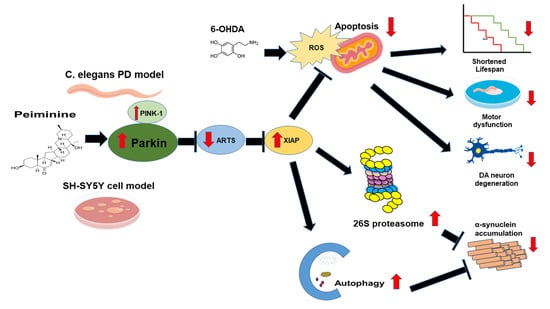
Peiminine Reduces ARTS-Mediated Degradation of XIAP by Modulating the PINK1/Parkin Pathway to Ameliorate 6-Hydroxydopamine Toxicity and α-Synuclein Accumulation in Parkinson’s Disease Models In Vivo and In Vitro
Abstract
:1. Introduction
2. Results
2.1. Toxicity of Peiminine in Worms
2.2. PMN Pretreatment Significantly Reduces Dopaminergic Neuron Degeneration of 6-Hydroxydopamine-Exposed BZ555 Worms
2.3. Food-Sensing Behavioral Defects of 6-OHDA-Exposed Worms Are Restored by PMN Pretreatment
2.4. Lifespan of 6-OHDA-Exposed Worms Is Extended by PMN Pretreatment
2.5. Accumulation of Human α-Synuclein in Muscle Cells of NL5901 Worms Is Reduced by PMN Treatment
2.6. PMN Pretreatment Decreases the Level of Reactive Oxygen Species in 6-OHDA-Exposed N2 Worms and Enhances the Expression of Pink1 and Pdr-1
2.7. PMN Treatment Enhances Proteasome Activity, Autophagy, and expression of Pdr-1 in NL5901 Worms
2.8. Inhibiting the Expression of Pdr-1 in Worms Can Reverse the Ability of PMN to Improve PD Pathology
2.9. PMN Treatment Improves the Toxicity of 6-OHDA Exposure and α-Synuclein Overexpression in the SH-SY5Y Cell Line
2.10. Down-Regulation of Parkin Abolishes the Anti-Apoptotic ability of PMN in 6-OHDA-Exposed SH-SY5Y Cells
2.11. Down-Regulation of Parkin Can Reverse the Ability of PMN to Enhance Ubiquitin-Proteasome System Activity and Autophagy in α-Synuclein-Overexpressing SH-SY5Y Cells
2.12. PMN May Contribute to Anti-Parkinson Activity by Up-Regulating Parkin Performance, Leading to a Diminution of Apoptosis-Related Protein in the TGF-β Signaling Pathway (ARTS) and a Rise in X-Linked Inhibitor of Apoptosis (XIAP)
3. Discussion
4. Materials and Methods
4.1. Chemicals, C. elegans Strains and Synchronization
4.2. Food Clearance Assay for Worms
4.3. 6-OHDA Exposure and PMN Pretreatment of Worms
4.4. Quantification of DA Neuron Degeneration in Worms
4.5. Food Sensitivity Behavior Test in Worms
4.6. Lifespan Test in Worms
4.7. Quantification of Accumulation of Human α-Synuclein in Worm Muscle Cells
4.8. Analysis of Protein Expression in Worms
4.9. Determination of Reactive Oxygen Species Content in Worms
4.10. Total RNA Extraction and qPCR of Worms
4.11. Determination of the Proteasome Activity of Worms
4.12. Determination of the Autophagy Activity of Worms
4.13. RNA Interference of Worm
4.14. PMN Pretreatment and 6-OHDA Exposure of SH-SY5Y Cell Line
4.15. Preparation of SH-SY5Y Cell Line Transiently Overexpressing α-Synuclein
4.16. Immunofluorescence Staining of SH-SY5Y Cells
4.17. Cytotoxicity Analysis of PMN
4.18. Western Blot Analysis of the SH-SY5Y Cell Line
4.19. Measurement of Mitochondrial Membrane Potential in SH-SY5Y Cell Line
4.20. Staining of Hoechst 33258 in SH-SY5Y Cell Line
4.21. Apoptosis Assay by Flow Cytometry
4.22. RNA Interference of SH-SY5Y Cell Line
4.23. Determination of Proteasome Activity in SH-SY5Y Cell Line
4.24. Acidic Vesicular Organelle Staining in SH-SY5Y Cell Line
4.25. Determination of Autophilic Activity in SH-SY5Y Cell Line
4.26. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tarakad, A.; Jankovic, J. Recent Advances in Understanding and Treatment of Parkinson’s Disease. Fac. Rev. 2020, 9, 6. [Google Scholar] [CrossRef]
- Balestrino, R.; Schapira, A.H.V. Parkinson Disease. Eur. J. Neurol. 2020, 27, 27–42. [Google Scholar] [CrossRef] [PubMed]
