Flavonoids: Nutraceuticals for Rheumatic Diseases via Targeting of Inflammasome Activation
Abstract
:1. Introduction
2. Inflammasome-Induced Inflammatory Responses
2.1. Structures and Activation of Canonical Inflammasomes
2.2. Structures and Activation of Caspase-11 Non-Canonical Inflammasomes
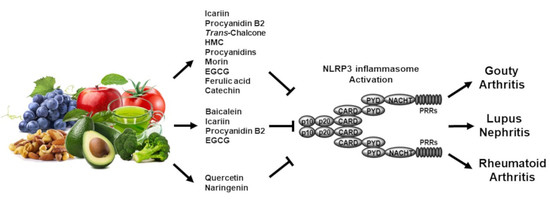
3. Regulatory Roles of Flavonoids in Inflammasome-Mediated Rheumatic Diseases
3.1. Gouty Arthritis
3.2. Systemic Lupus Erythematosus
3.3. Rheumatoid Arthritis
4. Conclusions and Perspectives
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| IL | Interleukin |
| PRR | Pattern recognition receptor |
| TLR | Toll-like receptor |
| PAMP | Pattern-associated molecular pattern |
| DAMP | Danger-associated molecular pattern |
| NLR | NOD-like receptor |
| RLR | Retinoic acid-inducible gene I-like receptor |
| AIM2 | Absent in melanoma 2 |
| ALR | AIM2-like receptor |
| LPS | Lipopolysaccharide |
| GSDMD | Gasdermin D |
| NACHT | Nucleotide-binding and oligomerization domain |
| LRR | Leucine-rich repeat |
| FIIND | Functional-to-find domain |
| OMV | Outer membrane vesicle |
| GA | Gouty arthritis |
| SLE | Systemic lupus erythematosus |
| LN | Lupus nephritis |
| RA | Rheumatoid arthritis |
| MSU | Monosodium urate |
| EGCG | Epigallocatechin-3-gallate |
References
- Janeway, C.A., Jr.; Medzhitov, R. Innate immune recognition. Annu. Rev. Immunol. 2002, 20, 197–216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takeuchi, O.; Akira, S. Pattern recognition receptors and inflammation. Cell 2010, 140, 805–820. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, L.; Deng, H.; Cui, H.; Fang, J.; Zuo, Z.; Deng, J.; Li, Y.; Wang, X.; Zhao, L. Inflammatory responses and inflammation-associated diseases in organs. Oncotarget 2018, 9, 7204–7218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yi, Y.S. Folate Receptor-Targeted Diagnostics and Therapeutics for Inflammatory Diseases. Immune Netw. 2016, 16, 337–343. [Google Scholar] [CrossRef] [PubMed]
- Duan, L.; Rao, X.; Sigdel, K.R. Regulation of Inflammation in Autoimmune Disease. J. Immunol. Res. 2019, 2019, 7403796. [Google Scholar] [CrossRef] [Green Version]
- Singh, R.; Mishra, M.K.; Aggarwal, H. Inflammation, Immunity, and Cancer. Mediat. Inflamm. 2017, 2017, 6027305. [Google Scholar] [CrossRef]
- Yi, Y.S.; Son, Y.J.; Ryou, C.; Sung, G.H.; Kim, J.H.; Cho, J.Y. Functional roles of Syk in macrophage-mediated inflammatory responses. Mediat. Inflamm. 2014, 2014, 270302. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Kim, S.C.; Yu, T.; Yi, Y.S.; Rhee, M.H.; Sung, G.H.; Yoo, B.C.; Cho, J.Y. Functional roles of p38 mitogen-activated protein kinase in macrophage-mediated inflammatory responses. Mediat. Inflamm. 2014, 2014, 352371. [Google Scholar] [CrossRef] [Green Version]
- Yu, T.; Yi, Y.S.; Yang, Y.; Oh, J.; Jeong, D.; Cho, J.Y. The pivotal role of TBK1 in inflammatory responses mediated by macrophages. Mediat. Inflamm. 2012, 2012, 979105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, Y.K.; Kang, M.; Choi, E.Y. TLR/MyD88-mediated Innate Immunity in Intestinal Graft-versus-Host Disease. Immune Netw. 2017, 17, 144–151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xue, Y.; Enosi Tuipulotu, D.; Tan, W.H.; Kay, C.; Man, S.M. Emerging Activators and Regulators of Inflammasomes and Pyroptosis. Trends Immunol. 2019, 40, 1035–1052. [Google Scholar] [CrossRef]
