F.; White, R.R.; Gurley, S.B.; et al. Targeting ENOS in Pancreatic Cancer. Cancer Res. 2012, 72, 4472–4482. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hodis, E.; Watson, I.R.; Kryukov, G.V.; Arold, S.T.; Imielinski, M.; Theurillat, J.P.; Nickerson, E.; Auclair, D.; Li, L.; Place, C.; et al. A Landscape of Driver Mutations in Melanoma. Cell 2012, 150, 251–263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Montor, W.R.; Salas, A.R.O.S.E.; de Melo, F.H.M. Receptor Tyrosine Kinases and Downstream Pathways as Druggable Targets for Cancer Treatment: The Current Arsenal of Inhibitors. Mol. Cancer 2018, 17, 55. [Google Scholar] [CrossRef] [PubMed]
- Paluncic, J.; Kovacevic, Z.; Jansson, P.J.; Kalinowski, D.; Merlot, A.M.; Huang, M.L.H.; Lok, H.C.; Sahni, S.; Lane, D.J.R.; Richardson, D.R. Roads to Melanoma: Key Pathways and Emerging Players in Melanoma Progression and Oncogenic Signaling. Biochim. Biophys. Acta Mol. Cell Res. 2016, 1863, 770–784. [Google Scholar] [CrossRef]
- Barbieri, A.; Palma, G.; Rosati, A.; Giudice, A.; Falco, A.; Petrillo, A.; Petrillo, M.; Bimonte, S.; Di Benedetto, M.; Esposito, G.; et al. Role of Endothelial Nitric Oxide Synthase (ENOS) in Chronic Stress-Promoted Tumour Growth. J. Cell. Mol. Med. 2012, 16, 920–926. [Google Scholar] [CrossRef]
- Teng, R.J.; Du, J.; Xu, H.; Bakhutashvili, I.; Eis, A.; Shi, Y.; Pritchard, K.A.; Konduri, G.G. Sepiapterin Improves Angiogenesis of Pulmonary Artery Endothelial Cells with in Utero Pulmonary Hypertension by Recoupling Endothelial Nitric Oxide Synthase. Am. J. Physiol. Lung Cell. Mol. Physiol. 2011, 301, 334–345. [Google Scholar] [CrossRef] [Green Version]
- Cardnell, R.J.G.; Rabender, C.S.; Ross, G.R.; Guo, C.; Howlett, E.L.; Alam, A.; Wang, X.Y.; Akbarali, H.I.; Mikkelsen, R.B. Sepiapterin Ameliorates Chemically Induced Murine Colitis and Azoxymethane-Induced Colon Cancer. J. Pharmacol. Exp. Ther. 2013, 347, 117–125. [Google Scholar] [CrossRef] [Green Version]
- Rabender, C.S.; Alam, A.; Sundaresan, G.; Cardnell, R.J.; Yakovlev, V.A.; Mukhopadhyay, N.D.; Graves, P.; Zweit, J.; Mikkelsen, R.B. The Role of Nitric Oxide Synthase Uncoupling in Tumor Progression. Mol. Cancer Res. 2015, 13, 1034–1043. [Google Scholar] [CrossRef] [Green Version]
- Fernandes, D.C.; Wosniak, J.; Pescatore, L.A.; Bertoline, M.A.; Liberman, M.; Laurindo, F.R.M.; Santos, C.X.C. Analysis of DHE-Derived Oxidation Products by HPLC in the Assessment of Superoxide Production and NADPH Oxidase Activity in Vascular Systems. Am. J. Physiol. Cell Physiol. 2007, 292. [Google Scholar] [CrossRef] [PubMed]
- Marinos, R.S.; Zhang, W.; Wu, G.; Kelly, K.A.; Meininger, C.J. Tetrahydrobiopterin Levels Regulate Endothelial Cell Proliferation. Am. J. Physiol. Hear. Circ. Physiol. 2001, 281, H482–H489. [Google Scholar] [CrossRef] [PubMed]










Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Melo, F.H.M.d.; Gonçalves, D.A.; Sousa, R.X.d.; Icimoto, M.Y.; Fernandes, D.d.C.; Laurindo, F.R.M.; Jasiulionis, M.G. Metastatic Melanoma Progression Is Associated with Endothelial Nitric Oxide Synthase Uncoupling Induced by Loss of eNOS:BH4 Stoichiometry. Int. J. Mol. Sci. 2021, 22, 9556. https://doi.org/10.3390/ijms22179556
Melo FHMd, Gonçalves DA, Sousa RXd, Icimoto MY, Fernandes DdC, Laurindo FRM, Jasiulionis MG. Metastatic Melanoma Progression Is Associated with Endothelial Nitric Oxide Synthase Uncoupling Induced by Loss of eNOS:BH4 Stoichiometry. International Journal of Molecular Sciences. 2021; 22(17):9556. https://doi.org/10.3390/ijms22179556
Chicago/Turabian StyleMelo, Fabiana Henriques Machado de, Diego Assis Gonçalves, Ricardo Xisto de Sousa, Marcelo Yudi Icimoto, Denise de Castro Fernandes, Francisco R. M. Laurindo, and Miriam Galvonas Jasiulionis. 2021. "Metastatic Melanoma Progression Is Associated with Endothelial Nitric Oxide Synthase Uncoupling Induced by Loss of eNOS:BH4 Stoichiometry" International Journal of Molecular Sciences 22, no. 17: 9556. https://doi.org/10.3390/ijms22179556
APA StyleMelo, F. H. M. d., Gonçalves, D. A., Sousa, R. X. d., Icimoto, M. Y., Fernandes, D. d. C., Laurindo, F. R. M., & Jasiulionis, M. G. (2021). Metastatic Melanoma Progression Is Associated with Endothelial Nitric Oxide Synthase Uncoupling Induced by Loss of eNOS:BH4 Stoichiometry. International Journal of Molecular Sciences, 22(17), 9556. https://doi.org/10.3390/ijms22179556