Recent Advances in Phenolic Metabolites and Skin Cancer
Abstract
:1. Introduction
2. Methods
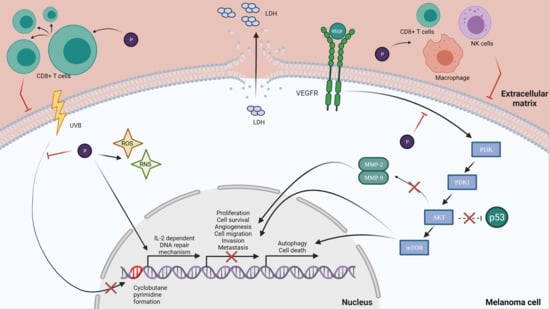
3. Skin Cancer
4. Polyphenols
4.1. Cytotoxic Effect of Polyphenols
4.2. Antiproliferative Effects
4.3. Protection from UV Radiation
4.4. Antioxidant Effects
4.5. Anti-Inflammatory Effect
4.6. Cell Cycle and Apoptosis
4.7. Autophagy
4.8. Tumor Metastasis
5. Polyphenols’ Limitations
6. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Di Lorenzo, C.; Colombo, F.; Biella, S.; Stockley, C.; Restani, P. Polyphenols and Human Health: The Role of Bioavailability. Nutrients 2021, 13, 273. [Google Scholar] [CrossRef]
- Afaq, F.; Katiyar, S.K. Polyphenols: Skin Photoprotection and Inhibition of Photocarcinogenesis. Mini-Rev. Med. Chem. 2012, 11, 1200–1215. [Google Scholar] [CrossRef] [Green Version]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer Statistics, 2016. CA Cancer J. Clin. 2016, 66, 7–30. [Google Scholar] [CrossRef] [Green Version]
- Food Phenolics: Sources, Chemistry, Effects, Applications (Book, 1995) [WorldCat.org]. Available online: https://www.worldcat.org/title/food-phenolics-sources-chemistry-effects-applications/oclc/33243502 (accessed on 12 May 2021).
- El-Harakeh, M.; Al-Ghadban, S.; Safi, R. Medicinal Plants Towards Modeling Skin Cancer. Curr. Drug Targets 2020, 22, 148–161. [Google Scholar] [CrossRef]
- Narayanan, D.L.; Saladi, R.N.; Fox, J.L. Ultraviolet Radiation and Skin Cancer. Int. J. Dermatol. 2010, 49, 978–986. [Google Scholar] [CrossRef]
- O’Driscoll, L.; McMorrow, J.; Doolan, P.; McKiernan, E.; Mehta, J.P.; Ryan, E.; Gammell, P.; Joyce, H.; O’Donovan, N.; Walsh, N.; et al. Investigation of the Molecular Profile of Basal Cell Carcinoma Using Whole Genome Microarrays. Mol. Cancer 2006, 5. [Google Scholar] [CrossRef] [Green Version]
- Suárez, B.; López-Abente, G.; Martínez, C.; Navarro, C.; Tormo, M.J.; Rosso, S.; Schraub, S.; Gafà, L.; Sancho-Garnier, H.; Wechsler, J.; et al. Occupation and Skin Cancer: The Results of the HELIOS-I Multicenter Case-Control Study. BMC Public Health 2007, 7, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Lomas, A.; Leonardi-Bee, J.; Bath-Hextall, F. A Systematic Review of Worldwide Incidence of Nonmelanoma Skin Cancer. Br. J. Dermatol. 2012, 166, 1069–1080. [Google Scholar] [CrossRef]
- Bennardo, L.; Bennardo, F.; Giudice, A.; Passante, M.; Dastoli, S.; Morrone, P.; Provenzano, E.; Patruno, C.; Nisticò, S.P. Local Chemotherapy as an Adjuvant Treatment in Unresectable Squamous Cell Carcinoma: What Do We Know So Far? Curr. Oncol. 2021, 28, 2317–2325. [Google Scholar] [CrossRef] [PubMed]
- Gloster, H.M.; Neal, K. Skin Cancer in Skin of Color. J. Am. Acad. Dermatol. 2006, 55, 741–760. [Google Scholar] [CrossRef]
- Lombardo, N.; Della Corte, M.; Pelaia, C.; Piazzetta, G.; Lobello, N.; Del Duca, E.; Bennardo, L.; Nisticò, S.P. Primary Mucosal Melanoma Presenting with a Unilateral Nasal Obstruction of the Left Inferior Turbinate. Medicina 2021, 57, 359. [Google Scholar] [CrossRef]
- Sini, M.C.; Doneddu, V.; Paliogiannis, P.; Casula, M.; Colombino, M.; Manca, A.; Botti, G.; Ascierto, P.A.; Lissia, A.; Cossu, A.; et al. Genetic Alterations in Main Candidate Genes during Melanoma Progression. Oncotarget 2018, 9, 8531. [Google Scholar] [CrossRef] [Green Version]
- Budden, T.; Bowden, N.A. The Role of Altered Nucleotide Excision Repair and UVB-Induced DNA Damage in Melanomagenesis. Int. J. Mol. Sci. 2013, 14, 1132–1151. [Google Scholar] [CrossRef] [Green Version]
- Mazouzi, A.; Vigouroux, A.; Aikeshev, B.; Brooks, P.J.; Saparbaev, M.K.; Morera, S.; Ishchenko, A.A. Insight into Mechanisms of 3′-5′ Exonuclease Activity and Removal of Bulky 8,5′-Cyclopurine Adducts by Apurinic/Apyrimidinic Endonucleases. Proc. Natl. Acad. Sci. USA 2013, 110, E3071–E3080. [Google Scholar] [CrossRef] [Green Version]
- Bachelor, M.A.; Bowden, G.T. UVA-Mediated Activation of Signaling Pathways Involved in Skin Tumor Promotion and Progression. Semin. Cancer Biol. 2004, 14, 131–138. [Google Scholar] [CrossRef]
- Rastogi, R.P.; Richa; Kumar, A.; Tyagi, M.B.; Sinha, R.P. Molecular Mechanisms of Ultraviolet Radiation-Induced DNA Damage and Repair. J. Nucleic Acids 2010, 2010, 592980. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Benetou, V.; Lagiou, A.; Lagiou, P. Chemoprevention of Cancer: Current Evidence and Future Prospects. F1000Research 2015, 4, 916. [Google Scholar] [CrossRef] [Green Version]
- Simões, M.C.F.; Sousa, J.J.S.; Pais, A.A.C.C. Skin Cancer and New Treatment Perspectives: A Review. Cancer Lett. 2015, 367, 8–42. [Google Scholar] [CrossRef]
- Briguglio, G.; Costa, C.; Pollicino, M.; Giambò, F.; Catania, S.; Fenga, C. Polyphenols in Cancer Prevention: New Insights (Review). Int. J. Funct. Nutr. 2020, 1. [Google Scholar] [CrossRef]
- Nowak, R.; Olech, M.; Nowacka, N. Plant Polyphenols as Chemopreventive Agents. Polyphen. Hum. Health Dis. 2013, 2, 1289–1307. [Google Scholar] [CrossRef]
- Beckman, C.H. Phenolic-Storing Cells: Keys to Programmed Cell Death and Periderm Formation in Wilt Disease Resistance and in General Defence Responses in Plants? Physiol. Mol. Plant Pathol. 2000, 57, 101–110. [Google Scholar] [CrossRef]