- Yi, Y.S. Caspase-11 non-canonical inflammasome: A critical sensor of intracellular lipopolysaccharide in macrophage-mediated inflammatory responses. Immunology 2017, 152, 207–217. [Google Scholar] [CrossRef]
- Xia, S.; Hollingsworth, L.R.; Wu, H. Mechanism and Regulation of Gasdermin-Mediated Cell Death. Cold Spring Harb. Perspect. Biol. 2019, 12, a036400. [Google Scholar] [CrossRef] [Green Version]
- Yi, Y.S. Regulatory Roles of the Caspase-11 Non-Canonical Inflammasome in Inflammatory Diseases. Immune Netw. 2018, 18, e41. [Google Scholar] [CrossRef]
- Yi, Y.S. Functional crosstalk between non-canonical caspase-11 and canonical NLRP3 inflammasomes during infection-mediated inflammation. Immunology 2020, 159, 142–155. [Google Scholar] [CrossRef] [Green Version]
- Yun, M.; Yi, Y.S. Regulatory roles of ginseng on inflammatory caspases, executioners of inflammasome activation. J. Ginseng Res. 2020, 44, 373–385. [Google Scholar] [CrossRef]
- Guo, H.; Callaway, J.B.; Ting, J.P. Inflammasomes: Mechanism of action, role in disease, and therapeutics. Nat. Med. 2015, 21, 677–687. [Google Scholar] [CrossRef] [Green Version]
- Wilson, S.P.; Cassel, S.L. Inflammasome-mediated autoinflammatory disorders. Postgrad. Med. 2010, 122, 125–133. [Google Scholar] [CrossRef]
- Yi, Y.S. Role of inflammasomes in inflammatory autoimmune rheumatic diseases. Korean J. Physiol. Pharmacol. 2018, 22, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Kayagaki, N.; Warming, S.; Lamkanfi, M.; Vande Walle, L.; Louie, S.; Dong, J.; Newton, K.; Qu, Y.; Liu, J.; Heldens, S.; et al. Non-canonical inflammasome activation targets caspase-11. Nature 2011, 479, 117–121. [Google Scholar] [CrossRef]
- Shi, J.; Zhao, Y.; Wang, Y.; Gao, W.; Ding, J.; Li, P.; Hu, L.; Shao, F. Inflammatory caspases are innate immune receptors for intracellular LPS. Nature 2014, 514, 187–192. [Google Scholar] [CrossRef]
- Yi, Y.S. Caspase-11 Non-Canonical Inflammasome: Emerging Activator and Regulator of Infection-Mediated Inflammatory Responses. Int. J. Mol. Sci. 2020, 21, 2736. [Google Scholar] [CrossRef] [Green Version]
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An overview. J. Nutr. Sci. 2016, 5, e47. [Google Scholar] [CrossRef] [Green Version]
- Kay, C.D. The future of flavonoid research. Br. J. Nutr. 2010, 104 (Suppl. S3), S91–S95. [Google Scholar] [CrossRef]
- Maleki, S.J.; Crespo, J.F.; Cabanillas, B. Anti-inflammatory effects of flavonoids. Food Chem. 2019, 299, 125124. [Google Scholar] [CrossRef]
- Serafini, M.; Peluso, I.; Raguzzini, A. Flavonoids as anti-inflammatory agents. Proc. Nutr. Soc. 2010, 69, 273–278. [Google Scholar] [CrossRef] [Green Version]
- Choy, K.W.; Murugan, D.; Leong, X.F.; Abas, R.; Alias, A.; Mustafa, M.R. Flavonoids as Natural Anti-Inflammatory Agents Targeting Nuclear Factor-Kappa B (NFkappaB) Signaling in Cardiovascular Diseases: A Mini Review. Front. Pharmacol. 2019, 10, 1295. [Google Scholar] [CrossRef] [Green Version]
- Bai, L.; Bai, Y.; Yang, Y.; Zhang, W.; Huang, L.; Ma, R.; Wang, L.; Duan, H.; Wan, Q. Baicalin alleviates collageninduced arthritis and suppresses TLR2/MYD88/NFkappaB p65 signaling in rats and HFLSRAs. Mol. Med. Rep. 2020, 22, 2833–2841. [Google Scholar] [CrossRef]
