Sodium 4-Phenylbutyrate Reduces Ocular Hypertension by Degrading Extracellular Matrix Deposition via Activation of MMP9
Abstract
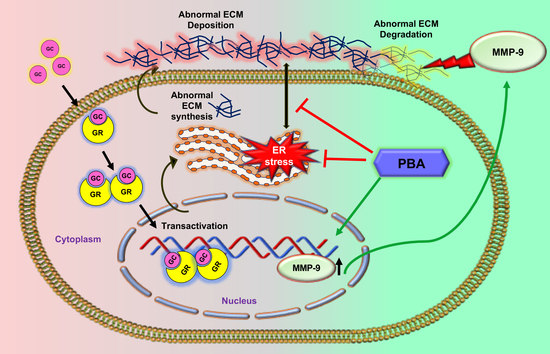
:1. Introduction
2. Results
2.1. PBA Reduces Dexamethasone 21-Acetate (Dex)-Induced OHT and Decreases ECM Deposition and ER Stress in TM Tissue
2.2. PBA Reduces Dex-Induced Synthesis of ECM Proteins in Primary Human TM Cells
2.3. PBA Reduces Dex Induced ECM Deposition in Primary Human TM Cells
2.4. PBA Prevents Abnormal ECM Induced ER Stress in Primary Human TM Cells
2.5. PBA Rescues Primary TM Cells from Exogenous Cellular Fibronectin (cFN) Induced ER Stress
2.6. PBA Degrades ECM by Upregulating MMP9 Gene and Protein Expression
2.7. Inhibition of MMPs Abrogates PBA’s Effect on ECM Degradation and Its Associated ER Stress
2.8. Effect of PBA on Dex Induced ECM Deposition and ER Stress in Ex Vivo Cultured Human Corneoscleral Segment Tissue
3. Discussion
4. Methods and Materials
4.1. Experimental Animals
4.2. Antibodies and Reagents
4.3. GC-Induced Mouse Model of OHT
4.4. IOP Measurements
4.5. Topical Ocular Eye Drops of PBA
4.6. Human Primary TM Cells
4.7. Exogenous Cellular Fibronectin (cFN) Treatment
4.8. Ex Vivo Human Corneoscleral Segments Cultures
4.9. Decellularization
4.10. Immunostaining
Primary Human TM Cells
4.11. Western Blot Analysis
4.12. Gelatin Zymography
4.13. Quantitative Real Time Polymerase Chain Reaction (qPCR) Analysis
4.14. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Overby, D.R.; Clark, A.F. Animal models of glucocorticoid-induced glaucoma. Exp. Eye Res. 2015, 141, 15–22. [Google Scholar] [CrossRef] [Green Version]
- Feroze, K.B.; Khazaeni, L. Steroid Induced Glaucoma; StatPearls: Treasure Island, FL, USA, 2021. [Google Scholar]
- Tripathi, R.C.; Parapuram, S.K.; Tripathi, B.J.; Zhong, Y.; Chalam, K.V. Corticosteroids and Glaucoma Risk. Drugs Aging 1999, 15, 439–450. [Google Scholar] [CrossRef]
- van Boxtel, L.A.; Hardus, P.L.; Al Hassan, W.S.; van Voorst Vader, P.C.; Jansonium, N.M. Corticosteroids and the risk of glaucoma. Ned Tijdschr Geneeskd. 2005, 149, 2485–2489. [Google Scholar] [PubMed]
- Jones, R., III; Rhee, D.J. Corticosteroid-induced ocular hypertension and glaucoma: A brief review and update of the literature. Curr. Opin. Ophthalmol. 2006, 17, 163–167. [Google Scholar] [PubMed]
- Kersey, J.P.; Broadway, D.C. Corticosteroid-induced glaucoma: A review of the literature. Eye 2006, 20, 407–416. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roberti, G.; Oddone, F.; Agnifili, L.; Katsanos, A.; Michelessi, M.; Mastropasqua, L.; Quaranta, L.; Riva, I.; Tanga, L.; Manni, G. Steroid-induced glaucoma: Epidemiology, pathophysiology, and clinical management. Surv. Ophthalmol. 2020, 65, 458–472. [Google Scholar] [CrossRef]
- Armaly, M.F. Effect of Corticosteroids on Intraocular Pressure and Fluid Dynamics. I. The Effect of Dexamethasone in the Normal Eye. Arch. Ophthalmol. 1963, 70, 482–491. [Google Scholar] [CrossRef]
- Armaly, M.F. Effect of Corticosteroids on Intraocular Pressure and Fluid Dynamics. Ii. The Effect of Dexamethasone in the Glaucomatous Eye. Arch. Ophthalmol. 1963, 70, 492–499. [Google Scholar] [CrossRef]
- Spaeth, G.L.; Rodrigues, M.M.; Weinreb, S. Steroid-induced glaucoma: A. Persistent elevation of intraocular pressure B. Histopathological aspects. Trans. Am. Ophthalmol. Soc. 1977, 75, 353–381. [Google Scholar]