- Scalbert, A.; Manach, C.; Morand, C.; Rémésy, C.; Jiménez, L. Dietary Polyphenols and the Prevention of Diseases. Crit. Rev. Food Sci. Nutr. 2005, 45, 287–306. [Google Scholar] [CrossRef]
- Spencer, J.P.E.; Abd El Mohsen, M.M.; Minihane, A.M.; Mathers, J.C. Biomarkers of the Intake of Dietary Polyphenols: Strengths, Limitations and Application in Nutrition Research. Br. J. Nutr. 2008, 99, 12–22. [Google Scholar] [CrossRef] [Green Version]
- Adlercreutz, H.; Mazur, W. Phyto-Oestrogens and Western Diseases. Ann. Med. 1997, 29, 95–120. [Google Scholar] [CrossRef]
- Soleymani, S.; Habtemariam, S.; Rahimi, R.; Nabavi, S.M. The What and Who of Dietary Lignans in Human Health: Special Focus on Prooxidant and Antioxidant Effects. Trends Food Sci. Technol. 2020, 382–390. [Google Scholar] [CrossRef]
- Imran, M.; Ahmad, N.; Anjum, F.M.; Khan, M.K.; Mushtaq, Z.; Nadeem, M.; Hussain, S. Potential Protective Properties of Flax Lignan Secoisolariciresinol Diglucoside. Nutr. J. 2015, 14, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Mukker, J.K.; Singh, R.S.P.; Muir, A.D.; Krol, E.S.; Alcorn, J. Comparative Pharmacokinetics of Purified Flaxseed and Associated Mammalian Lignans in Male Wistar Rats. Br. J. Nutr. 2015, 113, 749–757. [Google Scholar] [CrossRef]
- Pilar, B.; Güllich, A.; Oliveira, P.; Ströher, D.; Piccoli, J.; Manfredini, V. Protective Role of Flaxseed Oil and Flaxseed Lignan Secoisolariciresinol Diglucoside Against Oxidative Stress in Rats with Metabolic Syndrome. J. Food Sci. 2017, 82, 3029–3036. [Google Scholar] [CrossRef]
- Karasawa, M.M.G.; Mohan, C. Fruits as Prospective Reserves of Bioactive Compounds: A Review. Nat. Prod. Bioprospect. 2018, 8, 335–346. [Google Scholar] [CrossRef] [Green Version]
- Stuper-Szablewska, K.; Perkowski, J. Phenolic Acids in Cereal Grain: Occurrence, Biosynthesis, Metabolism and Role in Living Organisms. Crit. Rev. Food Sci. Nutr. 2019, 59, 664–675. [Google Scholar] [CrossRef] [PubMed]
- Anantharaju, P.G.; Gowda, P.C.; Vimalambike, M.G.; Madhunapantula, S.V. An Overview on the Role of Dietary Phenolics for the Treatment of Cancers. Nutr. J. 2016, 15, 1–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, N.; Goel, N. Phenolic Acids: Natural Versatile Molecules with Promising Therapeutic Applications. Biotechnol. Rep. 2019, 24, e00370. [Google Scholar] [CrossRef] [PubMed]
- Pereira, D.M.; Valentão, P.; Pereira, J.A.; Andrade, P.B. Phenolics: From Chemistry to Biology. Molecules 2009, 14, 2202–2211. [Google Scholar] [CrossRef]
- Clifford, M.N. Review Chlorogenic Acids and Other Cinnamates-Nature, Occurrence and Dietary Burden. J. Sci. Food Agric. 1999, 79, 362–372. [Google Scholar] [CrossRef]
- Rashmi, H.B.; Negi, P.S. Phenolic Acids from Vegetables: A Review on Processing Stability and Health Benefits. Food Res. Int. 2020, 136, 109298. [Google Scholar] [CrossRef]
- Plant Biochemistry—1st Edition. Available online: https://www.elsevier.com/books/plant-biochemistry/dey/978-0-12-214674-9 (accessed on 13 May 2021).
- Khoddami, A.; Wilkes, M.A.; Roberts, T.H. Techniques for Analysis of Plant Phenolic Compounds. Molecules 2013, 18, 2328–2375. [Google Scholar] [CrossRef]
- D’Archivio, M.; Filesi, C.; Di Benedetto, R.; Gargiulo, R.; Giovannini, C.; Masella, R. Polyphenols, Dietary Sources and Bioavailability. Ann. Ist. Super. Sanita 2007, 43, 348. [Google Scholar]
- Neveu, V.; Perez-Jiménez, J.; Vos, F.; Crespy, V.; du Chaffaut, L.; Mennen, L.; Knox, C.; Eisner, R.; Cruz, J.; Wishart, D.; et al. Phenol-Explorer: An Online Comprehensive Database on Polyphenol Contents in Foods. Database (Oxford) 2010, 2010. [Google Scholar] [CrossRef]
- Vang, O.; Ahmad, N.; Baile, C.A.; Baur, J.A.; Brown, K.; Csiszar, A.; Das, D.K.; Delmas, D.; Gottfried, C.; Lin, H.Y.; et al. What Is New for an Old Molecule? Systematic Review and Recommendations on the Use of Resveratrol. PLoS ONE 2011, 6, e19881. [Google Scholar] [CrossRef] [Green Version]
- (PDF) Resveratrol in Berries: A Review. Available online: https://www.researchgate.net/publication/286495158_Resveratrol_in_berries_A_review (accessed on 13 May 2021).
- Review of Rhubarbs: Chemistry and Pharmacology | Request PDF. Available online: https://www.researchgate.net/publication/257680683_Review_of_Rhubarbs_Chemistry_and_Pharmacology (accessed on 13 May 2021).
- Chiva-Blanch, G.; Urpi-Sarda, M.; Rotchés-Ribalta, M.; Zamora-Ros, R.; Llorach, R.; Lamuela-Raventós, R.M.; Estruch, R.; Andrés-Lacueva, C. Determination of Resveratrol and Piceid in Beer Matrices by Solid-Phase Extraction and Liquid Chromatography-Tandem Mass Spectrometry. J. Chromatogr. A 2011, 1218, 698–705. [Google Scholar] [CrossRef]
- Determination of Trans-Resveratrol Levels in Different Fruits, Vegetables and Their Skin by HPLC | Request PDF. Available online: https://www.researchgate.net/publication/286015812_Determination_of_Trans-Resveratrol_Levels_in_Different_Fruits_Vegetables_and_Their_Skin_by_HPLC (accessed on 13 May 2021).
