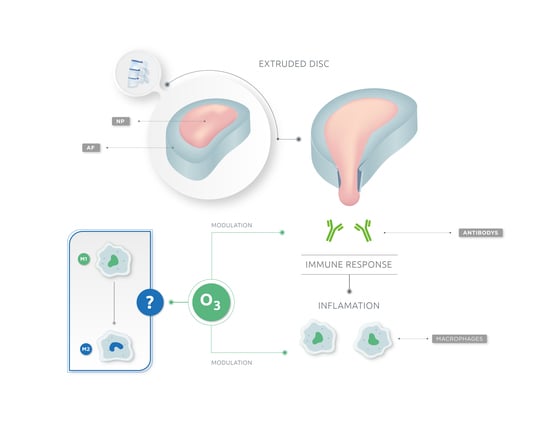
Ozone as Modulator of Resorption and Inflammatory Response in Extruded Nucleus Pulposus Herniation. Revising Concepts
Abstract
:1. Intervertebral Disc
2. Low Back Pain as a Consequence of Disc Herniation
3. Disc Herniation: Immune Phenomenon
4. Natural Evolution of Extruded Disc Herniation
5. Ozone Therapy and Its Implication on Extruded Disc Herniation Resorption. Macrophages: The Master Key
6. Ozone Therapy as Immunomodulator in Extruded Disc Herniation
7. What Is the Role of Ozone Therapy in this Pathology?
8. Discussion
9. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Shapiro, I.M.; Vresilovic, E.J.; Risbud, M.V. Is the spinal motion segment a diarthrodial polyaxially joint: What a nice nucleus like you doing in a joint like this? Bone 2012, 50, 771–776. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jahnke, M.R.; McDevitt, C.A. Proteoglycans of the human intervertebral disc. Electrophoretic heterogeneity of the aggregating proteoglycans of the nucleus pulposus. Biochem. J. 1988, 251, 347–356. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rider, S.M.; Mizuno, S.; Kang, J.D. Molecular Mechanisms of Intervertebral Disc Degeneration. Spine Surg. Relat. Res. 2019, 3, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adams, M.A.; Roughley, P.J. What is Intervertebral Disc Degeneration, and What Causes It? Spine 2006, 31, 2151–2161. [Google Scholar] [CrossRef] [Green Version]
- Wang, F.; Gao, Z.X.; Cai, F.; Sinkemani, A.; Xie, Z.Y.; Shi, R.; Wei, J.N.; Wu, X.T. Formation, function, and exhaustion of notochordal cytoplasmic vacuoles within intervertebral disc: Current understanding and speculation. Oncotarget 2017, 8, 57800–57812. [Google Scholar] [CrossRef]
- Boos, N.; Weissbach, S.; Rohrbach, H.; Weiler, C.; Spratt, K.F.; Nerlich, A.G. Classification of age-related changes in lumbar intervertebral discs: 2002 Volvo Award in basic science. Spine 2002, 27, 2631–2644. [Google Scholar] [CrossRef]
- Sun, Z.; Liu, B.; Luo, Z.J. The Immune Privilege of the Intervertebral Disc: Implications for Intervertebral Disc Degeneration Treatment. Int. J. Med. Sci. 2020, 17, 685–692. [Google Scholar] [CrossRef] [Green Version]
- Takada, T.; Nishida, K.; Doita, M.; Kurosaka, M. Fas ligand exists on intervertebral disc cells: A potential molecular mechanism for immune privilege of the disc. Spine 2002, 27, 1526–1530. [Google Scholar] [CrossRef]
- Kaneyama, S.; Nishida, K.; Takada, T.; Suzuki, T.; Shimomura, T.; Maeno, K.; Kurosaka, M.; Doita, M. Fas ligand expression on human nucleus pulposus cells decreases with disc degeneration processes. J. Orthop. Sci. 2008, 13, 130–135. [Google Scholar] [CrossRef]