- Bras, I.C.; Outeiro, T.F. Alpha-Synuclein: Mechanisms of Release and Pathology Progression in Synucleinopathies. Cells 2021, 10, 375. [Google Scholar] [CrossRef] [PubMed]
- Ray, B.; Mahalakshmi, A.M.; Tuladhar, S.; Bhat, A.; Srinivasan, A.; Pellegrino, C.; Kannan, A.; Bolla, S.R.; Chidambaram, S.B.; Sakharkar, M.K. “Janus-Faced” Alpha-Synuclein: Role in Parkinson’s Disease. Front. Cell Dev. Biol. 2021, 9, 673395. [Google Scholar] [CrossRef]
- He, S.; Zhong, S.; Liu, G.; Yang, J. Alpha-Synuclein: The Interplay of Pathology, Neuroinflammation, and Environmental Factors in Parkinson’s Disease. Neurodegener. Dis. 2020, 20, 55–64. [Google Scholar] [CrossRef]
- Stanojlovic, M.; Pallais, J.P.; Kotz, C.M. Inhibition of Orexin/Hypocretin Neurons Ameliorates Elevated Physical Activity and Energy Expenditure in the A53T Mouse Model of Parkinson’s Disease. Int. J. Mol. Sci. 2021, 22, 795. [Google Scholar] [CrossRef]
- Nascimento, A.C.; Erustes, A.G.; Reckziegel, P.; Bincoletto, C.; Ureshino, R.P.; Pereira, G.J.S.; Smaili, S.S. Alpha-Synuclein Overexpression Induces Lysosomal Dysfunction and Autophagy Impairment in Human Neuroblastoma SH-SY5Y. Neurochem. Res. 2020, 45, 2749–2761. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.; Jo, M.G.; Kim, S.Y.; Chung, C.G.; Lee, S.B. Dietary Antioxidants and the Mitochondrial Quality Control: Their Potential Roles in Parkinson’s Disease Treatment. Antioxidants 2020, 9, 1056. [Google Scholar] [CrossRef] [PubMed]
- Dionisio, P.A.; Amaral, J.D.; Rodrigues, C.M.P. Oxidative Stress and Regulated Cell Death in Parkinson’s Disease. Ageing Res. Rev. 2021, 67, 101263. [Google Scholar] [CrossRef]
- Paul, S.; Pickrell, A.M. Hidden Phenotypes of PINK1/Parkin Knockout Mice. Biochim. Biophys. Acta Gen. Subj. 2021, 1865, 129871. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, K. The PINK1-Parkin Axis: An Overview. Neurosci. Res. 2020, 159, 9–15. [Google Scholar] [CrossRef]
- Rakovic, A.; Ziegler, J.; Martensson, C.U.; Prasuhn, J.; Shurkewitsch, K.; Konig, P.; Paulson, H.L.; Klein, C. PINK1-Dependent Mitophagy Is Driven by the UPS and Can Occur Independently of LC3 Conversion. Cell Death Differ. 2019, 26, 1428–1441. [Google Scholar] [CrossRef] [PubMed]
- Arena, G.; Gelmetti, V.; Torosantucci, L.; Vignone, D.; Lamorte, G.; De Rosa, P.; Cilia, E.; Jonas, E.A.; Valente, E.M. PINK1 Protects Against Cell Death Induced by Mitochondrial Depolarization, by Phosphorylating Bcl-xL and Impairing Its Pro-Apoptotic Cleavage. Cell Death Differ. 2013, 20, 920–930. [Google Scholar] [CrossRef] [Green Version]
- Bernardini, J.P.; Brouwer, J.M.; Tan, I.K.; Sandow, J.J.; Huang, S.; Stafford, C.A.; Bankovacki, A.; Riffkin, C.D.; Wardak, A.Z.; Czabotar, P.E.; et al. Parkin Inhibits BAK and BAX Apoptotic Function by Distinct Mechanisms During Mitophagy. EMBO J. 2019, 38, e99916. [Google Scholar] [CrossRef]
- Tu, H.; Costa, M. XIAP’s Profile in Human Cancer. Biomolecules 2020, 10, 1493. [Google Scholar] [CrossRef] [PubMed]
- Galban, S.; Duckett, C.S. XIAP as A Ubiquitin Ligase in Cellular Signaling. Cell Death Differ. 2010, 17, 54–60. [Google Scholar] [CrossRef] [Green Version]
- Cheung, C.H.A.; Chang, Y.C.; Lin, T.Y.; Cheng, S.M.; Leung, E. Anti-apoptotic Proteins in the Autophagic World: An Update on Functions of XIAP, Survivin, and BRUCE. J. Biomed. Sci. 2020, 27, 31. [Google Scholar] [CrossRef] [PubMed]