- Peng, X.L.; Xu, J.; Sun, X.F.; Ying, C.J.; Hao, L.P. Analysis of Trans-Resveratrol and Trans-Piceid in Vegetable Foods Using High-Performance Liquid Chromatography. Int. J. Food Sci. Nutr. 2015, 66, 729–735. [Google Scholar] [CrossRef]
- Matsui, Y.; Sugiyama, K.; Kamei, M.; Takahashi, T.; Suzuki, T.; Katagata, Y.; Ito, T. Extract of Passion Fruit (Passiflora Edulis) Seed Containing High Amounts of Piceatannol Inhibits Melanogenesis and Promotes Collagen Synthesis. J. Agric. Food Chem. 2010, 58, 11112–11118. [Google Scholar] [CrossRef]
- Nabavi, S.M.; Šamec, D.; Tomczyk, M.; Milella, L.; Russo, D.; Habtemariam, S.; Suntar, I.; Rastrelli, L.; Daglia, M.; Xiao, J.; et al. Flavonoid Biosynthetic Pathways in Plants: Versatile Targets for Metabolic Engineering. Biotechnol. Adv. 2020, 38, 107316. [Google Scholar] [CrossRef] [PubMed]
- Vukics, V.; Guttman, A. Structural Characterization of Flavonoid Glycosides by Multi-Stage Mass Spectrometry. Mass Spectrom. Rev. 2010, 29, 1–16. [Google Scholar] [CrossRef]
- Parihar, A.; Grotewold, E.; Doseff, A.I. Flavonoid Dietetics: Mechanisms and Emerging Roles of Plant Nutraceuticals. In Pigments in Fruits and Vegetables; Springer: New York, NY, USA, 2015; pp. 93–126. [Google Scholar] [CrossRef]
- Ahn-Jarvis, J.H.; Parihar, A.; Doseff, A.I. Dietary Flavonoids for Immunoregulation and Cancer: Food Design for Targeting Disease. Antioxidants 2019, 8, 202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, J.; Wang, X.; Yong, H.; Kan, J.; Jin, C. Recent Advances in Flavonoid-Grafted Polysaccharides: Synthesis, Structural Characterization, Bioactivities and Potential Applications. Int. J. Biol. Macromol. 2018, 116, 1011–1025. [Google Scholar] [CrossRef] [PubMed]
- Quality Control and Evaluation of Herbal Drugs: Evaluating Natural Products …—Pulok K. Mukherjee—Google Books. Available online: https://books.google.ro/books?hl=en&lr=&id=ReGaDwAAQBAJ&oi=fnd&pg=PP1&dq=Mukherjee,+P.+K.+(2019).+Bioassay-Guided+Isolation+and+Evaluation+of+Herbal+Drugs.+Quality+Control+and+Evaluation+of+Herbal+Drugs,&ots=gXKmJbE0HY&sig=neFK8AeRGupD8Gy3TOUpbhEuFVM&redir_esc=y#v=onepage&q=Mukherjee%2CP.K.(2019).Bioassay-GuidedIsolationandEvaluationofHerbalDrugs.QualityControlandEvaluationofHerbalDrugs%2C&f=false (accessed on 13 May 2021).
- Mantena, S.K.; Meeran, S.M.; Elmets, C.A.; Katiyar, S.K. Orally Administered Green Tea Polyphenols Prevent Ultraviolet Radiation-Induced Skin Cancer in Mice through Activation of Cytotoxic T Cells and Inhibition of Angiogenesis in Tumors. J. Nutr. 2005, 135, 2871–2877. [Google Scholar] [CrossRef]
- Wang, E.; Liu, Y.; Xu, C.; Liu, J. Antiproliferative and Proapoptotic Activities of Anthocyanin and Anthocyanidin Extracts from Blueberry Fruits on B16-F10 Melanoma Cells. Food Nutr. Res. 2017, 61. [Google Scholar] [CrossRef] [Green Version]
- Sassi, A.; Maatouk, M.; El gueder, D.; Bzéouich, I.M.; Abdelkefi-Ben Hatira, S.; Jemni-Yacoub, S.; Ghedira, K.; Chekir-Ghedira, L. Chrysin, a Natural and Biologically Active Flavonoid Suppresses Tumor Growth of Mouse B16F10 Melanoma Cells: In Vitro and in Vivo Study. Chem. Biol. Interact. 2018, 283, 10–19. [Google Scholar] [CrossRef]
- Bano, S.; Ahmed, F.; Khan, F.; Chaudhary, S.C.; Samim, M. Enhancement of the Cancer Inhibitory Effect of the Bioactive Food Component Resveratrol by Nanoparticle Based Delivery. Food Funct. 2020, 11, 3213–3226. [Google Scholar] [CrossRef]
- Osmond, G.W.; Augustine, C.K.; Zipfel, P.A.; Padussis, J.; Tyler, D.S. Enhancing Melanoma Treatment with Resveratrol. J. Surg. Res. 2012, 172, 109–115. [Google Scholar] [CrossRef]
- Rugină, D.; Hanganu, D.; Diaconeasa, Z.; Tăbăran, F.; Coman, C.; Leopold, L.; Bunea, A.; Pintea, A. Antiproliferative and Apoptotic Potential of Cyanidin-Based Anthocyanins on Melanoma Cells. Int. J. Mol. Sci. 2017, 18, 949. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Diaconeasa, Z.; Ayvaz, H.; Ruginǎ, D.; Leopold, L.; Stǎnilǎ, A.; Socaciu, C.; Tăbăran, F.; Luput, L.; Mada, D.C.; Pintea, A.; et al. Melanoma Inhibition by Anthocyanins Is Associated with the Reduction of Oxidative Stress Biomarkers and Changes in Mitochondrial Membrane Potential. Plant Foods Hum. Nutr. 2017, 72, 404–410. [Google Scholar] [CrossRef] [PubMed]
- Yáñez, J.; Vicente, V.; Alcaraz, M.; Castillo, J.; Benavente-García, O.; Canteras, M.; Lozano Teruel, J.A. Cytotoxicity and Antiproliferative Activities of Several Phenolic Compounds against Three Melanocytes Cell Lines: Relationship between Structure and Activity. Nutr. Cancer 2004, 49, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Dana, N.; Javanmard, S.H.; Rafiee, L. Antiangiogenic and Antiproliferative Effects of Black Pomegranate Peel Extract on Melanoma Cell Line. Res. Pharm. Sci. 2015, 10, 117. [Google Scholar]
- Khonkarn, R.; Okonogi, S.; Ampasavate, C.; Anuchapreeda, S. Investigation of Fruit Peel Extracts as Sources for Compounds with Antioxidant and Antiproliferative Activities against Human Cell Lines. Food Chem. Toxicol. 2010, 48, 2122–2129. [Google Scholar] [CrossRef]
- Aggarwal, B.; Kumar, A.; Bharti, A. Anticancer Potential of Curcumin: Preclinical and Clinical Studies. Anticancer. Res. 2003, 23, 363–398. [Google Scholar]
- Abusnina, A.; Keravis, T.; Yougbaré, I.; Bronner, C.; Lugnier, C. Anti-Proliferative Effect of Curcumin on Melanoma Cells Is Mediated by PDE1A Inhibition That Regulates the Epigenetic Integrator UHRF1. Mol. Nutr. Food Res. 2011, 55, 1677–1689. [Google Scholar] [CrossRef]
- Hu, S.; Zhang, X.; Chen, F.; Wang, M. Dietary Polyphenols as Photoprotective Agents against UV Radiation. J. Funct. Foods 2017, 30, 108–118. [Google Scholar] [CrossRef]