- Bach, F.C.; Tellegen, A.R.; Beukers, M.; Miranda-Bedate, A.; Teunissen, M.; de Jong, W.A.; de Vries, S.A.; Creemers, L.B.; Benz, K.; Meij, B.P.; et al. Biologic canine and human intervertebral disc repair by notochordal cell-derived matrix: From bench towards bedside. Oncotarget 2018, 9, 26507–26526. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.H.; Moon, H.J.; Lee, J.H.; Kim, J.H.; Kwon, T.H.; Park, Y.K. Rabbit notochordal cells modulate the expression of inflammatory mediators by human annulus fibrosus cells cocultured with activated macrophage-like THP-1 cells. Spine 2012, 37, 1856–1864. [Google Scholar] [CrossRef]
- Sun, Z.; Wan, Z.Y.; Guo, Y.S.; Wang, H.Q.; Luo, Z.J. FasL on human nucleus pulposus cells prevents angiogenesis in the disc by inducing Fas-mediated apoptosis of vascular endothelial cells. Int. J. Clin. Exp. Path. 2013, 6, 2376–2385. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z.; Sun, Z.; Wang, H.-Q.; Ge, J.; Jiang, T.-S.; Chen, Y.-F.; Ma, Y.; Wang, C.; Hu, S.; Samartzis, D.; et al. FasL Expression on Human Nucleus Pulposus Cells Contributes to the Immune Privilege of Intervertebral Disc by Interacting with Immunocytes. Int. J. Med. Sci. 2013, 10, 1053–1060. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- García-Cosamalón, J.; del Valle, M.E.; Calavia, M.G.; García-Suárez, O.; López-Muñiz, A.; Otero, J.; Vega, J.A. Intervertebral disc, sensory nerves and neurotrophins: Who is who in discogenic pain? J. Anat. 2010, 217, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Giurazza, F.; Guarnieri, G.; Murphy, K.J.; Muto, M. Intradiscal O2O3, Rationale, Injection Technique, Short- and Long-term Outcomes for the Treatment of Low Back Pain Due to Disc Herniation. Can. Assoc. Radiol. J. 2017, 68, 171–177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fardon, D.F. Nomenclature and classification of lumbar disc pathology. Spine 2001, 26, 461–462. [Google Scholar] [CrossRef]
- Fardon, D.F.; Williams, A.L.; Dohring, E.J.; Murtagh, F.R.; Rothman, S.L.G.; Sze, G.K. Lumbar disc nomenclature: Version 2.0, Recommendations of the combined task forces of the North American Spine Society, the American Society of Spine Radiology and the American Society of Neuroradiology. Spine J. Off. J. N. Am. Spine Soc. 2014, 14, 2525–2545. [Google Scholar] [CrossRef] [Green Version]
- Xu, Z.; Zhou, X.; Chen, G. Expression and Mechanism of Interleukin 1 (IL-1), Interleukin 2 (IL-2), Interleukin 8 (IL-8), BMP, Fibroblast Growth Factor 1 (FGF1), and Insulin-Like Growth Factor (IGF-1) in Lumbar Disc Herniation. Med. Sci. Monit. 2019, 25, 984–990. [Google Scholar] [CrossRef]
- Doita, M.; Kanatani, T.; Ozaki, T.; Matsui, N.; Kurosaka, M.; Yoshiya, S. Influence of macrophage infiltration of herniated disc tissue on the production of matrix metalloproteinases leading to disc resorption. Spine 2001, 26, 1522–1527. [Google Scholar] [CrossRef]
- Grönblad, M.; Virri, J.; Tolonen, J.; Seitsalo, S.; Kääpä, E.; Kankare, J.; Myllynen, P.; Karaharju, E.O. A controlled immunohistochemical study of inflammatory cells in disc herniation tissue. Spine 1994, 19, 2744–2751. [Google Scholar] [CrossRef]
- O’Donnell, J.L.; O’Donnell, A.L. Prostaglandin E2 content in herniated lumbar disc disease. Spine 1996, 21, 1653–1655. [Google Scholar] [CrossRef]