- Shahar, N.; Larisch, S. Inhibiting the Inhibitors: Targeting Anti-Apoptotic Proteins in Cancer and Therapy Resistance. Drug Resist. Updat. 2020, 52, 100712. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Xu, F.; Lu, S.; Zhang, N.; Sun, Y. Septin4 as an Autophagy Modulator Regulates Angiotensin-II Mediated VSMCs Proliferation and Migration. Biochem. Biophys. Res. Commun. 2020, 525, 272–279. [Google Scholar] [CrossRef]
- Kemeny, S.; Dery, D.; Loboda, Y.; Rovner, M.; Lev, T.; Zuri, D.; Finberg, J.P.; Larisch, S. Parkin Promotes Degradation of the Mitochondrial Pro-Apoptotic ARTS Protein. PLoS ONE 2012, 7, e38837. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Hung, A.; Li, M.; Yang, A.W.H. Fritillariae Thunbergii Bulbus: Traditional Uses, Phytochemistry, Pharmacodynamics, Pharmacokinetics and Toxicity. Int. J. Mol. Sci. 2019, 20, 1667. [Google Scholar] [CrossRef] [Green Version]
- Du, B.; Cao, L.; Wang, K.; Miu, J.; Yao, L.; Xu, Z.; Song, J. Peiminine Attenuates Acute Lung Injury Induced by LPS Through Inhibiting Lipid Rafts Formation. Inflammation 2020, 43, 1110–1119. [Google Scholar] [CrossRef]
- Luo, Z.; Zheng, B.; Jiang, B.; Xue, X.; Xue, E.; Zhou, Y. Peiminine Inhibits the IL-1beta Induced Inflammatory Response in Mouse Articular Chondrocytes and Ameliorates Murine Osteoarthritis. Food Funct. 2019, 10, 2198–2208. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Liu, J.; Jiang, L.; Ran, X.; He, D.; Li, Y.; Huang, B.; Wang, W.; Liu, D.; Fu, S. Peiminine Protects Dopaminergic Neurons from Inflammation-Induced Cell Death by Inhibiting the ERK1/2 and NF-kappaB Signalling Pathways. Int. J. Mol. Sci. 2018, 19, 821. [Google Scholar] [CrossRef] [Green Version]
- Caldwell, K.A.; Willicott, C.W.; Caldwell, G.A. Modeling Neurodegeneration in Caenorhabditis elegans. Dis. Model Mech. 2020, 13, dmm046110. [Google Scholar] [CrossRef] [PubMed]
- Brunetti, G.; Di Rosa, G.; Scuto, M.; Leri, M.; Stefani, M.; Schmitz-Linneweber, C.; Calabrese, V.; Saul, N. Healthspan Maintenance and Prevention of Parkinson’s-like Phenotypes with Hydroxytyrosol and Oleuropein Aglycone in C. elegans. Int. J. Mol. Sci. 2020, 21, 2588. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bagoudou, A.F.; Zheng, Y.; Nakabayashi, M.; Rawdkuen, S.; Park, H.Y.; Vattem, D.A.; Sato, K.; Nakamura, S.; Katayama, S. Glochidion littorale Leaf Extract Exhibits Neuroprotective Effects in Caenorhabditis elegans via DAF-16 Activation. Molecules 2021, 26, 2958. [Google Scholar] [CrossRef]
- Lee, H.S.; Kim, E.N.; Jeong, G.S. Lupenone Protects Neuroblastoma SH-SY5y Cells Against Methamphetamine-Induced Apoptotic Cell Death via PI3K/Akt/mTOR Signaling Pathway. Int. J. Mol. Sci. 2020, 21, 1617. [Google Scholar] [CrossRef] [Green Version]
- Tian, W.; Heo, S.; Kim, D.W.; Kim, I.S.; Ahn, D.; Tae, H.J.; Kim, M.K.; Park, B.Y. Ethanol Extract of Maclura tricuspidata Fruit Protects SH-SY5Y Neuroblastoma Cells Against H2O2-Induced Oxidative Damage via Inhibiting MAPK and NF-kappaB Signaling. Int. J. Mol. Sci. 2021, 22, 6946. [Google Scholar] [CrossRef]
- Chen, J.H.; Ou, H.P.; Lin, C.Y.; Lin, F.J.; Wu, C.R.; Chang, S.W.; Tsai, C.W. Carnosic Acid Prevents 6-Hydroxydopamine-Induced Cell Death in SH-SY5Y Cells via Mediation of Glutathione Synthesis. Chem. Res. Toxicol. 2012, 25, 1893–1901. [Google Scholar] [CrossRef]
- Tsai, R.T.; Tsai, C.W.; Liu, S.P.; Gao, J.X.; Kuo, Y.H.; Chao, P.M.; Hung, H.S.; Shyu, W.C.; Lin, S.Z.; Fu, R.H. Maackiain Ameliorates 6-Hydroxydopamine and SNCA Pathologies by Modulating the PINK1/Parkin Pathway in Models of Parkinson’s Disease in Caenorhabditis elegans and the SH-SY5Y Cell Line. Int. J. Mol. Sci. 2020, 21, 4455. [Google Scholar] [CrossRef]