- Kim, J.; Hwang, J.S.; Cho, Y.K.; Han, Y.; Jeon, Y.J.; Yang, K.H. Protective Effects of (-)-Epigallocatechin-3-Gallate on UVA- and UVB-Induced Skin Damage. Skin Pharmacol. Appl. Skin Physiol. 2001, 14, 11–19. [Google Scholar] [CrossRef]
- Katiyar, S.K. Skin Photoprotection by Green Tea: Antioxidant and Immunomodulatory Effects. Curr. Drug Targets Immune Endocr. Metab. Disord. 2003, 3, 234–242. [Google Scholar] [CrossRef]
- Katiyar, S.K.; Perez, A.; Mukhtar, H. Green Tea Polyphenol Treatment to Human Skin Prevents Formation of Ultraviolet Light B-Induced Pyrimidine Dimers in DNA. Clin. Cancer Res. 2000, 6, 3864–3869. [Google Scholar]
- Katiyar, S.K.; Afaq, F.; Perez, A.; Mukhtar, H. Green Tea Polyphenol (-)-Epigallocatechin-3-Gallate Treatment of Human Skin Inhibits Ultraviolet Radiation-Induced Oxidative Stress. Carcinogenesis 2001, 22, 287–294. [Google Scholar] [CrossRef]
- Stevanato, R.; Bertelle, M.; Fabris, S. Photoprotective Characteristics of Natural Antioxidant Polyphenols. Regul. Toxicol. Pharmacol. 2014, 69, 71–77. [Google Scholar] [CrossRef]
- Markiewicz, E.; Idowu, O.C. DNA Damage in Human Skin and the Capacities of Natural Compounds to Modulate the Bystander Signalling. Open Biol. 2019, 9, 190208. [Google Scholar] [CrossRef] [Green Version]
- Schwarz, A.; Maeda, A.; Gan, D.; Mammone, T.; Matsui, M.S.; Schwarz, T. Green Tea Phenol Extracts Reduce UVB-Induced DNA Damage in Human Cells via Interleukin-12. Photochem. Photobiol. 2008, 84, 350–355. [Google Scholar] [CrossRef] [PubMed]
- Zillich, O.V.; Schweiggert-Weisz, U.; Eisner, P.; Kerscher, M. Polyphenols as Active Ingredients for Cosmetic Products. Int. J. Cosmet. Sci. 2015, 37, 455–464. [Google Scholar] [CrossRef]
- Kumar, Y.; Bhatia, A. Polyphenols and Skin Cancers. Polyphen. Hum. Health Dis. 2013, 1, 643–653. [Google Scholar] [CrossRef]
- Perron, N.R.; Brumaghim, J.L. A Review of the Antioxidant Mechanisms of Polyphenol Compounds Related to Iron Binding. Cell Biochem. Biophys. 2009, 53, 75–100. [Google Scholar] [CrossRef] [PubMed]
- Bunea, A.; Ruginǎ, D.; Sconţa, Z.; Pop, R.M.; Pintea, A.; Socaciu, C.; Tǎbǎran, F.; Grootaert, C.; Struijs, K.; VanCamp, J. Anthocyanin Determination in Blueberry Extracts from Various Cultivars and Their Antiproliferative and Apoptotic Properties in B16-F10 Metastatic Murine Melanoma Cells. Phytochemistry 2013, 95, 436–444. [Google Scholar] [CrossRef]
- Pietta, P.G. Flavonoids as Antioxidants. J. Nat. Prod. 2000, 63, 1035–1042. [Google Scholar] [CrossRef]
- Liu-Smith, F.; Meyskens, F.L. Molecular Mechanisms of Flavonoids in Melanin Synthesis and the Potential for the Prevention and Treatment of Melanoma. Mol. Nutr. Food Res. 2016, 60, 1264–1274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Awad, H.M.; Boersma, M.G.; Vervoort, J.; Rietjens, I.M.C.M. Peroxidase-Catalyzed Formation of Quercetin Quinone Methide-Glutathione Adducts. Arch. Biochem. Biophys. 2000, 378, 224–233. [Google Scholar] [CrossRef] [PubMed]
- Metodiewa, D.; Jaiswal, A.K.; Cenas, N.; Dickancaité, E.; Segura-Aguilar, J. Quercetin May Act as a Cytotoxic Prooxidant after Its Metabolic Activation to Semiquinone and Quinoidal Product. Free Radic. Biol. Med. 1999, 26, 107–116. [Google Scholar] [CrossRef]
- Thangasamy, T.; Sittadjody, S.; Lanza-Jacoby, S.; Wachsberger, P.R.; Limesand, K.H.; Burd, R. Quercetin Selectively Inhibits Bioreduction and Enhances Apoptosis in Melanoma Cells That Overexpress Tyrosinase. Nutr. Cancer 2007, 59, 258–268. [Google Scholar] [CrossRef]
- Amawi, H.; Ashby, C.R.; Samuel, T.; Peraman, R.; Tiwari, A.K. Polyphenolic Nutrients in Cancer Chemoprevention and Metastasis: Role of the Epithelial-to-Mesenchymal (EMT) Pathway. Nutrients 2017, 9, 911. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mena, P.; Domínguez-Perles, R.; Gironés-Vilaplana, A.; Baenas, N.; García-Viguera, C.; Villaño, D. Flavan-3-Ols, Anthocyanins, and Inflammation. IUBMB Life 2014, 66, 745–758. [Google Scholar] [CrossRef]
- Chapple, K.S.; Cartwright, E.J.; Hawcroft, G.; Tisbury, A.; Bonifer, C.; Scott, N.; Windsor, A.C.J.; Guillou, P.J.; Markham, A.F.; Coletta, P.L.; et al. Localization of Cyclooxygenase-2 in Human Sporadic Colorectal Adenomas. Am. J. Pathol. 2000, 156, 545–553. [Google Scholar] [CrossRef]
- Meeran, S.M.; Akhtar, S.; Katiyar, S.K. Inhibition of UVB-Induced Skin Tumor Development by Drinking Green Tea Polyphenols Is Mediated through DNA Repair and Subsequent Inhibition of Inflammation. J. Investig. Dermatol. 2009, 129, 1258–1270. [Google Scholar] [CrossRef] [Green Version]
- Katiyar, S.K.; Matsui, M.S.; Elmets, C.A.; Mukhtar, H. Polyphenolic Antioxidant (-)-Epigallocatechin-3-Gallate from Green Tea Reduces UVB-Induced Inflammatory Responses and Infiltration of Leukocytes in Human Skin. Photochem. Photobiol. 1999, 69, 148–153. [Google Scholar] [CrossRef]
- Mnich, C.D.; Hoek, K.S.; Virkki, L.V.; Farkas, A.; Dudli, C.; Laine, E.; Urosevic, M.; Dummer, R. Green Tea Extract Reduces Induction of P53 and Apoptosis in UVB-Irradiated Human Skin Independent of Transcriptional Controls. Exp. Dermatol. 2009, 18, 69–77. [Google Scholar] [CrossRef]
- Afaq, F.; Adhami, V.M.; Ahmad, N. Prevention of Short-Term Ultraviolet B Radiation-Mediated Damages by Resveratrol in SKH-1 Hairless Mice. Toxicol. Appl. Pharmacol. 2003, 186, 28–37. [Google Scholar] [CrossRef]
- Aziz, M.; Afaq, F.; Ahmad, N. Prevention of Ultraviolet B Radiation—Damage by Resveratrol in Mouse Skin Is Mediated via Modulation in Survivin. Photochem. Photobiol. 2004, 81, 25–31. [Google Scholar] [CrossRef]
- Ramsewak, R.S.; DeWitt, D.L.; Nair, M.G. Cytotoxicity, Antioxidant and Anti-Inflammatory Activities of Curcumins I–III from Curcuma Longa. Phytomedicine 2000, 7, 303–308. [Google Scholar] [CrossRef]
- Fortes, C. Are Anti-Inflammatory Foods Associated with a Protective Effect for Cutaneous Melanoma? Eur. J. Cancer Prev. 2020, 29, 466–469. [Google Scholar] [CrossRef] [PubMed]