- Maddineni, P.; Kasetti, R.B.; Patel, P.D.; Millar, J.C.; Kiehlbauch, C.; Clark, A.F.; Zode, G.S. CNS axonal degeneration and transport deficits at the optic nerve head precede structural and functional loss of retinal ganglion cells in a mouse model of glaucoma. Mol. Neurodegener. 2020, 15, 1–20. [Google Scholar] [CrossRef]
- Kasetti, R.B.; Maddineni, P.; Millar, J.C.; Clark, A.F.; Zode, G.S. Increased synthesis and deposition of extracellular matrix proteins leads to endoplasmic reticulum stress in the trabecular meshwork. Sci. Rep. 2017, 7, 14951. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kasetti, R.B.; Maddineni, P.; Patel, P.D.; Searby, C.; Sheffield, V.C.; Zode, G.S. Transforming growth factor beta2 (TGFbeta2) signaling plays a key role in glucocorticoid-induced ocular hypertension. J. Biol. Chem. 2018, 293, 9854–9868. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patel, G.C.; Phan, T.N.; Maddineni, P.; Kasetti, R.B.; Millar, J.C.; Clark, A.F.; Zode, G.S. Dexamethasone-Induced Ocular Hypertension in Mice: Effects of Myocilin and Route of Administration. Am. J. Pathology 2017, 187, 713–723. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peters, J.C.; Bhattacharya, S.K.; Clark, A.F.; Zode, G.S. Increased Endoplasmic Reticulum Stress in Human Glaucomatous Trabecular Meshwork Cells and Tissues. Investig. Opthalmol. Vis. Sci. 2015, 56, 3860–3868. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vranka, J.A.; Kelley, M.J.; Acott, T.S.; Keller, K.E. Extracellular matrix in the trabecular meshwork: Intraocular pressure regulation and dysregulation in glaucoma. Exp. Eye Res. 2015, 133, 112–125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, K.; Read, A.T.; Sulchek, T.; Ethier, C.R. Trabecular meshwork stiffness in glaucoma. Exp. Eye Res. 2017, 158, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Zode, G.S.; Sharma, A.B.; Lin, X.; Searby, C.; Bugge, K.; Kim, G.H.; Clark, A.F.; Sheffield, V.C. Ocular-specific ER stress reduction rescues glaucoma in murine glucocorticoid-induced glaucoma. J. Clin. Investig. 2014, 124, 1956–1965. [Google Scholar] [CrossRef] [Green Version]
- Clark, A.F.; Wilson, K.; de Kater, A.W.; Allingham, R.R.; McCartney, M.D. Dexamethasone-induced ocular hypertension in perfusion-cultured human eyes. Investig. Ophthalmol. Vis. Sci. 1995, 36, 478–489. [Google Scholar]
- Briggs, A.E.L.; Toh, T.; Eri, R.; Hewitt, A.W.; Cook, A.L. TIMP1, TIMP2, and TIMP4 are increased in aqueous humor from primary open angle glaucoma patients. Mol. Vis. 2015, 21, 1162–1172. [Google Scholar]
- Badier-Commander, C.; Verbeuren, T.; Lebard, C.; Michel, J.B.; Jacob, M.P. Increased TIMP/MMP ratio in varicose veins: A possible explanation for extracellular matrix accumulation. J. Pathol. 2000, 192, 105–112. [Google Scholar] [CrossRef]
- el-Shabrawi, Y.; Eckhardt, M.; Berghold, A.; Faulborn, J.; Auboeck, L.; Mangge, H.; Ardjomand, N. Synthesis pattern of matrix metalloproteinases (MMPs) and inhibitors (TIMPs) in human explant organ cultures after treatment with latanoprost and dexamethasone. Eye 2000, 14, 375–383. [Google Scholar] [CrossRef]
- Nga, A.D.; Yap, S.-L.; Samsudin, A.; Abdul-Rahman, P.S.; Hashim, O.H.; Mimiwati, Z. Matrix metalloproteinases and tissue inhibitors of metalloproteinases in the aqueous humour of patients with primary angle closure glaucoma—a quantitative study. BMC Ophthalmol. 2014, 14, 33. [Google Scholar] [CrossRef] [Green Version]