- Li, J.; Qin, Y.; Wang, W.; Yang, K.; Zhang, M. Peiminine Inhibits the Progression of Colorectal Cancer Through Up-Regulating MiR-760 via Declining the Expression of Long Noncoding RNA LINC00659. Anticancer Drugs 2021, 32, 148–156. [Google Scholar] [CrossRef] [PubMed]
- Morais, M.G.; Saldanha, A.A.; Costa Rodrigues, J.P.; Cotta Mendes, I.; Ferreira, L.M.; Avelar Amado, P.; de Souza Farias, K.; Samudio Santos Zanuncio, V.; Brentan da Silva, D.; Carmo Horta Pinto, F.; et al. Chemical Composition, Antioxidant, Anti-inflammatory and Antinociceptive Activities of the Ethanol Extract of Ripe Fruits of Solanum lycocarpum St. Hil. (Solanaceae). J. Ethnopharmacol. 2020, 262, 113125. [Google Scholar] [CrossRef] [PubMed]
- Brown, S.J.; Boussaad, I.; Jarazo, J.; Fitzgerald, J.C.; Antony, P.; Keatinge, M.; Blechman, J.; Schwamborn, J.C.; Kruger, R.; Placzek, M.; et al. PINK1 Deficiency Impairs Adult Neurogenesis of Dopaminergic Neurons. Sci. Rep. 2021, 11, 6617. [Google Scholar] [CrossRef]
- Bus, C.; Zizmare, L.; Feldkaemper, M.; Geisler, S.; Zarani, M.; Schaedler, A.; Klose, F.; Admard, J.; Mageean, C.J.; Arena, G.; et al. Human Dopaminergic Neurons Lacking PINK1 Exhibit Disrupted Dopamine Metabolism Related to Vitamin B6 Co-Factors. iScience 2020, 23, 101797. [Google Scholar] [CrossRef]
- Qiao, J.D.; Mao, Y.L. Knockout of PINK1 Altered the Neural Connectivity of Drosophila Dopamine PPM3 Neurons at Input and Output Sites. Invert. Neurosci. 2020, 20, 11. [Google Scholar] [CrossRef]
- Creed, R.B.; Goldberg, M.S. Enhanced Susceptibility of PINK1 Knockout Rats to Alpha-Synuclein Fibrils. Neuroscience 2020, 437, 64–75. [Google Scholar] [CrossRef] [PubMed]
- Maynard, M.E.; Redell, J.B.; Kobori, N.; Underwood, E.L.; Fischer, T.D.; Hood, K.N.; LaRoche, V.; Waxham, M.N.; Moore, A.N.; Dash, P.K. Loss of PTEN-Induced Kinase 1 (Pink1) Reduces Hippocampal Tyrosine Hydroxylase and Impairs Learning and Memory. Exp. Neurol. 2020, 323, 113081. [Google Scholar] [CrossRef]
- Li, J.; Xue, C.; Gao, Q.; Tan, J.; Wan, Z. Mitochondrial DNA Heteroplasmy Rises in Substantial Nigra of Aged PINK1 KO Mice. Biochem. Biophys. Res. Commun. 2020, 521, 1024–1029. [Google Scholar] [CrossRef] [PubMed]
- Furlong, R.M.; O’Keeffe, G.W.; O’Neill, C.; Sullivan, A.M. Alterations in Alpha-Synuclein and PINK1 Expression Reduce Neurite Length and Induce Mitochondrial Fission and Golgi Fragmentation in Midbrain Neurons. Neurosci. Lett. 2020, 720, 134777. [Google Scholar] [CrossRef]
- Han, H.; Tan, J.; Wang, R.; Wan, H.; He, Y.; Yan, X.; Guo, J.; Gao, Q.; Li, J.; Shang, S.; et al. PINK1 Phosphorylates Drp1(S616) to Regulate Mitophagy-Independent Mitochondrial Dynamics. EMBO Rep. 2020, 21, e48686. [Google Scholar] [CrossRef]
- Noda, S.; Sato, S.; Fukuda, T.; Tada, N.; Uchiyama, Y.; Tanaka, K.; Hattori, N. Loss of Parkin Contributes to Mitochondrial Turnover and Dopaminergic Neuronal Loss in Aged Mice. Neurobiol. Dis. 2020, 136, 104717. [Google Scholar] [CrossRef]
- Baaske, M.K.; Kramer, E.R.; Meka, D.P.; Engler, G.; Engel, A.K.; Moll, C.K.E. Parkin Deficiency Perturbs Striatal Circuit Dynamics. Neurobiol. Dis. 2020, 137, 104737. [Google Scholar] [CrossRef]