- Won, Y.S.; Seo, K.I. Sanggenol L Induces Apoptosis and Cell Cycle Arrest via Activation of P53 and Suppression of PI3k/Akt/MTOR Signaling in Human Prostate Cancer Cells. Nutrients 2020, 12, 488. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elmore, S. Apoptosis: A Review of Programmed Cell Death. Toxicol. Pathol. 2007, 35, 495–516. [Google Scholar] [CrossRef]
- George, J.; Singh, M.; Srivastava, A.K.; Bhui, K.; Shukla, Y. Synergistic Growth Inhibition of Mouse Skin Tumors by Pomegranate Fruit Extract and Diallyl Sulfide: Evidence for Inhibition of Activated MAPKs/NF-ΚB and Reduced Cell Proliferation. Food Chem. Toxicol. 2011, 49, 1511–1520. [Google Scholar] [CrossRef]
- Prasad, R.; Katiyar, S.K. Polyphenols from Green Tea Inhibit the Growth of Melanoma Cells through Inhibition of Class I Histone Deacetylases and Induction of DNA Damage. Genes Cancer 2015, 6, 49–61. [Google Scholar] [CrossRef] [PubMed]
- Davoodvandi, A.; Darvish, M.; Borran, S.; Nejati, M.; Mazaheri, S.; Reza Tamtaji, O.; Hamblin, M.R.; Masoudian, N.; Mirzaei, H. The Therapeutic Potential of Resveratrol in a Mouse Model of Melanoma Lung Metastasis. Int. Immunopharmacol. 2020, 88, 106905. [Google Scholar] [CrossRef] [PubMed]
- Gong, C.; Xia, H. Resveratrol Suppresses Melanoma Growth by Promoting Autophagy through Inhibiting the PI3K/AKT/MTOR Signaling Pathway. Exp. Ther. Med. 2019, 19, 1878–1886. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jensen, J.D.; Dunn, J.H.; Luo, Y.; Liu, W.; Fujita, M.; Dellavalle, R.P. Ellagic Acid Inhibits Melanoma Growth in Vitro. Dermatol. Rep. 2011, 3. [Google Scholar] [CrossRef] [PubMed]
- Lo, C.; Lai, T.-Y.; Yang, J.-S.; Yang, J.-H.; Ma, Y.-S.; Weng, S.-W.; Lin, H.-Y.; Chen, H.-Y.; Lin, J.-G.; Chung, J.-G. Gallic Acid Inhibits the Migration and Invasion of A375.S2 Human Melanoma Cells through the Inhibition of Matrix Metalloproteinase-2 and Ras. Melanoma Res. 2011, 21, 267–273. [Google Scholar] [CrossRef]
- Pelinson, L.P.; Assmann, C.E.; Palma, T.V.; da Cruz, I.B.M.; Pillat, M.M.; Mânica, A.; Stefanello, N.; Weis, G.C.C.; de Oliveira Alves, A.; de Andrade, C.M.; et al. Antiproliferative and Apoptotic Effects of Caffeic Acid on SK-Mel-28 Human Melanoma Cancer Cells. Mol. Biol. Rep. 2019, 46, 2085–2092. [Google Scholar] [CrossRef]
- Kim, S.H.; Yoo, E.S.; Woo, J.S.; Han, S.H.; Lee, J.H.; Jung, S.H.; Kim, H.J.; Jung, J.Y. Antitumor and Apoptotic Effects of Quercetin on Human Melanoma Cells Involving JNK/P38 MAPK Signaling Activation. Eur. J. Pharmacol. 2019, 860. [Google Scholar] [CrossRef]
- Yao, X.; Jiang, W.; Yu, D.; Yan, Z. Luteolin Inhibits Proliferation and Induces Apoptosis of Human Melanoma Cells: In Vivo and in Vitro by Suppressing MMP-2 and MMP-9 through the PI3K/AKT Pathway. Food Funct. 2019, 10, 703–712. [Google Scholar] [CrossRef]
- Hearing, V.J.; Leong, S.P.L. From Melanocytes to Melanoma: The Progression to Malignancy; Humana Press: Totowa, NJ, USA, 2006. [Google Scholar] [CrossRef]
- Di Leo, L.; Bodemeyer, V.; De Zio, D. The Complex Role of Autophagy in Melanoma Evolution: New Perspectives from Mouse Models. Front. Oncol. 2020, 9, 1506. [Google Scholar] [CrossRef] [Green Version]
- Musial, C.; Siedlecka-Kroplewska, K.; Kmiec, Z.; Gorska-Ponikowska, M. Modulation of Autophagy in Cancer Cells by Dietary Polyphenols. Antioxidants 2021, 10, 123. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; Han, X.; Zheng, S.; Li, Z.; Sha, Y.; Ni, J.; Sun, Z.; Qiao, S.; Song, Z. Curcumin Induces Autophagy, Inhibits Proliferation and Invasion by Downregulating AKT/MTOR Signaling Pathway in Human Melanoma Cells. Oncol. Rep. 2016, 35, 1065–1074. [Google Scholar] [CrossRef] [Green Version]
- Chiu, C.-T.; Hsuan, S.-W.; Lin, H.-H.; Hsu, C.-C.; Chou, F.-P.; Chen, J.-H. Hibiscus Sabdariffa Leaf Polyphenolic Extract Induces Human Melanoma Cell Death, Apoptosis, and Autophagy. J. Food Sci. 2015, 80, H649–H658. [Google Scholar] [CrossRef]
- Prieto, K.M.; Urueña, C.; Fiorentino, S.; Barreto, A. Abstract 1226: Polyphenol Rich Extract from Caesalpinia Spinosa Plant Delays Cell Death through Autophagy Induction to Enhance Immunogenic Signals in Melanoma Cells. In Proceedings of the Annual Meeting of the American Association for Cancer Research, Philadelphia, PA, USA, 24–29 April 2020; p. 1226. [Google Scholar] [CrossRef]
- Rosenfeldt, M.T.; O’Prey, J.; Lindsay, C.R.; Nixon, C.; Roth, S.; Sansom, O.J.; Ryan, K.M. Loss of Autophagy Affects Melanoma Development in a Manner Dependent on PTEN Status. Cell Death Differ. 2021, 28, 1437–1439. [Google Scholar] [CrossRef]
- Chin, L.; Garraway, L.A.; Fisher, D.E. Malignant Melanoma: Genetics and Therapeutics in the Genomic Era. Genes Dev. 2006, 20, 2149–2182. [Google Scholar] [CrossRef] [Green Version]
- Fidler, I.J. The Organ Microenvironment and Cancer Metastasis. Differentiation 2002, 70, 498–505. [Google Scholar] [CrossRef]
- Jour, G.; Ivan, D.; Aung, P.P. Angiogenesis in Melanoma: An Update with a Focus on Current Targeted Therapies. J. Clin. Pathol. 2016, 69, 472–483. [Google Scholar] [CrossRef]
- Su, C.C.; Wang, C.J.; Huang, K.H.; Lee, Y.J.; Chan, W.M.; Chang, Y.C. Anthocyanins from Hibiscus Sabdariffa Calyx Attenuate in Vitro and in Vivo Melanoma Cancer Metastasis. J. Funct. Foods 2018, 48, 614–631. [Google Scholar] [CrossRef]
- Menon, L.G.; Kuttan, R.; Kuttan, G. Inhibition of Lung Metastasis in Mice Induced by B16F10 Melanoma Cells by Polyphenolic Compounds. Cancer Lett. 1995, 95, 221–225. [Google Scholar] [CrossRef]
- Olivas-Aguirre, F.; Rodrigo-García, J.; Martínez-Ruiz, N.; Cárdenas-Robles, A.; Mendoza-Díaz, S.; Álvarez-Parrilla, E.; González-Aguilar, G.; de la Rosa, L.; Ramos-Jiménez, A.; Wall-Medrano, A. Cyanidin-3-O-Glucoside: Physical-Chemistry, Foodomics and Health Effects. Molecules 2016, 21, 1264. [Google Scholar] [CrossRef] [Green Version]