- Weinreb, R.N.; Robinson, M.R.; Dibas, M.; Stamer, W.D. Matrix Metalloproteinases and Glaucoma Treatment. J. Ocul. Pharmacol. Ther. 2020, 36, 208–228. [Google Scholar] [CrossRef]
- Raghunathan, V.K.; Benoit, J.; Kasetti, R.; Zode, G.; Salemi, M.; Phinney, B.; Keller, K.E.; Staverosky, J.A.; Murphy, C.J.; Acott, T.; et al. Glaucomatous cell derived matrices differentially modulate non-glaucomatous trabecular meshwork cellular behavior. Acta Biomater. 2018, 71, 444–459. [Google Scholar] [CrossRef]
- Zode, G.S.; Bugge, K.E.; Mohan, K.; Grozdanic, S.D.; Peters, J.C.; Koehn, D.R.; Anderson, M.; Kardon, R.; Stone, E.M.; Sheffield, V.C. Topical Ocular Sodium 4-Phenylbutyrate Rescues Glaucoma in a Myocilin Mouse Model of Primary Open-Angle Glaucoma. Investig. Opthalmol. Vis. Sci. 2012, 53, 1557–1565. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.; Rhead, W.; Diaz, G.A.; Scharschmidt, B.F.; Mian, A.; Shchelochkov, O.; Marier, J.F.; Beliveau, M.; Mauney, J.; Dickinson, K.; et al. Phase 2 comparison of a novel ammonia scavenging agent with sodium phenylbutyrate in patients with urea cycle disorders: Safety, pharmacokinetics and ammonia control. Mol. Genet. Metab. 2010, 100, 221–228. [Google Scholar] [CrossRef] [Green Version]
- Ricobaraza, A.; Cuadrado-Tejedor, M.; Perez-Mediavilla, A.; Frechilla, D.; Del Rio, J.; Garcia-Osta, A. Phenylbutyrate ameliorates cognitive deficit and reduces tau pathology in an Alzheimer’s disease mouse model. Neuropsychopharmacology 2009, 34, 1721–1732. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bauer, D.; Kasper, M.; Walscheid, K.; Koch, J.M.; Müther, P.S.; Kirchhof, B.; Heiligenhaus, A.; Heinz, C. Alteration of MCP-1 and MMP-9 in Aqueous Humor Is Associated with Secondary Glaucoma in Fuchs Uveitis Syndrome. Ocul. Immunol. Inflamm. 2020, 28, 688–698. [Google Scholar] [CrossRef] [PubMed]
- Thakur, N.; Kupani, M.; Pandey, R.; Mannan, R.; Pruthi, A.; Mehrotra, S. Genetic association of −1562C > T polymorphism in the MMP9 gene with primary glaucoma in a north Indian population. PLoS ONE 2018, 13, e0192636. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patel, G.C.; Millar, J.C.; Clark, A.F. Glucocorticoid Receptor Transactivation Is Required for Glucocorticoid-Induced Ocular Hypertension and Glaucoma. Investig. Opthalmol. Vis. Sci. 2019, 60, 1967–1978. [Google Scholar] [CrossRef] [Green Version]
- Shafiee, A.; Bucolo, C.; Budzynski, E.; Ward, K.W.; Lopez, F.J. In vivo ocular efficacy profile of mapracorat, a novel selective glucocorticoid receptor agonist, in rabbit models of ocular disease. Investig. Opthalmol. Vis. Sci. 2011, 52, 1422–1430. [Google Scholar] [CrossRef] [Green Version]
- Clark, A.F.; Wordinger, R.J. The role of steroids in outflow resistance. Exp. Eye. Res. 2009, 88, 752–759. [Google Scholar] [CrossRef]
- Phulke, S.; Kaushik, S.; Kaur, S.; Pandav, S.S. Steroid-induced Glaucoma: An Avoidable Irreversible Blindness. J. Curr. Glaucoma Pract. 2017, 11, 67–72. [Google Scholar] [PubMed]
- Rezkallah, A.; Kodjikian, L.; Malclès, A.; Dot, C. DEX implant intravitreal injection, sustained intraocular hypertension, and steroid-induced glaucoma in patients with no risk factors. Graefe’s Arch. Clin. Exp. Ophthalmol. 2018, 256, 219–220. [Google Scholar] [CrossRef]