- Jo, A.; Lee, Y.; Park, C.H.; Shin, J.H. Deubiquitinase USP29 Governs MYBBP1A in the Brains of Parkinson’s Disease Patients. J. Clin. Med. 2019, 9, 52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilkaniec, A.; Lenkiewicz, A.M.; Babiec, L.; Murawska, E.; Jesko, H.M.; Cieslik, M.; Culmsee, C.; Adamczyk, A. Exogenous Alpha-Synuclein Evoked Parkin Downregulation Promotes Mitochondrial Dysfunction in Neuronal Cells. Implications for Parkinson’s Disease Pathology. Front. Aging Neurosci. 2021, 13, 591475. [Google Scholar] [CrossRef]
- Yan, Y.Q.; Fang, Y.; Zheng, R.; Pu, J.L.; Zhang, B.R. NLRP3 Inflammasomes in Parkinson’s Disease and Their Regulation by Parkin. Neuroscience 2020, 446, 323–334. [Google Scholar] [CrossRef]
- Tokarew, J.M.; El-Kodsi, D.N.; Lengacher, N.A.; Fehr, T.K.; Nguyen, A.P.; Shutinoski, B.; O’Nuallain, B.; Jin, M.; Khan, J.M.; Ng, A.C.H.; et al. Age-Associated Insolubility of Parkin in Human Midbrain is Linked to Redox Balance and Sequestration of Reactive Dopamine Metabolites. Acta Neuropathol. 2021, 141, 725–754. [Google Scholar] [CrossRef] [PubMed]
- Ganguly, U.; Banerjee, A.; Chakrabarti, S.S.; Kaur, U.; Sen, O.; Cappai, R.; Chakrabarti, S. Interaction of Alpha-synuclein and Parkin in Iron Toxicity on SH-SY5Y Cells: Implications in the Pathogenesis of Parkinson’s Disease. Biochem. J. 2020, 477, 1109–1122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gholkar, A.A.; Schmollinger, S.; Velasquez, E.F.; Lo, Y.C.; Cohn, W.; Capri, J.; Dharmarajan, H.; Deardorff, W.J.; Gao, L.W.; Abdusamad, M.; et al. Regulation of Iron Homeostasis Through Parkin-Mediated Lactoferrin Ubiquitylation. Biochemistry 2020, 59, 2916–2921. [Google Scholar] [CrossRef] [PubMed]
- Bi, M.; Du, X.; Jiao, Q.; Liu, Z.; Jiang, H. Alpha-Synuclein Regulates Iron Homeostasis via Preventing Parkin-Mediated DMT1 Ubiquitylation in Parkinson’s Disease Models. ACS Chem. Neurosci. 2020, 11, 1682–1691. [Google Scholar] [CrossRef]
- Zhong, Y.; Li, X.; Du, X.; Bi, M.; Ma, F.; Xie, J.; Jiang, H. The S-Nitrosylation of Parkin Attenuated the Ubiquitination of Divalent Metal Transporter 1 in MPP(+)-Treated SH-SY5Y Cells. Sci. Rep. 2020, 10, 15542. [Google Scholar] [CrossRef]
- Cahill, C.M.; Lahiri, D.K.; Huang, X.; Rogers, J.T. Amyloid Precursor Protein and Alpha Synuclein Translation, Implications for Iron and Inflammation in Neurodegenerative Diseases. Biochim. Biophys. Acta. 2009, 1790, 615–628. [Google Scholar] [CrossRef] [Green Version]
- Schwartzentruber, A.; Boschian, C.; Lopes, F.M.; Myszczynska, M.A.; New, E.J.; Beyrath, J.; Smeitink, J.; Ferraiuolo, L.; Mortiboys, H. Oxidative Switch Drives Mitophagy Defects in Dopaminergic Parkin Mutant Patient Neurons. Sci. Rep. 2020, 10, 15485. [Google Scholar] [CrossRef] [PubMed]
- Okarmus, J.; Bogetofte, H.; Schmidt, S.I.; Ryding, M.; Garcia-Lopez, S.; Ryan, B.J.; Martinez-Serrano, A.; Hyttel, P.; Meyer, M. Lysosomal Perturbations in Human Dopaminergic Neurons Derived from Induced Pluripotent Stem Cells with PARK2 Mutation. Sci. Rep. 2020, 10, 10278. [Google Scholar] [CrossRef] [PubMed]
- Pu, J.; Gao, T.; Zheng, R.; Fang, Y.; Ruan, Y.; Jin, C.; Shen, T.; Tian, J.; Zhang, B. Parkin Mutation Decreases Neurite Complexity and Maturation in Neurons Derived from Human Fibroblasts. Brain Res. Bull. 2020, 159, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Pirooznia, S.K.; Yuan, C.; Khan, M.R.; Karuppagounder, S.S.; Wang, L.; Xiong, Y.; Kang, S.U.; Lee, Y.; Dawson, V.L.; Dawson, T.M. PARIS Induced Defects in Mitochondrial Biogenesis Drive Dopamine Neuron Loss Under Conditions of Parkin or PINK1 Deficiency. Mol. Neurodegener. 2020, 15, 17. [Google Scholar] [CrossRef] [Green Version]