- Park, J.B.; Chang, H.; Kim, Y.S. The pattern of interleukin-12 and T-helper types 1 and 2 cytokine expression in herniated lumbar disc tissue. Spine 2002, 27, 2125–2128. [Google Scholar] [CrossRef]
- Shamji, M.F.; Setton, L.A.; Jarvis, W.; So, S.; Chen, J.; Jing, L.; Bullock, R.; Isaacs, R.E.; Brown, C.; Richardson, W.J. Proinflammatory cytokine expression profile in degenerated and herniated human intervertebral disc tissues. Arthritis Rheum. 2010, 62, 1974–1982. [Google Scholar] [CrossRef] [Green Version]
- Djuric, N.; Yang, X.; El Barzouhi, A.; Ostelo, R.; van Duinen, S.G.; Lycklama À Nijeholt, G.J.; van der Kallen, B.F.W.; Peul, W.C.; Vleggeert-Lankamp, C.L.A. Lumbar disc extrusions reduce faster than bulging discs due to an active role of macrophages in sciatica. Acta Neurochir. 2020, 162, 79–85. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bobechko, W.P.; Hirsch, C. Auto-immune response to nucleus pulposus in the rabbit. J. Bone Jt. Surg. Br. 1965, 47, 574–580. [Google Scholar] [CrossRef]
- Habtemariam, A.; Gronblad, M.; Virri, J.; Seitsalo, S.; Ruuskanen, M.; Karaharju, E. Immunocytochemical localization of immunoglobulins in disc herniations. Spine 1996, 21, 1864–1869. [Google Scholar] [CrossRef]
- Satoh, K.; Konno, S.; Nishiyama, K.; Olmarker, K.; Kikuchi, S. Presence and distribution of antigen-antibody complexes in the herniated nucleus pulposus. Spine 1999, 24, 1980–1984. [Google Scholar] [CrossRef] [PubMed]
- Gronblad, M.; Habtemariam, A.; Virri, J.; Seitsalo, S.; Vanharanta, H.; Guyer, R.D. Complement membrane attack complexes in pathologic disc tissues. Spine 2003, 28, 114–118. [Google Scholar] [CrossRef]
- Nygaard, O.P.; Mellgren, S.I.; Osterud, B. The inflammatory properties of contained and noncontained lumbar disc herniation. Spine 1997, 22, 2484–2488. [Google Scholar] [CrossRef]
- Autio, R.A.; Karppinen, J.; Niinimäki, J.; Ojala, R.; Kurunlahti, M.; Haapea, M.; Vanharanta, H.; Tervonen, O. Determinants of spontaneous resorption of intervertebral disc herniations. Spine 2006, 31, 1247–1252. [Google Scholar] [CrossRef]
- Martinez, F.O.; Gordon, S. The M1 and M2 paradigm of macrophage activation: Time for reassessment. F1000Prime Rep. 2014, 6, 13. [Google Scholar] [CrossRef] [Green Version]
- Pedersen, L.M.; Schistad, E.; Jacobsen, L.M.; Røe, C.; Gjerstad, J. Serum levels of the pro-inflammatory interleukins 6 (IL-6) and -8 (IL-8) in patients with lumbar radicular pain due to disc herniation: A 12-month prospective study. Brain Behav. Immun. 2015, 46, 132–136. [Google Scholar] [CrossRef] [PubMed]
- Martinez, F.O.; Helming, L.; Gordon, S. Alternative activation of macrophages: An immunologic functional perspective. Annu. Rev. Immunol. 2009, 27, 451–483. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Djuric, N.; Lafeber, G.C.M.; Vleggeert-Lankamp, C.L.A. The contradictory effect of macrophage-related cytokine expression in lumbar disc herniations: A systematic review. Eur. Spine J. 2020, 29, 1649–1659. [Google Scholar] [CrossRef] [Green Version]