- Russell, P.; Johnson, M. Elastic modulus determination of normal and glaucomatous human trabecular meshwork. Investig. Ophthalmol. Vis. Sci. 2012, 53, 117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, G.; Lee, C.; Agrahari, V.; Wang, K.; Navarro, I.; Sherwood, J.M.; Crews, K.; Farsiu, S.; Gonzalez, P.; Lin, C.W.; et al. In vivo measurement of trabecular meshwork stiffness in a corticosteroid-induced ocular hypertensive mouse model. Proc. Natl. Acad. Sci. USA 2019, 116, 1714–1722. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vahabikashi, A.; Gelman, A.; Dong, B.; Gong, L.; Cha, E.D.K.; Schimmel, M.; Tamm, E.R.; Perkumas, K.; Stamer, W.D.; Sun, C.; et al. Increased stiffness and flow resistance of the inner wall of Schlemm’s canal in glaucomatous human eyes. Proc. Natl. Acad. Sci. USA. 2019, 116, 26555–26563. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Johnstone, M.A.; Xin, C.; Song, S.; Padilla, S.; Vranka, J.A.; Acott, T.S.; Zhou, K.; Schwaner, S.A.; Wang, R.; et al. Estimating Human Trabecular Meshwork Stiffness by Numerical Modeling and Advanced OCT Imaging. Investig. Opthalmol. Vis. Sci. 2017, 58, 4809–4817. [Google Scholar] [CrossRef]
- De Groef, L.; Van Hove, I.; Dekeyster, E.; Stalmans, I.; Moons, L. MMPs in the trabecular meshwork: Promising targets for future glaucoma therapies? Investig. Ophthalmol. Vis. Sci. 2013, 54, 7756–7763. [Google Scholar] [CrossRef] [Green Version]
- O’Callaghan, J.; Crosbie, D.E.; Cassidy, P.; Sherwood, J.M.; Flügel-Koch, C.; Lütjen-Drecoll, E.; Humphries, M.M.; Reina-Torres, E.; Wallace, D.; Kiang, A.-S.; et al. Therapeutic potential of AAV-mediated MMP-3 secretion from corneal endothelium in treating glaucoma. Hum. Mol. Genet. 2017, 26, 1230–1246. [Google Scholar] [CrossRef]
- Bradley, J.M.; Vranka, J.; Colvis, C.M.; Conger, D.M.; Alexander, J.P.; Fisk, A.S.; Samples, J.R.; Acott, T.S. Effect of matrix metalloproteinases activity on outflow in perfused human organ culture. Investig. Ophthalmol. Vis. Sci. 1998, 39, 2649–2658. [Google Scholar]
- Parshley, D.E.; Bradley, J.M.; Samples, J.R.; Van Buskirk, E.M.; Acott, T.S. Early changes in matrix metalloproteinases and inhibitors after in vitro laser treatment to the trabecular meshwork. Curr. Eye Res. 1995, 14, 537–544. [Google Scholar] [CrossRef]
- Oh, D.-J.; Kang, M.H.; Ooi, Y.H.; Choi, K.R.; Sage, E.H.; Rhee, D.J. Overexpression of SPARC in Human Trabecular Meshwork Increases Intraocular Pressure and Alters Extracellular Matrix. Investig. Opthalmol. Vis. Sci. 2013, 54, 3309–3319. [Google Scholar] [CrossRef] [Green Version]
- Guo, M.-S.; Wu, Y.-Y.; Liang, Z.-B. Hyaluronic acid increases MMP-2 and MMP-9 expressions in cultured trabecular meshwork cells from patients with primary open-angle glaucoma. Mol. Vis. 2012, 18, 1175–1181. [Google Scholar]
- De Groef, L.; Andries, L.; Siwakoti, A.; Geeraerts, E.; Bollaerts, I.; Noterdaeme, L.; Etienne, I.; Papageorgiou, A.P.; Stalmans, I.; Billen, J.; et al. Aberrant Collagen Composition of the Trabecular Meshwork Results in Reduced Aqueous Humor Drainage and Elevated IOP in MMP-9 Null Mice. Investig. Ophthalmol. Vis. Sci. 2016, 57, 5984–5995. [Google Scholar] [CrossRef] [PubMed]
- Yabluchanskiy, A.; Ma, Y.; Iyer, R.P.; Hall, M.E.; Lindsey, M.L. Matrix Metalloproteinase-9: Many Shades of Function in Cardiovascular Disease. Physiology 2013, 28, 391–403. [Google Scholar] [CrossRef] [Green Version]