- Liu, M.; Du, Y.; Li, H.; Wang, L.; Ponikwicka-Tyszko, D.; Lebiedzinska, W.; Pilaszewicz-Puza, A.; Liu, H.; Zhou, L.; Fan, H.; et al. Cyanidin-3-o-Glucoside Pharmacologically Inhibits Tumorigenesis via Estrogen Receptor β in Melanoma Mice. Front. Oncol. 2019, 9, 1110. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Lei, Z.; Huang, Z.; Zhang, X.; Zhou, Y.; Luo, Z.; Zeng, W.; Su, J.; Peng, C.; Chen, X. Epigallocatechin-3-Gallate(EGCG) Suppresses Melanoma Cell Growth and Metastasis by Targeting TRAF6 Activity. Oncotarget 2016, 7, 79557. [Google Scholar] [CrossRef] [Green Version]
- Nihal, M.; Ahmad, N.; Mukhtar, H.; Wood, G.S. Anti-Proliferative and Proapoptotic Effects of (-)-Epigallocatechin-3-Gallate on Human Melanoma: Possible Implications for the Chemoprevention of Melanoma. Int. J. Cancer 2005, 114, 513–521. [Google Scholar] [CrossRef]
- Zhang, L.; Yan, B.; Meng, S.; Zhou, L.; Xu, Y.; Du, W.; Shan, L. Theaflavin Induces Apoptosis of A375 Human Melanoma Cells and Inhibits Tumor Growth in Xenograft Zebrafishes Through P53- and JNK-Related Mechanism. Front. Pharmacol. 2020, 11. [Google Scholar] [CrossRef]
- Huang, Y.C.; Liu, K.C.; Chiou, Y.L. Melanogenesis of Murine Melanoma Cells Induced by Hesperetin, a Citrus Hydrolysate-Derived Flavonoid. Food Chem. Toxicol. 2012, 50, 653–659. [Google Scholar] [CrossRef] [PubMed]
- Ohguchi, K.; Akao, Y.; Nozawa, Y. Stimulation of Melanogenesis by the Citrus Flavonoid Naringenin in Mouse B16 Melanoma Cells. Biosci. Biotechnol. Biochem. 2006, 70, 1499–1501. [Google Scholar] [CrossRef]
- Shankar, E.; Goel, A.; Gupta, K.; Gupta, S. Plant Flavone Apigenin: An Emerging Anticancer Agent. Curr. Pharmacol. Rep. 2017, 3, 423–446. [Google Scholar] [CrossRef]
- Xu, L.; Zhang, Y.; Tian, K.; Chen, X.; Zhang, R.; Mu, X.; Wu, Y.; Wang, D.; Wang, S.; Liu, F.; et al. Apigenin Suppresses PD-L1 Expression in Melanoma and Host Dendritic Cells to Elicit Synergistic Therapeutic Effects. J. Exp. Clin. Cancer Res. 2018, 37, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Woo, J.S.; Choo, G.S.; Yoo, E.S.; Kim, S.H.; Lee, J.H.; Han, S.H.; Kim, H.J.; Jung, S.H.; Park, Y.S.; Kim, B.S.; et al. Apigenin Induces Apoptosis by Regulating Akt and MAPK Pathways in Human Melanoma Cell A375SM. Mol. Med. Rep. 2020, 22, 4877–4889. [Google Scholar] [CrossRef]
- Zhao, G.; Han, X.; Cheng, W.; Ni, J.; Zhang, Y.; Lin, J.; Song, Z. Apigenin Inhibits Proliferation and Invasion, and Induces Apoptosis and Cell Cycle Arrest in Human Melanoma Cells. Oncol. Rep. 2017, 37, 2277–2285. [Google Scholar] [CrossRef] [Green Version]
- Ghiƫu, A.; Pavel, I.Z.; Avram, S.; Kis, B.; Minda, D.; Dehelean, C.A.; Buda, V.; Folescu, R.; Danciu, C. An In Vitro-In Vivo Evaluation of the Antiproliferative and Antiangiogenic Effect of Flavone Apigenin against SK-MEL-24 Human Melanoma Cell Line. Anal. Cell. Pathol. 2021, 2021, 1–11. [Google Scholar] [CrossRef]
- Huang, L.; Peng, B.; Nayak, Y.; Wang, C.; Si, F.; Liu, X.; Dou, J.; Xu, H.; Peng, G. Baicalein and Baicalin Promote Melanoma Apoptosis and Senescence via Metabolic Inhibition. Front. Cell Dev. Biol. 2020, 8, 836. [Google Scholar] [CrossRef]
- Pichichero, E.; Cicconi, R.; Mattei, M.; Canini, A. Chrysin-Induced Apoptosis Is Mediated through P38 and Bax Activation in B16-F1 and A375 Melanoma Cells. Int. J. Oncol. 2011, 38, 473–483. [Google Scholar] [CrossRef] [Green Version]
- Chen, H.-Y.; Jiang, Y.-W.; Kuo, C.-L.; Way, T.-D.; Chou, Y.-C.; Chang, Y.-S.; Chung, J.-G. Chrysin Inhibit Human Melanoma A375.S2 Cell Migration and Invasion via Affecting MAPK Signaling and NF-ΚB Signaling Pathway in Vitro. Environ. Toxicol. 2019, 34, 434–442. [Google Scholar] [CrossRef]
- Del Río, J.A.; Fuster, M.D.; Gómez, P.; Porras, I.; García-Lidón, A.; Ortuño, A. Citrus Limon: A Source of Flavonoids of Pharmaceutical Interest. Food Chem. 2004, 84, 457–461. [Google Scholar] [CrossRef]
- Conesa, C.M.; Ortega, V.V.; Yáñez Gascón, M.J.; Baños, M.A.; Jordana, M.C.; Benavente-García, O.; Castillo, J. Treatment of Metastatic Melanoma B16F10 by the Flavonoids Tangeretin, Rutin, and Diosmin. J. Agric. Food Chem. 2005, 53, 6791–6797. [Google Scholar] [CrossRef]
- Martínez, C.; Vicente Ortega, V.; Yáñez, J.; Alcaraz, M.; Castellas, M.T.; Canteras, M.; Benavente-García, O.; Castillo, J. The Effect of the Flavonoid Diosmin, Grape Seed Extract and Red Wine on the Pulmonary Metastatic B16F10 Melanoma. Histol. Histopathol. 2005, 20. [Google Scholar] [CrossRef]
- Dabeek, W.M.; Marra, M.V. Dietary Quercetin and Kaempferol: Bioavailability and Potential Cardiovascular-Related Bioactivity in Humans. Nutrients 2019, 11, 2288. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.; Xiao, P.; Sun, J.; Guo, L. Anticancer Effects of Kaempferol in A375 Human Malignant Melanoma Cells Are Mediated via Induction of Apoptosis, Cell Cycle Arrest, Inhibition of Cell Migration and Downregulation of m-TOR/PI3K/AKT Pathway. J. Buon 2018, 23, 218–223. [Google Scholar]
- Soll, F.; Ternent, C.; Berry, I.M.; Kumari, D.; Moore, T.C. Quercetin Inhibits Proliferation and Induces Apoptosis of B16 Melanoma Cells In Vitro. Assay Drug Dev. Technol. 2020, 18, 261–268. [Google Scholar] [CrossRef]
- Zhang, X.; Xu, Q.; Saiki, I. Quercetin Inhibits the Invasion and Mobility of Murine Melanoma B16-BL6 Cells through Inducing Apoptosis via Decreasing Bcl-2 Expression. Clin. Exp. Metastasis 2000, 18, 415–421. [Google Scholar] [CrossRef]
- Caltagirone, S.; Rossi, C.; Poggi, A.; Ranelletti, F.O.; Natali, P.G.; Brunetti, M.; Aiello, F.B.; Piantelli, M. Flavonoids Apigenin and Quercetin Inhibit Melanoma Growth and Metastatic Potential. Int. J. Cancer 2000, 87, 595–600. [Google Scholar] [CrossRef]