- Feng, H.; He, Y.; La, L.; Hou, C.; Song, L.; Yang, Q.; Wu, F.; Liu, W.; Hou, L.; Li, Y.; et al. The flavonoid-enriched extract from the root of Smilax china L. inhibits inflammatory responses via the TLR-4-mediated signaling pathway. J. Ethnopharmacol. 2020, 256, 112785. [Google Scholar] [CrossRef]
- Yahfoufi, N.; Alsadi, N.; Jambi, M.; Matar, C. The Immunomodulatory and Anti-Inflammatory Role of Polyphenols. Nutrients 2018, 10, 1618. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Yoon, J.H.; Won, H.J.; Ji, H.S.; Yuk, H.J.; Park, K.H.; Park, H.Y.; Jeong, T.S. Isotrifoliol inhibits pro-inflammatory mediators by suppression of TLR/NF-kappaB and TLR/MAPK signaling in LPS-induced RAW264.7 cells. Int. Immunopharmacol. 2017, 45, 110–119. [Google Scholar] [CrossRef] [PubMed]
- Yi, Y.S. Regulatory Roles of Flavonoids on Inflammasome Activation during Inflammatory Responses. Mol. Nutr. Food Res. 2018, 62, e1800147. [Google Scholar] [CrossRef] [PubMed]
- Owona, B.A.; Abia, W.A.; Moundipa, P.F. Natural compounds flavonoids as modulators of inflammasomes in chronic diseases. Int. Immunopharmacol. 2020, 84, 106498. [Google Scholar] [CrossRef] [PubMed]
- Martinez, G.; Mijares, M.R.; De Sanctis, J.B. Effects of Flavonoids and Its Derivatives on Immune Cell Responses. Recent Pat. Inflamm. Allergy Drug Discov. 2019, 13, 84–104. [Google Scholar] [CrossRef] [PubMed]
- Lim, H.; Heo, M.Y.; Kim, H.P. Flavonoids: Broad Spectrum Agents on Chronic Inflammation. Biomol. Ther. 2019, 27, 241–253. [Google Scholar] [CrossRef]
- de Carvalho, J.F.; da Silva, F.F.; Ferreira de Andrade, C.A.; Argolo, J.D.; da Mota, L.M.H. Famous Artists Who Suffer(ed) From Rheumatic Diseases: A Systematic Review. J. Rheumatol. 2020. [Google Scholar] [CrossRef]
- Yi, Y.S. Ameliorative effects of ginseng and ginsenosides on rheumatic diseases. J. Ginseng Res. 2019, 43, 335–341. [Google Scholar] [CrossRef]
- Yi, Y.S. Roles of ginsenosides in inflammasome activation. J. Ginseng Res. 2019, 43, 172–178. [Google Scholar] [CrossRef]
- Gegner, J.A.; Ulevitch, R.J.; Tobias, P.S. Lipopolysaccharide (LPS) signal transduction and clearance. Dual roles for LPS binding protein and membrane CD14. J. Biol. Chem. 1995, 270, 5320–5325. [Google Scholar] [CrossRef] [Green Version]
- Deng, M.; Tang, Y.; Li, W.; Wang, X.; Zhang, R.; Zhang, X.; Zhao, X.; Liu, J.; Tang, C.; Liu, Z.; et al. The Endotoxin Delivery Protein HMGB1 Mediates Caspase-11-Dependent Lethality in Sepsis. Immunity 2018, 49, 740–753.e7. [Google Scholar] [CrossRef] [Green Version]
- Vanaja, S.K.; Russo, A.J.; Behl, B.; Banerjee, I.; Yankova, M.; Deshmukh, S.D.; Rathinam, V.A.K. Bacterial Outer Membrane Vesicles Mediate Cytosolic Localization of LPS and Caspase-11 Activation. Cell 2016, 165, 1106–1119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruhl, S.; Broz, P. Caspase-11 activates a canonical NLRP3 inflammasome by promoting K(+) efflux. Eur. J. Immunol. 2015, 45, 2927–2936. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cunha, L.D.; Silva, A.L.N.; Ribeiro, J.M.; Mascarenhas, D.P.A.; Quirino, G.F.S.; Santos, L.L.; Flavell, R.A.; Zamboni, D.S. AIM2 Engages Active but Unprocessed Caspase-1 to Induce Noncanonical Activation of the NLRP3 Inflammasome. Cell Rep. 2017, 20, 794–805. [Google Scholar] [CrossRef] [PubMed]