- Snyder, R.W.; Stamer, W.D.; Kramer, T.R.; Seftor, R.E. Corticosteroid Treatment and Trabecular Meshwork Proteases in Cell and Organ Culture Supernatants. Exp. Eye Res. 1993, 57, 461–468. [Google Scholar] [CrossRef] [PubMed]
- Bucolo, C.; Campana, G.; Di Toro, R.; Cacciaguerra, S.; Spampinato, S. Sigma1 recognition sites in rabbit iris-ciliary body: Topical sigma1-site agonists lower intraocular pressure. J. Pharmacol. Exp. Ther. 1999, 289, 1362–1369. [Google Scholar]
- Sanchez, B.P.; Pozo, R.A.; Merlos, M.; Garzon, J. The Sigma-1 Receptor Antagonist, S1RA, Reduces Stroke Damage, Ameliorates Post-Stroke Neurological Deficits and Suppresses the Overexpression of MMP-9. Mol. Neurobiol. 2018, 55, 4940–4951. [Google Scholar] [CrossRef] [Green Version]
- Li, T.; Lindsley, K.; Rouse, B.; Hong, H.; Shi, Q.; Friedman, D.S.; Wormald, R.; Dickersin, K. Comparative Effectiveness of First-Line Medications for Primary Open-Angle Glaucoma: A Systematic Review and Network Meta-analysis. Ophthalmology 2016, 123, 129–140. [Google Scholar] [CrossRef] [Green Version]
- Zeitlin, P.L.; Diener-West, M.; Rubenstein, R.C.; Boyle, M.; Lee, C.K.; Brass-Ernst, L. Evidence of CFTR Function in Cystic Fibrosis after Systemic Administration of 4-Phenylbutyrate. Mol. Ther. 2002, 6, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Stamer, W.D.; Clark, A.F. The many faces of the trabecular meshwork cell. Exp. Eye Res. 2017, 158, 112–123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kasetti, R.B.; Patel, P.D.; Maddineni, P.; Zode, G.S. Ex-vivo cultured human corneoscleral segment model to study the effects of glaucoma factors on trabecular meshwork. PLoS ONE 2020, 15, e0232111. [Google Scholar]
- Rueden, C.T.; Schindelin, J.; Hiner, M.C.; DeZonia, B.E.; Walter, A.E.; Arena, E.T.; Eliceiri, K.W. ImageJ2: ImageJ for the next generation of scientific image data. BMC Bioinform. 2017, 18, 529. [Google Scholar] [CrossRef] [PubMed]
- Maddineni, P.; Kasetti, R.B.; Zode, G.S. Methods for Analyzing Endoplasmic Reticulum Stress in the Trabecular Meshwork of Glaucoma Models. Methods Mol. Biol. 2018, 1695, 121–134. [Google Scholar]
- GraphPad Software. GraphPad Prism Version 9.00 for Windows; GraphPad Software: San Diego, CA, USA, 2021. [Google Scholar]








Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maddineni, P.; Kasetti, R.B.; Kodati, B.; Yacoub, S.; Zode, G.S. Sodium 4-Phenylbutyrate Reduces Ocular Hypertension by Degrading Extracellular Matrix Deposition via Activation of MMP9. Int. J. Mol. Sci. 2021, 22, 10095. https://doi.org/10.3390/ijms221810095
Maddineni P, Kasetti RB, Kodati B, Yacoub S, Zode GS. Sodium 4-Phenylbutyrate Reduces Ocular Hypertension by Degrading Extracellular Matrix Deposition via Activation of MMP9. International Journal of Molecular Sciences. 2021; 22(18):10095. https://doi.org/10.3390/ijms221810095
Chicago/Turabian StyleMaddineni, Prabhavathi, Ramesh B. Kasetti, Bindu Kodati, Sam Yacoub, and Gulab S. Zode. 2021. "Sodium 4-Phenylbutyrate Reduces Ocular Hypertension by Degrading Extracellular Matrix Deposition via Activation of MMP9" International Journal of Molecular Sciences 22, no. 18: 10095. https://doi.org/10.3390/ijms221810095
APA StyleMaddineni, P., Kasetti, R. B., Kodati, B., Yacoub, S., & Zode, G. S. (2021). Sodium 4-Phenylbutyrate Reduces Ocular Hypertension by Degrading Extracellular Matrix Deposition via Activation of MMP9. International Journal of Molecular Sciences, 22(18), 10095. https://doi.org/10.3390/ijms221810095