- Cao, H.H.; Cheng, C.Y.; Su, T.; Fu, X.Q.; Guo, H.; Li, T.; Tse, A.K.W.; Kwan, H.Y.; Yu, H.; Yu, Z.L. Quercetin Inhibits HGF/c-Met Signaling and HGFstimulated Melanoma Cell Migration and Invasion. Mol. Cancer 2015, 14, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.Z.; Zhang, Y.; Xie, L.P.; Yu, X.Y.; Zhang, R.Q. Effects of Genistein and Daidzein on the Cell Growth, Cell Cycle, and Differentiation of Human and Murine Melanoma Cells. J. Nutr. Biochem. 2002, 13, 421–426. [Google Scholar] [CrossRef]
- Pramanik, K.C.; Kudugunti, S.K.; Fofaria, N.M.; Moridani, M.Y.; Srivastava, S.K. Caffeic Acid Phenethyl Ester Suppresses Melanoma Tumor Growth by Inhibiting PI3K/AKT/XIAP Pathway. Carcinogenesis 2013, 34, 2061–2070. [Google Scholar] [CrossRef] [Green Version]
- Niero, E.L.D.O.; MacHado-Santelli, G.M. Cinnamic Acid Induces Apoptotic Cell Death and Cytoskeleton Disruption in Human Melanoma Cells. J. Exp. Clin. Cancer Res. 2013, 32, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Daglia, M.; Lorenzo, A.; Nabavi, S.; Talas, Z.; Nabavi, S. Polyphenols: Well Beyond The Antioxidant Capacity: Gallic Acid and Related Compounds as Neuroprotective Agents: You Are What You Eat! Curr. Pharm. Biotechnol. 2014, 15, 362–372. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.-G.; Lo, C.; Lai, T.-Y.; Yang, J.-H.; Yang, J.-S.; Ma, Y.-S.; Weng, S.-W.; Chen, Y.-Y.; Chung, J.-G. Gallic Acid Induces Apoptosis in A375.S2 Human Melanoma Cells through Caspase-Dependent and -Independent Pathways. Int. J. Oncol. 2010, 37, 377–385. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.; Lin, J.J.; Yang, Z.Y.; Tsai, C.C.; Hsu, J.L.; Wu, Y.J. Proteomic Study Reveals a Co-Occurrence of Gallic Acid-Induced Apoptosis and Glycolysis in B16F10 Melanoma Cells. J. Agric. Food Chem. 2014, 62, 11672–11680. [Google Scholar] [CrossRef]
- Li, D.; Yee, J.A.; Thompson, L.U.; Yan, L. Dietary Supplementation with Secoisolariciresinol Diglycoside (SDG) Reduces Experimental Metastasis of Melanoma Cells in Mice. Cancer Lett. 1999, 142, 91–96. [Google Scholar] [CrossRef]
- Zhao, H.; Han, L.; Jian, Y.; Ma, Y.; Yan, W.; Chen, X.; Xu, H.; Li, L. Resveratrol Induces Apoptosis in Human Melanoma Cell through Negatively Regulating Erk/PKM2/Bcl-2 Axis. OncoTargets Ther. 2018, 11, 8995–9006. [Google Scholar] [CrossRef] [Green Version]
- Bhattacharya, S.; Darjatmoko, S.R.; Polans, A.S. Resveratrol Modulates the Malignant Properties of Cutaneous Melanoma through Changes in the Activation and Attenuation of the Antiapoptotic Protooncogenic Protein Akt/PKB. Melanoma Res. 2011, 21, 180. [Google Scholar] [CrossRef] [Green Version]
- Zhao, S.; Tang, L.; Chen, W.; Su, J.; Li, F.; Chen, X.; Wu, L. Resveratrol-Induced Apoptosis Is Associated with Regulating the MiR-492/CD147 Pathway in Malignant Melanoma Cells. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2021, 394, 797–807. [Google Scholar] [CrossRef]
- Platella, C.; Guida, S.; Bonmassar, L.; Aquino, A.; Bonmassar, E.; Ravagnan, G.; Montesarchio, D.; Roviello, G.N.; Musumeci, D.; Fuggetta, M.P. Antitumour Activity of Resveratrol on Human Melanoma Cells: A Possible Mechanism Related to Its Interaction with Malignant Cell Telomerase. Biochim. Biophys. Acta-Gen. Subj. 2017, 1861, 2843–2851. [Google Scholar] [CrossRef]
- Dei Cas, M.; Ghidoni, R. Cancer Prevention and Therapy with Polyphenols: Sphingolipid-Mediated Mechanisms. Nutrients 2018, 10, 940. [Google Scholar] [CrossRef] [Green Version]
- Srini Srinivasan, V. Bioavailability of Nutrients: A Practical Approach to in Vitro Demonstration of the Availability of Nutrients in Multivitamin-Mineral Combination Products. J. Nutr. 2001, 131, 1349S–1350S. [Google Scholar] [CrossRef]
- Eran Nagar, E.; Okun, Z.; Shpigelman, A. Digestive Fate of Polyphenols: Updated View of the Influence of Chemical Structure and the Presence of Cell Wall Material. Curr. Opin. Food Sci. 2020, 31, 38–46. [Google Scholar] [CrossRef]
- Castello, F.; Costabile, G.; Bresciani, L.; Tassotti, M.; Naviglio, D.; Luongo, D.; Ciciola, P.; Vitale, M.; Vetrani, C.; Galaverna, G.; et al. Bioavailability and Pharmacokinetic Profile of Grape Pomace Phenolic Compounds in Humans. Arch. Biochem. Biophys. 2018, 646, 1–9. [Google Scholar] [CrossRef]
- Pereira-Caro, G.; Gaillet, S.; Ordóñez, J.L.; Mena, P.; Bresciani, L.; Bindon, K.A.; Del Rio, D.; Rouanet, J.-M.; Moreno-Rojas, J.M.; Crozier, A. Bioavailability of Red Wine and Grape Seed Proanthocyanidins in Rats. Food Funct. 2020, 11, 3986–4001. [Google Scholar] [CrossRef]
- Annunziata, G.; Jiménez-García, M.; Capó, X.; Moranta, D.; Arnone, A.; Tenore, G.C.; Sureda, A.; Tejada, S. Microencapsulation as a Tool to Counteract the Typical Low Bioavailability of Polyphenols in the Management of Diabetes. Food Chem. Toxicol. 2020, 139, 111248. [Google Scholar] [CrossRef]
- Vittorio, O.; Curcio, M.; Cojoc, M.; Goya, G.F.; Hampel, S.; Iemma, F.; Dubrovska, A.; Cirillo, G. Polyphenols Delivery by Polymeric Materials: Challenges in Cancer Treatment. Drug Deliv. 2017, 24, 162–180. [Google Scholar] [CrossRef] [Green Version]
- Yang, B.; Dong, Y.; Wang, F.; Zhang, Y. Nanoformulations to Enhance the Bioavailability and Physiological Functions of Polyphenols. Molecules 2020, 24, 4613. [Google Scholar] [CrossRef]
- Heenatigala Palliyage, G.; Singh, S.; Ashby, C.R.; Tiwari, A.K.; Chauhan, H. Pharmaceutical Topical Delivery of Poorly Soluble Polyphenols: Potential Role in Prevention and Treatment of Melanoma. AAPS PharmSciTech 2019, 20, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Wei, Q.-Y.; He, K.-M.; Chen, J.-L.; Xu, Y.-M.; Lau, A.T.Y. Phytofabrication of Nanoparticles as Novel Drugs for Anticancer Applications. Molecules 2019, 24, 4246. [Google Scholar] [CrossRef] [PubMed] [Green Version]