- Pellegrini, C.; Antonioli, L.; Lopez-Castejon, G.; Blandizzi, C.; Fornai, M. Canonical and Non-Canonical Activation of NLRP3 Inflammasome at the Crossroad between Immune Tolerance and Intestinal Inflammation. Front. Immunol. 2017, 8, 36. [Google Scholar] [CrossRef] [Green Version]
- Man, S.M.; Kanneganti, T.D. Regulation of inflammasome activation. Immunol. Rev. 2015, 265, 6–21. [Google Scholar] [CrossRef] [Green Version]
- Mattiuzzi, C.; Lippi, G. Recent updates on worldwide gout epidemiology. Clin. Rheumatol. 2020, 39, 1061–1063. [Google Scholar] [CrossRef]
- Cao, Y. Icariin alleviates MSU-induced rat GA models through NF-kappaB/NALP3 pathway. Cell Biochem. Funct. 2020. [Google Scholar] [CrossRef]
- Qiao, C.Y.; Li, Y.; Shang, Y.; Jiang, M.; Liu, J.; Zhan, Z.Y.; Ye, H.; Lin, Y.C.; Jiao, J.Y.; Sun, R.H.; et al. Management of Gout-associated MSU crystals-induced NLRP3 inflammasome activation by procyanidin B2: Targeting IL-1beta and Cathepsin B in macrophages. Inflammopharmacology 2020, 28, 1481–1493. [Google Scholar] [CrossRef]
- Staurengo-Ferrari, L.; Ruiz-Miyazawa, K.W.; Pinho-Ribeiro, F.A.; Fattori, V.; Zaninelli, T.H.; Badaro-Garcia, S.; Borghi, S.M.; Carvalho, T.T.; Alves-Filho, J.C.; Cunha, T.M.; et al. Trans-Chalcone Attenuates Pain and Inflammation in Experimental Acute Gout Arthritis in Mice. Front. Pharmacol. 2018, 9, 1123. [Google Scholar] [CrossRef]
- Ruiz-Miyazawa, K.W.; Pinho-Ribeiro, F.A.; Borghi, S.M.; Staurengo-Ferrari, L.; Fattori, V.; Amaral, F.A.; Teixeira, M.M.; Alves-Filho, J.C.; Cunha, T.M.; Cunha, F.Q.; et al. Hesperidin Methylchalcone Suppresses Experimental Gout Arthritis in Mice by Inhibiting NF-kappaB Activation. J. Agric. Food Chem. 2018, 66, 6269–6280. [Google Scholar] [CrossRef]
- Liu, H.J.; Pan, X.X.; Liu, B.Q.; Gui, X.; Hu, L.; Jiang, C.Y.; Han, Y.; Fan, Y.X.; Tang, Y.L.; Liu, W.T. Grape seed-derived procyanidins alleviate gout pain via NLRP3 inflammasome suppression. J. Neuroinflamm. 2017, 14, 74. [Google Scholar] [CrossRef] [PubMed]
- Dhanasekar, C.; Rasool, M. Morin, a dietary bioflavonol suppresses monosodium urate crystal-induced inflammation in an animal model of acute gouty arthritis with reference to NLRP3 inflammasome, hypo-xanthine phospho-ribosyl transferase, and inflammatory mediators. Eur. J. Pharmacol. 2016, 786, 116–127. [Google Scholar] [CrossRef] [PubMed]
- Jhang, J.J.; Lu, C.C.; Yen, G.C. Epigallocatechin gallate inhibits urate crystals-induced peritoneal inflammation in C57BL/6 mice. Mol. Nutr. Food Res. 2016, 60, 2297–2303. [Google Scholar] [CrossRef] [PubMed]
- Doss, H.M.; Dey, C.; Sudandiradoss, C.; Rasool, M.K. Targeting inflammatory mediators with ferulic acid, a dietary polyphenol, for the suppression of monosodium urate crystal-induced inflammation in rats. Life Sci. 2016, 148, 201–210. [Google Scholar] [CrossRef]
- Jhang, J.J.; Lu, C.C.; Ho, C.Y.; Cheng, Y.T.; Yen, G.C. Protective Effects of Catechin against Monosodium Urate-Induced Inflammation through the Modulation of NLRP3 Inflammasome Activation. J. Agric. Food Chem. 2015, 63, 7343–7352. [Google Scholar] [CrossRef]
- Wu, J.F.; Dong, J.C.; Xu, C.Q. Effects of icariin on inflammation model stimulated by lipopolysaccharide in vitro and in vivo. Chin. J. Integr. Tradit. West. Med. 2009, 29, 330–334. [Google Scholar]
- Fang, J.; Zhang, Y. Icariin, an Anti-atherosclerotic Drug from Chinese Medicinal Herb Horny Goat Weed. Front. Pharmacol. 2017, 8, 734. [Google Scholar] [CrossRef] [Green Version]