- Martínez-Quiñones, J.V.; Aso-Escario, J.; Consolini, F.; Arregui-Calvo, R. Regresión espontánea de hernias discales intervertebrales. A propósito de una serie de 37 casos. Neurocirugía 2010, 21, 108–117. [Google Scholar] [CrossRef] [Green Version]
- Di Mauro, R.; Cantarella, G.; Bernardini, R.; Di Rosa, M.; Barbagallo, I.; Distefano, A.; Longhitano, L.; Vicario, N.; Nicolosi, D.; Lazzarino, G.; et al. The Biochemical and Pharmacological Properties of Ozone: The Smell of Protection in Acute and Chronic Diseases. Int. J. Mol. Sci. 2019, 20, 634. [Google Scholar] [CrossRef] [Green Version]
- Galiè, M.; Covi, V.; Tabaracci, G.; Malatesta, M. The Role of Nrf2 in the Antioxidant Cellular Response to Medical Ozone Exposure. Int. J. Mol. Sci. 2019, 20, 4009. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bocci, V.; Di Paolo, N. Oxygen-ozone therapy in medicine: An update. Blood Purif. 2009, 28, 373–376. [Google Scholar] [CrossRef]
- Bocci, V. Oxygen-Ozone Therapy: A Critical Evaluation; Kluwer Academic Publishers: Dordrechit, The Netherlands, 2002. [Google Scholar]
- Bocci, V.; Valacchi, G. Free radicals and antioxidants: How to reestablish redox homeostasis in chronic diseases? Curr. Med. Chem. 2013, 20, 3397–3415. [Google Scholar] [CrossRef] [PubMed]
- Bocci, V.A. Scientific and medical aspects of ozone therapy. State Art. Arch. Med. Res. 2006, 37, 425–435. [Google Scholar] [CrossRef]
- Bocci, V.; Borrelli, E.; Travagli, V.; Zanardi, I. The ozone paradox: Ozone is a strong oxidant as well as a medical drug. Med. Res. Rev. 2009, 29, 646–682. [Google Scholar] [CrossRef]
- Bocci, V. How a calculated oxidative stress can yield multiple therapeutic effects. Free Radic. Res. 2012, 46, 1068–1075. [Google Scholar] [CrossRef] [PubMed]
- de Sire, A.; Agostini, F.; Lippi, L.; Mangone, M.; Marchese, S.; Cisari, C.; Bernetti, A.; Invernizzi, M. Oxygen–Ozone Therapy in the Rehabilitation Field: State of the Art on Mechanisms of Action, Safety and Effectiveness in Patients with Musculoskeletal Disorders. Biomolecules 2021, 11, 356. [Google Scholar] [CrossRef]
- Smith, N.L.; Wilson, A.L.; Gandhi, J.; Vatsia, S.; Khan, S.A. Ozone therapy: An overview of pharmacodynamics, current research, and clinical utility. Med. Gas Res. 2017, 7, 212–219. [Google Scholar] [PubMed] [Green Version]
- Sagai, M.; Bocci, V. Mechanisms of Action Involved in Ozone Therapy: Is healing induced via a mild oxidative stress? Med. Gas Res. 2011, 1, 29. [Google Scholar] [CrossRef] [Green Version]
- Re, L.; Martínez-Sánchez, G.; Bordicchia, M.; Malcangi, G.; Pocognoli, A.; Morales-Segura, M.A.; Rothchild, J.; Rojas, A. Is ozone pre-conditioning effect linked to Nrf2/EpRE activation pathway in vivo? A preliminary result. Eur. J. Pharmacol. 2014, 742, 158–162. [Google Scholar] [CrossRef] [PubMed]