| Flavonoids | ||||||
|---|---|---|---|---|---|---|
| Subclass of Polyphenols | Compound | Sources | Study Type | Effect | Cell Lines | Ref |
| Anthocyanins/ Anthocyanidins | Cyanidin (Cyanidin-3-o glucoside) | Red to blue fruits | In vitro | ↑ apoptosis | B16-F10 | [117,118] |
| In vivo | ↓ tumor growth | SK-MEL 1 B16-F10 | ||||
| ↓ tumor growth ↓ tumor volume | B16-F10 B16-F10 luc | |||||
| Anthocyanins enrich extract (delphinidin, cyanidin, petunidin, peonidin and malvidin) | Berries | In vitro | ↓ cell growth ↓ cell proliferation ↓ viability ↑ apoptosis ↑ oxidative damage ↓ mitochondrial membrane potential | B16-F10 | [56,61] | |
| Flavanols | Catechin ((-)-epigallocatechin-3-gallate (EGCG)) | Green tea | In vitro | ↓ cell growth ↓ migration ↓ invasion | SK-MEL 5 SK-MEL 28 A375 G361 | [119] |
| In vivo | ↓ size and number of metastatic nodules | Lung metastasis mouse B16-F10 | ||||
| In vitro | ↓ cell viability ↓ cell proliferation ↑ apoptosis | A375 Hs-294T | [120] | |||
| Theaflavin | Tea | In vitro | ↓ cell proliferation ↑ apoptosis | A375 | [121] | |
| In vivo | ↓ tumor growth | A375 | ||||
| Flavanones | Hesperetin | Citrus | In vitro | ↑ melanogenesis | B16-F10 | [122] |
| Hesperidin | Citrus | In vitro | ↑ melanogenesis | B16 | [123] | |
| Flavones | Apigenin | Dried parsley, celery seeds, vine-spinach and dried oregano | In vitro | ↓ propagation of cells ↓ cell cycle at G2/M phase ↑ apoptosis | A375 A2058 RPMI-7951 | [124,125] |
| In vivo | ↓ tumor growth | B16-F10 | ||||
| In vitro | ↓ cell viability ↓ cell migration ↑ apoptosis ↓ tumor growth ↓ proliferation | A375P A375SM | [126] | |||
| In vivo | ↓ tumor volume ↑ apoptosis | A375SM | ||||
| In vitro | ↓ cell migration ↓ cell invasion ↑apoptosis | A375 C8161 | [127] | |||
| In vivo | ↓ proliferation ↓ cell migration ↓ tumor growth | SK-MEL 24 | [128] | |||
| Baicalein and Baicalin | Edible medicinal plants | In vitro | ↓ tumor growth ↓ proliferation ↑ apoptosis ↑ senescence | Mel586 SK-MEL 2 A375 B16-F0 | [129] | |
| In vivo | ↓ tumor growth ↓ tumor size ↑ apoptosis ↓ proliferation | B16F0 | ||||
| Chrysin | Honey, plants and propolis | In vitro | ↓ cell proliferation ↑ cells differentiation ↑ cell death | B16-F1 A375 | [130] | |
| In vitro | ↓ cell migration ↓ metastasis | A375.S2 | [131] | |||
| Diosmin | Citrus | In vivo | ↓ number of metastatic nodules ↓ growth ↓ invasion ↓proliferation | B16-F10 | [132,133] | |
| In vivo | ↓pulmonary metastasis | B16-F10 | [134] | |||
| Luteolin | Fruits, vegetables and herbs | In vitro | ↓ proliferation ↓ migration ↓invasion ↑apoptosis | A375 | [104] | |
| In vivo | ↓tumor growth | |||||
| Kaempferol | Green leafy vegetables (spinach, kale), herbs (dill, chives, tarragon) | In vitro | ↓proliferation ↑apoptosis ↓cell migratory potential ↓G2/M cell cycle | A375 | [135,136] | |
| Flavonols | Quercetin | Onion, asparagus, berries | In vitro | ↓viability ↓proliferation ↓proportion of the cell in the S and G2/M phase of cell cycle ↑apoptosis | B16 | [135,137] |
| In vitro | ↓ invasion ↓ mobility ↓ proliferation ↓ cell rate in S and G2/M phase of cell cycle ↑ apoptosis | B16-BL6 | [138,139] | |||
| In vivo | ↓ lung metastasis | |||||
| In vitro | ↓ migration ↓ invasion | A2058 A375 | [140] | |||
| Isoflavonoids | Daidzein | Soybean | In vitro | ↓growth | K1735M2 | [141] |
| PhenolicAcids | ||||||
| Hydroxycinnamic Acids | Caffeic acid | Coffee, apple, apricots, pears and berries | In vitro | ↓ cell viability ↑ apoptosis ↓ colony formation | SK-Mel28 | [102] |
| Caffeic acid phenethyl ester (CAPE) | Propolis | In vitro In vivo | ↓ growth ↑ apoptosis | SK-Mel28 B16-F0 | [142] | |
| Cinnamic acid | Coffee, apples, citric fruits, vegetable oils and nuts | In vitro | ↓ proliferation | HT-144 | [143] | |
| Hydroxybenzoic Acids | Gallic acid | Berries, plump, grapes, hazelnut, tea and wine | In vitro | ↓ cell viability ↑ apoptosis ↓ mitochondrial potential↓ invasion ↓metastasis | A375.S2 | [101,144,145] |
| In vitro | ↑ apoptosis | B16-F10 | [146] | |||
| Ellagic acid | Berries, pomegranates and nuts | In vitro | ↓ growth ↑ apoptosis ↓ cell proliferation in G1 phase | 1205LU WM852c A375 | [100] | |
| Lignans | ||||||
| Lignans | Secoisolariciresinol diglycoside | Flaxseed | In vivo | ↓ pulmonary metastasis ↓ growth of metastatic tumor | B16-BL6 | [147] |
| Stilbenes | ||||||
| Stilbenes | Resveratrol | Grapes and red wine | In vitro | ↓ cell proliferation ↑apoptosis | MV3 A375 | [148] |
| In vitro | ↓ cell viability ↑ apoptosis ↑ autophagy ↓ migration ↓ invasion | B16-F10 A375 | [99] | |||
| In vitro | ↓ migration ↓ invasion ↓ tumor volume | B16-F10 B16-BL6 | [149] | |||
| In vitro | ↑ apoptosis | A375 SK-Mel28 | [150] | |||
| Trans-resveratrol | In vitro | ↓ colony formation ↓ cell growth ↑ cytotoxicity | M14 SK-Mel28 | [151] | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pop, T.D.; Diaconeasa, Z. Recent Advances in Phenolic Metabolites and Skin Cancer. Int. J. Mol. Sci. 2021, 22, 9707. https://doi.org/10.3390/ijms22189707
Pop TD, Diaconeasa Z. Recent Advances in Phenolic Metabolites and Skin Cancer. International Journal of Molecular Sciences. 2021; 22(18):9707. https://doi.org/10.3390/ijms22189707
Chicago/Turabian StylePop, Teodora Daria, and Zorita Diaconeasa. 2021. "Recent Advances in Phenolic Metabolites and Skin Cancer" International Journal of Molecular Sciences 22, no. 18: 9707. https://doi.org/10.3390/ijms22189707
APA StylePop, T. D., & Diaconeasa, Z. (2021). Recent Advances in Phenolic Metabolites and Skin Cancer. International Journal of Molecular Sciences, 22(18), 9707. https://doi.org/10.3390/ijms22189707