- Zhou, J.; Wu, J.; Chen, X.; Fortenbery, N.; Eksioglu, E.; Kodumudi, K.N.; Pk, E.B.; Dong, J.; Djeu, J.Y.; Wei, S. Icariin and its derivative, ICT, exert anti-inflammatory, anti-tumor effects, and modulate myeloid derived suppressive cells (MDSCs) functions. Int. Immunopharmacol. 2011, 11, 890–898. [Google Scholar] [CrossRef] [Green Version]
- Basiorka, A.A.; McGraw, K.L.; Eksioglu, E.A.; Chen, X.; Johnson, J.; Zhang, L.; Zhang, Q.; Irvine, B.A.; Cluzeau, T.; Sallman, D.A.; et al. The NLRP3 inflammasome functions as a driver of the myelodysplastic syndrome phenotype. Blood 2016, 128, 2960–2975. [Google Scholar] [CrossRef]
- Liu, B.; Xu, C.; Wu, X.; Liu, F.; Du, Y.; Sun, J.; Tao, J.; Dong, J. Icariin exerts an antidepressant effect in an unpredictable chronic mild stress model of depression in rats and is associated with the regulation of hippocampal neuroinflammation. Neuroscience 2015, 294, 193–205. [Google Scholar] [CrossRef]
- Wang, Y.; Chung, S.J.; Song, W.O.; Chun, O.K. Estimation of daily proanthocyanidin intake and major food sources in the U.S. diet. J. Nutr. 2011, 141, 447–452. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rue, E.A.; Rush, M.D.; van Breemen, R.B. Procyanidins: A comprehensive review encompassing structure elucidation via mass spectrometry. Phytochem. Rev. 2018, 17, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Micaelo, N.; Gonzalez-Abuin, N.; Pinent, M.; Ardevol, A.; Blay, M. Procyanidin B2 inhibits inflammasome-mediated IL-1beta production in lipopolysaccharide-stimulated macrophages. Mol. Nutr. Food Res. 2015, 59, 262–269. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Xiao, L.; Yuan, Y.; Luo, X.; Jiang, M.; Ni, J.; Wang, N. Procyanidin B2 inhibits NLRP3 inflammasome activation in human vascular endothelial cells. Biochem. Pharmacol. 2014, 92, 599–606. [Google Scholar] [CrossRef]
- Williams, R.J.; Spencer, J.P.; Rice-Evans, C. Flavonoids: Antioxidants or signalling molecules? Free Radic. Biol. Med. 2004, 36, 838–849. [Google Scholar] [CrossRef]
- Cai, Y.; Kong, H.; Pan, Y.B.; Jiang, L.; Pan, X.X.; Hu, L.; Qian, Y.N.; Jiang, C.Y.; Liu, W.T. Procyanidins alleviates morphine tolerance by inhibiting activation of NLRP3 inflammasome in microglia. J. Neuroinflamm. 2016, 13, 53. [Google Scholar] [CrossRef] [Green Version]
- Kang, D.G.; Moon, M.K.; Sohn, E.J.; Lee, D.H.; Lee, H.S. Effects of morin on blood pressure and metabolic changes in fructose-induced hypertensive rats. Biol. Pharm. Bull. 2004, 27, 1779–1783. [Google Scholar] [CrossRef] [Green Version]
- Xie, M.X.; Long, M.; Liu, Y.; Qin, C.; Wang, Y.D. Characterization of the interaction between human serum albumin and morin. Biochim. Et Biophys. Acta 2006, 1760, 1184–1191. [Google Scholar] [CrossRef]
- Lotito, S.B.; Frei, B. Consumption of flavonoid-rich foods and increased plasma antioxidant capacity in humans: Cause, consequence, or epiphenomenon? Free Radic. Biol. Med. 2006, 41, 1727–1746. [Google Scholar] [CrossRef]
- Dhanasekar, C.; Kalaiselvan, S.; Rasool, M. Morin, a Bioflavonoid Suppresses Monosodium Urate Crystal-Induced Inflammatory Immune Response in RAW 264.7 Macrophages through the Inhibition of Inflammatory Mediators, Intracellular ROS Levels and NF-kappaB Activation. PLoS ONE 2015, 10, e0145093. [Google Scholar] [CrossRef] [Green Version]