- Galiè, M.; Costanzo, M.; Nodari, A.; Boschi, F.; Calderan, L.; Mannucci, S.; Covi, V.; Tabaracci, G.; Malatesta, M. Mild ozonisation activates antioxidant cell response by the Keap1/Nrf2 dependent pathway. Free Radic. Biol. Med. 2018, 124, 114–121. [Google Scholar] [CrossRef] [PubMed]
- Costanzo, M.; Cisterna, B.; Vella, A.; Cestari, T.; Covi, V.; Tabaracci, G.; Malatesta, M. Low ozone concentrations stimulate cytoskeletal organization, mitochondrial activity and nuclear transcription. Eur. J. Histochem. 2015, 59, 2515. [Google Scholar] [CrossRef] [Green Version]
- Viebahn-Hänsler, R.; Fernández, O.S.L.; Fahmy, Z. Ozone in Medicine: The Low-Dose Ozone Concept—Guidelines and Treatment Strategies. Ozone Sci. Eng. 2012, 34, 408–424. [Google Scholar] [CrossRef]
- Gallucci, M.; Limbucci, N.; Zugaro, L.; Barile, A.; Stavroulis, E.; Ricci, A.; Galzio, R.; Masciocchi, C. Sciatica: Treatment with intradiscal and intraforaminal injections of steroid and oxygen-ozone versus steroid only. Radiology 2007, 242, 907–913. [Google Scholar] [CrossRef]
- Wang, J.; Wu, M.; Lin, X.; Li, Y.; Fu, Z. Low-Concentration Oxygen/Ozone Treatment Attenuated Radiculitis and Mechanical Allodynia via PDE2A-cAMP/cGMP-NF-κB/p65 Signaling in Chronic Radiculitis Rats. Pain Res. Manag. 2018, 2018, 5192814. [Google Scholar] [CrossRef] [Green Version]
- Ozcan, S.; Muz, A.; Yildiz Altun, A.; Onal, S.A. Intradiscal ozone therapy for lumbar disc herniation. Cell Mol. Biol. 2018, 64, 52–55. [Google Scholar] [CrossRef] [PubMed]
- Ercalik, T.; Kilic, M. Efficacy of Intradiscal Ozone Therapy with or without Periforaminal Steroid Injection on Lumbar Disc Herniation: A Double-Blinded Controlled Study. Pain Physician. 2020, 23, 477–484. [Google Scholar] [CrossRef] [PubMed]
- Bocci, V.; Borrelli, E.; Zanardi, I.; Travagli, V. The usefulness of ozone treatment in spinal pain. Drug Des. Dev. Ther. 2015, 9, 2677–2685. [Google Scholar] [CrossRef] [Green Version]
- Paoloni, M.; Di Sante, L.; Cacchio, A.; Apuzzo, D.; Marotta, S.; Razzano, M.; Franzini, M.; Santilli, V. Intramuscular oxygen-ozone therapy in the treatment of acute back pain with lumbar disc herniation: A multicenter, randomized, double-blind, clinical trial of active and simulated lumbar paravertebral injection. Spine 2009, 34, 1337–1344. [Google Scholar] [CrossRef] [Green Version]
- Pellicanò, G.; Martinelli, F.; Tavanti, V.; Bonetti, M.; Leonardi, M.; Muto, M. The Italian Oxygen-Ozone Therapy Federation (FIO) study on oxygen-ozone treatment of herniated disc. Int. J. Ozone Ther. 2006, 6, 7–15. [Google Scholar]
- Bonetti, M.; Cotticelli, B.; Fontana, A.; Guindani, M.; Leonardi, M.; Volta, G.D. Intraforaminal O2O3 versus periradicular steroidal infiltrations in lower back pain: Randomized controlled study. AJNR Am. J. Neuroradiol. 2005, 26, 996–1000. [Google Scholar] [PubMed]
- Murphy, K.; Elias, G.; Steppan, J.; Boxley, C.; Balagurunathan, K.; Victor, X.; Meaders, T.; Muto, M. Percutaneous Treatment of Herniated Lumbar Discs with Ozone: Investigation of the Mechanisms of Action. J. Vasc. Interv. Radiol. 2016, 27, 1242–1250.e3. [Google Scholar] [CrossRef]