- Ham, S.J.; Lee, D.; Yoo, H.; Jun, K.; Shin, H.; Chung, J. Decision Between Mitophagy and Apoptosis by Parkin via VDAC1 Ubiquitination. Proc. Natl. Acad. Sci. USA 2020, 117, 4281–4291. [Google Scholar] [CrossRef]
- Borsche, M.; Konig, I.R.; Delcambre, S.; Petrucci, S.; Balck, A.; Bruggemann, N.; Zimprich, A.; Wasner, K.; Pereira, S.L.; Avenali, M.; et al. Mitochondrial Damage-Associated Inflammation Highlights Biomarkers in PRKN/PINK1 Parkinsonism. Brain 2020, 143, 3041–3051. [Google Scholar] [CrossRef]
- Barazzuol, L.; Giamogante, F.; Brini, M.; Cali, T. PINK1/Parkin Mediated Mitophagy, Ca(2+) Signalling, and ER-Mitochondria Contacts in Parkinson’s Disease. Int. J. Mol. Sci. 2020, 21, 1772. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kesh, S.; Kannan, R.R.; Balakrishnan, A. Naringenin Alleviates 6-Hydroxydopamine Induced Parkinsonism in SHSY5Y Cells and Zebrafish Model. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2021, 239, 108893. [Google Scholar] [CrossRef]
- Qi, H.; Shen, D.; Jiang, C.; Wang, H.; Chang, M. Ursodeoxycholic Acid Protects Dopaminergic Neurons from Oxidative Stress via Regulating Mitochondrial Function, Autophagy, and Apoptosis in MPTP/MPP(+)-Induced Parkinson’s Disease. Neurosci. Lett. 2021, 741, 135493. [Google Scholar] [CrossRef]
- Lin, M.W.; Lin, C.C.; Chen, Y.H.; Yang, H.B.; Hung, S.Y. Celastrol Inhibits Dopaminergic Neuronal Death of Parkinson’s Disease Through Activating Mitophagy. Antioxidants 2019, 9, 37. [Google Scholar] [CrossRef] [Green Version]
- Liu, M.; Yu, S.; Wang, J.; Qiao, J.; Liu, Y.; Wang, S.; Zhao, Y. Ginseng Protein Protects Against Mitochondrial Dysfunction and Neurodegeneration by Inducing Mitochondrial Unfolded Protein Response in Drosophila Melanogaster PINK1 Model of Parkinson’s Disease. J. Ethnopharmacol. 2020, 247, 112213. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.; Kwatra, M.; Ranjan Panda, S.; Murty, U.S.N.; Naidu, V.G.M. Andrographolide Suppresses NLRP3 Inflammasome Activation in Microglia Through Induction of Parkin-Mediated Mitophagy in In-Vitro and In-Vivo Models of Parkinson Disease. Brain Behav. Immun. 2021, 91, 142–158. [Google Scholar] [CrossRef]
- Huang, C.Y.; Sivalingam, K.; Shibu, M.A.; Liao, P.H.; Ho, T.J.; Kuo, W.W.; Chen, R.J.; Day, C.H.; Viswanadha, V.P.; Ju, D.T. Induction of Autophagy by Vasicinone Protects Neural Cells from Mitochondrial Dysfunction and Attenuates Paraquat-Mediated Parkinson’s Disease Associated alpha-Synuclein Levels. Nutrients 2020, 12, 1707. [Google Scholar] [CrossRef]
- Gundogdu, M.; Tadayon, R.; Salzano, G.; Shaw, G.S.; Walden, H. A Mechanistic Review of Parkin Activation. Biochim. Biophys. Acta Gen. Subj. 2021, 1865, 129894. [Google Scholar] [CrossRef] [PubMed]
- Larisch, S.; Yi, Y.; Lotan, R.; Kerner, H.; Eimerl, S.; Tony Parks, W.; Gottfried, Y.; Birkey Reffey, S.; de Caestecker, M.P.; Danielpour, D.; et al. A Novel Mitochondrial Septin-Like Protein, ARTS, Mediates Apoptosis Dependent on Its P-loop Motif. Nat. Cell Biol. 2000, 2, 915–921. [Google Scholar] [CrossRef] [PubMed]
- Shehadeh, L.; Mitsi, G.; Adi, N.; Bishopric, N.; Papapetropoulos, S. Expression of Lewy Body Protein Septin 4 in Postmortem Brain of Parkinson’s Disease and Control Subjects. Mov. Disord. 2009, 24, 204–210. [Google Scholar] [CrossRef]