- Mukai, K.; Mitani, S.; Ohara, K.; Nagaoka, S. Structure-activity relationship of the tocopherol-regeneration reaction by catechins. Free Radic. Biol. Med. 2005, 38, 1243–1256. [Google Scholar] [CrossRef] [PubMed]
- Rice-Evans, C.A.; Miller, N.J.; Bolwell, P.G.; Bramley, P.M.; Pridham, J.B. The relative antioxidant activities of plant-derived polyphenolic flavonoids. Free Radic. Res. 1995, 22, 375–383. [Google Scholar] [CrossRef] [PubMed]
- Granja, A.; Frias, I.; Neves, A.R.; Pinheiro, M.; Reis, S. Therapeutic Potential of Epigallocatechin Gallate Nanodelivery Systems. BioMed Res. Int. 2017, 2017, 5813793. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, M.; Sudheer, A.R.; Menon, V.P. Ferulic Acid: Therapeutic potential through its antioxidant property. J. Clin. Biochem. Nutr. 2007, 40, 92–100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mahmoud, A.M.; Hussein, O.E.; Hozayen, W.G.; Bin-Jumah, M.; Abd El-Twab, S.M. Ferulic acid prevents oxidative stress, inflammation, and liver injury via upregulation of Nrf2/HO-1 signaling in methotrexate-induced rats. Environ. Sci. Pollut. Res. Int. 2020, 27, 7910–7921. [Google Scholar] [CrossRef] [PubMed]
- Lampiasi, N.; Montana, G. The molecular events behind ferulic acid mediated modulation of IL-6 expression in LPS-activated Raw 264.7 cells. Immunobiology 2016, 221, 486–493. [Google Scholar] [CrossRef] [PubMed]
- Rahman, I.; Biswas, S.K.; Kirkham, P.A. Regulation of inflammation and redox signaling by dietary polyphenols. Biochem. Pharmacol. 2006, 72, 1439–1452. [Google Scholar] [CrossRef]
- Lisnevskaia, L.; Murphy, G.; Isenberg, D. Systemic lupus erythematosus. Lancet 2014, 384, 1878–1888. [Google Scholar] [CrossRef]
- Pons-Estel, G.J.; Ugarte-Gil, M.F.; Alarcon, G.S. Epidemiology of systemic lupus erythematosus. Expert Rev. Clin. Immunol. 2017, 13, 799–814. [Google Scholar] [CrossRef]
- Zhu, Q.; Zhuang, X.; Lu, J. Neuroprotective effects of baicalein in animal models of Parkinson’s disease: A systematic review of experimental studies. Phytomedicine 2019, 55, 302–309. [Google Scholar] [CrossRef]
- Dinda, B.; Dinda, S.; DasSharma, S.; Banik, R.; Chakraborty, A.; Dinda, M. Therapeutic potentials of baicalin and its aglycone, baicalein against inflammatory disorders. Eur. J. Med. Chem. 2017, 131, 68–80. [Google Scholar] [CrossRef]
- Tuli, H.S.; Aggarwal, V.; Kaur, J.; Aggarwal, D.; Parashar, G.; Parashar, N.C.; Tuorkey, M.; Kaur, G.; Savla, R.; Sak, K.; et al. Baicalein: A metabolite with promising antineoplastic activity. Life Sci. 2020, 259, 118183. [Google Scholar] [CrossRef]
- Li, D.; Shi, G.; Wang, J.; Zhang, D.; Pan, Y.; Dou, H.; Hou, Y. Baicalein ameliorates pristane-induced lupus nephritis via activating Nrf2/HO-1 in myeloid-derived suppressor cells. Arthritis Res. Ther. 2019, 21, 105. [Google Scholar] [CrossRef] [Green Version]
- Su, B.; Ye, H.; You, X.; Ni, H.; Chen, X.; Li, L. Icariin alleviates murine lupus nephritis via inhibiting NF-kappaB activation pathway and NLRP3 inflammasome. Life Sci. 2018, 208, 26–32. [Google Scholar] [CrossRef]
- He, J.; Sun, M.; Tian, S. Procyanidin B2 prevents lupus nephritis development in mice by inhibiting NLRP3 inflammasome activation. Innate Immun. 2018, 24, 307–315. [Google Scholar] [CrossRef]