- Buric, J.; Rigobello, L.; Hooper, D. Five- and ten-year follow-up on intradiscal ozone injection for disc herniation. Int. J. Spine Surg. 2014, 8, 17. [Google Scholar] [CrossRef] [Green Version]
- Grangeat, A.M. Inflammatory Mechanisms Involved in the Lumbar Disc Herniation with Hydrated Nucleus Pulposus. In Proceedings of the III Congress of the Mexican Association of Ozone Therapy, Cancun, Mexico, 10–12 November 2011. [Google Scholar]
- Grangeat, A.M.; Erario, M.A. Inflammatory Mechanisms Involved in the lumbar disc herniation with hydrated nucleus pulposus (acute herniated disc) and oxygen ozone therapy. A different viewpoint. Int. J. Ozone Ther. 2012, 11, 9–14. [Google Scholar]
- Ducusin, R.J.; Nishimura, M.; Sarashina, T.; Uzuka, Y.; Tanabe, S.; Otani, M. Phagocytosis of bovine blood and milk polymorphonuclear leukocytes after ozone gas administration in vitro. J. Vet. Med. Sci. 2003, 65, 535–539. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Volkhovskaya, N.B.; Tkachenko, S.B.; Belopolsky, A.A. Modulation of phagocytic activity of blood polynuclear leukocytes with ozonized physiological saline. Bull. Exp. Biol. Med. 2008, 146, 559–561. [Google Scholar] [CrossRef]
- Diaz-Luis, J.; Menéndez-Cepero, S.; Diaz-Luis, A.; Ascanio-García, Y. In vitro effect of ozone in phagocytic function of leucocytes in peripheral blood. J. Ozone Ther. 2015, 1, 4. [Google Scholar] [CrossRef] [Green Version]
- Menendez Cepero, S.A.; Gonzalez Alvarez, R.; Ledea Lozano, O.E.; Hernandez Rosales, F.A.; León Fernández, O.S.; Diaz Gómez, M.F. Ozono. Aspectos Básicos y Aplicaciones Clínicas. Primera Edicion; Editorial CENIC: La Habana, Cuba, 2008. [Google Scholar]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Erario, M.d.l.Á.; Croce, E.; Moviglia Brandolino, M.T.; Moviglia, G.; Grangeat, A.M. Ozone as Modulator of Resorption and Inflammatory Response in Extruded Nucleus Pulposus Herniation. Revising Concepts. Int. J. Mol. Sci. 2021, 22, 9946. https://doi.org/10.3390/ijms22189946
Erario MdlÁ, Croce E, Moviglia Brandolino MT, Moviglia G, Grangeat AM. Ozone as Modulator of Resorption and Inflammatory Response in Extruded Nucleus Pulposus Herniation. Revising Concepts. International Journal of Molecular Sciences. 2021; 22(18):9946. https://doi.org/10.3390/ijms22189946
Chicago/Turabian StyleErario, María de los Ángeles, Eduardo Croce, Maria Teresita Moviglia Brandolino, Gustavo Moviglia, and Aníbal M. Grangeat. 2021. "Ozone as Modulator of Resorption and Inflammatory Response in Extruded Nucleus Pulposus Herniation. Revising Concepts" International Journal of Molecular Sciences 22, no. 18: 9946. https://doi.org/10.3390/ijms22189946
APA StyleErario, M. d. l. Á., Croce, E., Moviglia Brandolino, M. T., Moviglia, G., & Grangeat, A. M. (2021). Ozone as Modulator of Resorption and Inflammatory Response in Extruded Nucleus Pulposus Herniation. Revising Concepts. International Journal of Molecular Sciences, 22(18), 9946. https://doi.org/10.3390/ijms22189946