- Munoz-Soriano, V.; Paricio, N. Overexpression of Septin 4, the Drosophila Homologue of Human CDCrel-1, Is Toxic for DopaMinergic Neurons. Eur. J. Neurosci. 2007, 26, 3150–3158. [Google Scholar] [CrossRef]
- Munoz-Soriano, V.; Nieto-Arellano, R.; Paricio, N. Septin 4, the Drosophila Ortholog of Human CDCrel-1, Accumulates in Parkin Mutant Brains and Is Functionally Related to the Nedd4 E3 Ubiquitin Ligase. J. Mol. Neurosci. 2012, 48, 136–143. [Google Scholar] [CrossRef]
- Hao, Q.; Chen, J.; Liao, J.; Huang, Y.; Gan, Y.; Larisch, S.; Zeng, S.X.; Lu, H.; Zhou, X. P53 Induces ARTS to Promote Mitochondrial Apoptosis. Cell Death Dis. 2021, 12, 204. [Google Scholar] [CrossRef]
- Crocker, S.J.; Liston, P.; Anisman, H.; Lee, C.J.; Smith, P.D.; Earl, N.; Thompson, C.S.; Park, D.S.; Korneluk, R.G.; Robertson, G.S. Attenuation of MPTP-induced Neurotoxicity and Behavioural Impairment in NSE-XIAP Transgenic Mice. Neurobiol. Dis. 2003, 12, 150–161. [Google Scholar] [CrossRef]
- Oh, C.K.; Choi, Y.K.; Hwang, I.Y.; Ko, Y.U.; Chung, I.K.; Yun, N.; Oh, Y.J. RING-finger Protein 166 Plays a Novel Pro-Apoptotic Role in Neurotoxin-Induced Neurodegeneration via Ubiquitination of XIAP. Cell Death Dis. 2020, 11, 939. [Google Scholar] [CrossRef] [PubMed]
- Fu, R.H.; Huang, L.C.; Lin, C.Y.; Tsai, C.W. Modulation of ARTS and XIAP by Parkin Is Associated with Carnosic Acid Protects SH-SY5Y Cells Against 6-Hydroxydopamine-Induced Apoptosis. Mol. Neurobiol. 2018, 55, 1786–1794. [Google Scholar] [CrossRef] [PubMed]
- Bornstein, B.; Edison, N.; Gottfried, Y.; Lev, T.; Shekhtman, A.; Gonen, H.; Rajalingam, K.; Larisch, S. X-linked Inhibitor of Apoptosis Protein Promotes the Degradation of Its Antagonist, the Pro-Apoptotic ARTS Protein. Int. J. Biochem. Cell Biol. 2012, 44, 489–495. [Google Scholar] [CrossRef] [PubMed]
- Van Den Berge, N.; Ferreira, N.; Gram, H.; Mikkelsen, T.W.; Alstrup, A.K.O.; Casadei, N.; Tsung-Pin, P.; Riess, O.; Nyengaard, J.R.; Tamguney, G.; et al. Evidence for Bidirectional and Trans-Synaptic Parasympathetic and Sympathetic Propagation of Alpha-synuclein in Rats. Acta Neuropathol. 2019, 138, 535–550. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferreira, N.; Goncalves, N.P.; Jan, A.; Jensen, N.M.; van der Laan, A.; Mohseni, S.; Vaegter, C.B.; Jensen, P.H. Trans-Synaptic Spreading of Alpha-Synuclein Pathology Through Sensory Afferents leads to Sensory Nerve Degeneration and Neuropathic pain. Acta Neuropathol. Commun. 2021, 9, 31. [Google Scholar] [CrossRef]
- Ferreira, N.; Gram, H.; Sorrentino, Z.A.; Gregersen, E.; Schmidt, S.I.; Reimer, L.; Betzer, C.; Perez-Gozalbo, C.; Beltoja, M.; Nagaraj, M.; et al. Multiple System Atrophy-Associated Oligodendroglial Protein p25Alpha Stimulates Formation of Novel Alpha-Synuclein Strain With Enhanced Neurodegenerative Potential. Acta Neuropathol. 2021, 142, 87–115. [Google Scholar] [CrossRef]
- Guo, H.; Ji, F.; Liu, B.; Chen, X.; He, J.; Gong, J. Peiminine Ameliorates Bleomycin-Induced Acute Lung Injury in Rats. Mol. Med. Rep. 2013, 7, 1103–1110. [Google Scholar] [CrossRef] [Green Version]
- Lim, J.M.; Lee, B.; Min, J.H.; Kim, E.Y.; Kim, J.H.; Hong, S.; Kim, J.J.; Sohn, Y.; Jung, H.S. Effect of Peiminine on DNCB-Induced atopic Dermatitis by Inhibiting Inflammatory Cytokine Expression in Vivo and in Vitro. Int. Immunopharmacol. 2018, 56, 135–142. [Google Scholar] [CrossRef]