- Tsai, P.Y.; Ka, S.M.; Chang, J.M.; Chen, H.C.; Shui, H.A.; Li, C.Y.; Hua, K.F.; Chang, W.L.; Huang, J.J.; Yang, S.S.; et al. Epigallocatechin-3-gallate prevents lupus nephritis development in mice via enhancing the Nrf2 antioxidant pathway and inhibiting NLRP3 inflammasome activation. Free Radic. Biol. Med. 2011, 51, 744–754. [Google Scholar] [CrossRef]
- Oton, T.; Carmona, L. The epidemiology of established rheumatoid arthritis. Best Pract. Res. Clin. Rheumatol. 2019, 33, 101477. [Google Scholar] [CrossRef]
- Hasan, U.H.; Uttra, A.M.; Qasim, S.; Ikram, J.; Saleem, M.; Niazi, Z.R. Phytochemicals targeting matrix metalloproteinases regulating tissue degradation in inflammation and rheumatoid arthritis. Phytomedicine 2020, 66, 153134. [Google Scholar] [CrossRef]
- Basu, A.; Schell, J.; Scofield, R.H. Dietary fruits and arthritis. Food Funct. 2018, 9, 70–77. [Google Scholar] [CrossRef]
- Singh, S.; Singh, T.G.; Mahajan, K.; Dhiman, S. Medicinal plants used against various inflammatory biomarkers for the management of rheumatoid arthritis. J. Pharm. Pharmacol. 2020, 72, 1306–1327. [Google Scholar] [CrossRef]
- Sato, S.; Mukai, Y. Modulation of Chronic Inflammation by Quercetin: The Beneficial Effects on Obesity. J. Inflamm. Res. 2020, 13, 421–431. [Google Scholar] [CrossRef] [PubMed]
- Jakaria, M.; Azam, S.; Jo, S.H.; Kim, I.S.; Dash, R.; Choi, D.K. Potential Therapeutic Targets of Quercetin and Its Derivatives: Its Role in the Therapy of Cognitive Impairment. J. Clin. Med. 2019, 8, 1789. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miltonprabu, S.; Tomczyk, M.; Skalicka-Wozniak, K.; Rastrelli, L.; Daglia, M.; Nabavi, S.F.; Alavian, S.M.; Nabavi, S.M. Hepatoprotective effect of quercetin: From chemistry to medicine. Food Chem. Toxicol. 2017, 108, 365–374. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimpour, S.; Zakeri, M.; Esmaeili, A. Crosstalk between obesity, diabetes, and alzheimer’s disease: Introducing quercetin as an effective triple herbal medicine. Ageing Res. Rev. 2020, 62, 101095. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.M.; Deng, X.T.; Zhou, J.; Li, Q.P.; Ge, X.X.; Miao, L. Pharmacological basis and new insights of quercetin action in respect to its anti-cancer effects. Biomed. Pharmacother. 2020, 121, 109604. [Google Scholar] [CrossRef] [PubMed]
- Domiciano, T.P.; Wakita, D.; Jones, H.D.; Crother, T.R.; Verri, W.A., Jr.; Arditi, M.; Shimada, K. Quercetin Inhibits Inflammasome Activation by Interfering with ASC Oligomerization and Prevents Interleukin-1 Mediated Mouse Vasculitis. Sci. Rep. 2017, 7, 41539. [Google Scholar] [CrossRef]
- Chanjitwiriya, K.; Roytrakul, S.; Kunthalert, D. Quercetin negatively regulates IL-1beta production in Pseudomonas aeruginosa-infected human macrophages through the inhibition of MAPK/NLRP3 inflammasome pathways. PLoS ONE 2020, 15, e0237752. [Google Scholar] [CrossRef]
- Lee, K.M.; Kang, J.H.; Yun, M.; Lee, S.B. Quercetin inhibits the poly(dA:dT)-induced secretion of IL-18 via down-regulation of the expressions of AIM2 and pro-caspase-1 by inhibiting the JAK2/STAT1 pathway in IFN-gamma-primed human keratinocytes. Biochem. Biophys. Res. Commun. 2018, 503, 116–122. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, X.; Xu, M.; Wu, X.; Zhao, F.; Zhao, C. Quercetin attenuates collagen-induced arthritis by restoration of Th17/Treg balance and activation of Heme Oxygenase 1-mediated anti-inflammatory effect. Int. Immunopharmacol. 2018, 54, 153–162. [Google Scholar] [CrossRef]
- Zobeiri, M.; Belwal, T.; Parvizi, F.; Naseri, R.; Farzaei, M.H.; Nabavi, S.F.; Sureda, A.; Nabavi, S.M. Naringenin and its Nano-formulations for Fatty Liver: Cellular Modes of Action and Clinical Perspective. Curr. Pharm. Biotechnol. 2018, 19, 196–205. [Google Scholar] [CrossRef]
- Salehi, B.; Fokou, P.V.T.; Sharifi-Rad, M.; Zucca, P.; Pezzani, R.; Martins, N.; Sharifi-Rad, J. The Therapeutic Potential of Naringenin: A Review of Clinical Trials. Pharmaceuticals 2019, 12, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, C.; Wei, Y.Z.; He, X.M.; Li, D.D.; Wang, G.Q.; Li, J.J.; Zhang, F. Naringenin Produces Neuroprotection Against LPS-Induced Dopamine Neurotoxicity via the Inhibition of Microglial NLRP3 Inflammasome Activation. Front. Immunol. 2019, 10, 936. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bussmann, A.J.C.; Borghi, S.M.; Zaninelli, T.H.; Dos Santos, T.S.; Guazelli, C.F.S.; Fattori, V.; Domiciano, T.P.; Pinho-Ribeiro, F.A.; Ruiz-Miyazawa, K.W.; Casella, A.M.B.; et al. The citrus flavanone naringenin attenuates zymosan-induced mouse joint inflammation: Induction of Nrf2 expression in recruited CD45(+) hematopoietic cells. Inflammopharmacology 2019, 27, 1229–1242. [Google Scholar] [CrossRef] [PubMed]

| Name | Group | MW | Chemical Formula |
|---|---|---|---|
| Icariin | Flavonol | 676.67 | C33H40O15 |
| Procyanidin B2 | Proanthocyanidin | 578.52 | C30H26O12 |
| Trans-Chalcone | Chalcone | 208.26 | C15H12O |
| HMC | Chalcone | 624.59 | C29H36O15 |
| Morin | Flavonol | 302.24 | C15H10O7 |
| EGCG | Flavanol | 458.37 | C22H18O11 |
| Ferulic acid | Polyphenol | 194.18 | C10H10O4 |
| Catechin | Flavanol | 290.27 | C15H14O6 |
| Baicalein | Falvone | 270.24 | C15H10O5 |
| Diseases | Flavonoids | Target Inflammasomes | Study Results | Experiment Models | Ref |
|---|---|---|---|---|---|
| GA | Icariin | NLRP3 |
|
| [47] |
| Procyanidin B2 | NLRP3 |
|
| [48] | |
| Trans-Chalcone | NLRP3 |
|
| [49] | |
| Hesperidin methylchalcone | NLRP3 |
|
| [50] | |
| Procyanidins | NLRP3 |
|
| [51] | |
| Morin | NLRP3 |
|
| [52] | |
| EGCG | NLRP3 |
|
| [53] | |
| Ferulic acid | NLRP3 |
|
| [54] | |
| Catechin | NLRP3 |
|
| [55] | |
| SLE | Baicalein | NLRP3 |
|
| [83] |
| Icariin | NLRP3 |
|
| [84] | |
| Procyanidin B2 | NLRP3 |
|
| [85] | |
| EGCG | NLRP3 |
|
| [86] | |
| RA | Quercetin | NLRP3 |
|
| [99] |
| Naringenin | NLRP3 |
|
| [103] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yi, Y.-S. Flavonoids: Nutraceuticals for Rheumatic Diseases via Targeting of Inflammasome Activation. Int. J. Mol. Sci. 2021, 22, 488. https://doi.org/10.3390/ijms22020488
Yi Y-S. Flavonoids: Nutraceuticals for Rheumatic Diseases via Targeting of Inflammasome Activation. International Journal of Molecular Sciences. 2021; 22(2):488. https://doi.org/10.3390/ijms22020488
Chicago/Turabian StyleYi, Young-Su. 2021. "Flavonoids: Nutraceuticals for Rheumatic Diseases via Targeting of Inflammasome Activation" International Journal of Molecular Sciences 22, no. 2: 488. https://doi.org/10.3390/ijms22020488
APA StyleYi, Y. -S. (2021). Flavonoids: Nutraceuticals for Rheumatic Diseases via Targeting of Inflammasome Activation. International Journal of Molecular Sciences, 22(2), 488. https://doi.org/10.3390/ijms22020488