- Chen, L.H.; Zhang, H.M.; Guan, Z.Y.; Zhu, W.F.; Yi, W.J.; Guan, Y.M.; Wang, S.; Liu, H.N. Sex Dependent Pharmacokinetics, Tissue Distribution and Excretion of Peimine and Peiminine in Rats Assessed by Liquid Chromatography-Tandem Mass Spectrometry. J. Ethnopharmacol. 2013, 145, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Duty, S.; Jenner, P. Animal Models of Parkinson’s Disease: A Source of Novel Treatments and Clues to the Cause of the Disease. Br. J. Pharmacol. 2011, 164, 1357–1391. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fu, R.H.; Wang, Y.C.; Chen, C.S.; Tsai, R.T.; Liu, S.P.; Chang, W.L.; Lin, H.L.; Lu, C.H.; Wei, J.R.; Wang, Z.W.; et al. Acetylcorynoline Attenuates Dopaminergic Neuron Degeneration and Alpha-Synuclein Aggregation in Animal Models of Parkinson’s Disease. Neuropharmacology 2014, 82, 108–120. [Google Scholar] [CrossRef] [PubMed]





















| Genes of C. elegans (Human) | Primer Sequences (5′-3′) | (Start→End) Size (bp) |
|---|---|---|
| Lrk-1 (LRRK1) | Forward: TTTCAACACCCAATCTCCAAC Reverse: TGATACTCGCTTGCCACAC | (1983→2092) 110 |
| Pdr-1 (PRKN) | Forward: TGCTCGTCAACCTCTGTTC Reverse: TCACTTTCTCCTTCCCATCAC | (376→601) 226 |
| Pink-1 (PINK1) | Forward: GAGACGATACCGACAAACAC Reverse: GGCATTTCCTCCAAGACTAAC | (882→1158) 277 |
| Djr-1.1 (PARK7) | Forward: CGGATTAGATGGAGCCGAAC Reverse: ATCAGCCCACCAGACTCTAC | (111→305) 195 |
| Djr-1.2 (PARK7) | Forward: GCTTTGATCCTTTTGCCACC Reverse: CTGCCAGTTTGCTACATCC | (19→247) 229 |
| Vps-35 (VPS35) | Forward: AACTCTGCTCAAAACTACTCAC Reverse: CCACAACCTTCTTCCCATTC | (1953→2146) 194 |
| Catp-6 (ATP13A3) | Forward: TCACACCATACCAACCTCC Reverse: GTTTCCAAGAGTCTTCAGAACC | (3092→3336) 245 |
| Dnj-27 (DNAJC10) | Forward: TCCACTTATTGCTCACATTGTC Reverse: TCCACCATCAACTCCACATC | (427→635) 209 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hsu, Y.-L.; Hung, H.-S.; Tsai, C.-W.; Liu, S.-P.; Chiang, Y.-T.; Kuo, Y.-H.; Shyu, W.-C.; Lin, S.-Z.; Fu, R.-H. Peiminine Reduces ARTS-Mediated Degradation of XIAP by Modulating the PINK1/Parkin Pathway to Ameliorate 6-Hydroxydopamine Toxicity and α-Synuclein Accumulation in Parkinson’s Disease Models In Vivo and In Vitro. Int. J. Mol. Sci. 2021, 22, 10240. https://doi.org/10.3390/ijms221910240
Hsu Y-L, Hung H-S, Tsai C-W, Liu S-P, Chiang Y-T, Kuo Y-H, Shyu W-C, Lin S-Z, Fu R-H. Peiminine Reduces ARTS-Mediated Degradation of XIAP by Modulating the PINK1/Parkin Pathway to Ameliorate 6-Hydroxydopamine Toxicity and α-Synuclein Accumulation in Parkinson’s Disease Models In Vivo and In Vitro. International Journal of Molecular Sciences. 2021; 22(19):10240. https://doi.org/10.3390/ijms221910240
Chicago/Turabian StyleHsu, Yu-Ling, Huey-Shan Hung, Chia-Wen Tsai, Shih-Ping Liu, Yu-Ting Chiang, Yun-Hua Kuo, Woei-Cherng Shyu, Shinn-Zong Lin, and Ru-Huei Fu. 2021. "Peiminine Reduces ARTS-Mediated Degradation of XIAP by Modulating the PINK1/Parkin Pathway to Ameliorate 6-Hydroxydopamine Toxicity and α-Synuclein Accumulation in Parkinson’s Disease Models In Vivo and In Vitro" International Journal of Molecular Sciences 22, no. 19: 10240. https://doi.org/10.3390/ijms221910240
APA StyleHsu, Y. -L., Hung, H. -S., Tsai, C. -W., Liu, S. -P., Chiang, Y. -T., Kuo, Y. -H., Shyu, W. -C., Lin, S. -Z., & Fu, R. -H. (2021). Peiminine Reduces ARTS-Mediated Degradation of XIAP by Modulating the PINK1/Parkin Pathway to Ameliorate 6-Hydroxydopamine Toxicity and α-Synuclein Accumulation in Parkinson’s Disease Models In Vivo and In Vitro. International Journal of Molecular Sciences, 22(19), 10240. https://doi.org/10.3390/ijms221910240