The Proteome and Citrullinome of Hippoglossus hippoglossus Extracellular Vesicles—Novel Insights into Roles of the Serum Secretome in Immune, Gene Regulatory and Metabolic Pathways
Abstract
:1. Introduction
2. Results
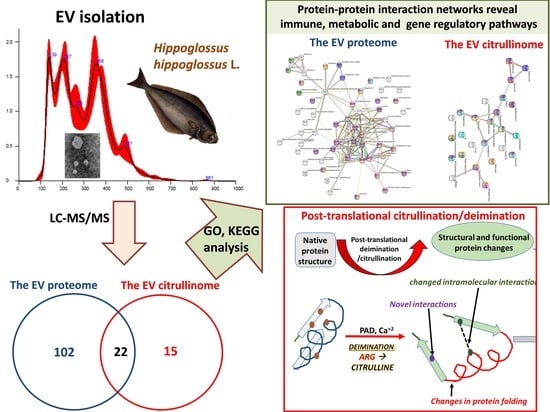
2.1. EV Profiling from Halibut Sera
2.2. The Proteome and Citrullinome of Halibut Serum EVs
2.3. Complement Component C3, C4 and Pentraxin-Like Protein Verified in Halibut EVs and F95 Enriched EV Protein Cargo Fractions Using Western Blotting
2.4. Protein–Protein Interaction Network Analysis for Halibut Serum-EV Protein Cargo: Deiminated and Total Protein Cargo
2.4.1. Protein Interaction Networks Enriched for Halibut Serum-EV Deiminated/Citrullinated Protein Cargo
2.4.2. Protein Interaction Networks Enriched for Halibut Serum-EV Total Protein Cargo
3. Discussion
4. Materials and Methods
4.1. Fish and Sampling
4.2. EV Isolation and Nanoparticle Tracking (NTA) Analysis
4.3. Transmission Electron Microscopy (TEM)
4.4. Proteomic Analysis and Protein Identification
4.5. Western Blotting
4.6. Silver Staining
4.7. Protein–Protein Interaction Network Analysis
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Russel, F.S. The Eggs and Planktonic Stages of British Marine Fishes; Academic Press: London, UK, 1976; ISBN 0-12-604050-8. [Google Scholar]
- Mangor-Jensen, A.; Harboe, T.; Shields, R.J.; Gara, B.; Naas, K.E. Atlantic halibut, Hippoglossus hippoglossus L.; larvae cultivation literature, including a bibliography. Aquac. Res. 1998, 29, 857–886. [Google Scholar] [CrossRef]
- Vossenaar, E.R.; Zendman, A.J.; van Venrooij, W.J.; Pruijn, G.J. PAD, a growing family of citrullinating enzymes: Genes, features and involvement in disease. Bioessays 2003, 25, 1106–1118. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Wang, Y. Peptidylarginine deiminases in citrullination, gene regulation, health and pathogenesis. Biochim. Biophys. Acta 2013, 1829, 1126–1135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Witalison, E.E.; Thompson, P.R.; Hofseth, L.J. Protein Arginine Deiminases and Associated Citrullination: Physiological Functions and Diseases Associated with Dysregulation. Curr. Drug Targets 2015, 16, 700–710. [Google Scholar] [CrossRef]
- György, B.; Toth, E.; Tarcsa, E.; Falus, A.; Buzas, E.I. Citrullination: A posttranslational modification in health and disease. Int. J. Biochem. Cell Biol. 2006, 38, 1662–1677. [Google Scholar] [CrossRef]
- Bicker, K.L.; Thompson, P.R. The protein arginine deiminases: Structure, function, inhibition, and disease. Biopolymers 2013, 99, 155–163. [Google Scholar] [CrossRef]
- Tarcsa, E.; Marekov, L.N.; Mei, G.; Melino, G.; Lee, S.C.; Steinert, P.M. Protein unfolding by peptidylarginine deiminase. Substrate specificity and structural relationships of the natural substrates trichohyalin and filaggrin. J. Biol. Chem. 1996, 271, 30709–30716. [Google Scholar] [CrossRef] [Green Version]
- Jeffrey, C.J. Protein moonlighting: What is it, and why is it important? Philos. Trans. R. Soc. B Biol. Sci. 2018, 373, 20160523. [Google Scholar] [CrossRef]
- Nomura, K. Specificity and mode of action of the muscle-type protein-arginine deiminase. Arch. Biochem. Biophys. 1992, 293, 362–369. [Google Scholar] [CrossRef]
- Rebl, A.; Köllner, B.; Anders, E.; Wimmers, K.; Goldammer, T. Peptidylarginine deiminase gene is differentially expressed in freshwater and brackish water rainbow trout. Mol. Biol. Rep. 2010, 37, 2333–2339. [Google Scholar] [CrossRef]
- Magnadottir, B.; Hayes, P.; Hristova, M.; Bragason, B.T.; Nicholas, A.P.; Dodds, A.W.; Gudmundsdottir, S.; Lange, S. Post-translational Protein Deimination in Cod (Gadus morhua L.) Ontogeny—Novel Roles in Tissue Remodelling and Mucosal Immune Defences? Dev. Comp. Immunol. 2018, 87, 157–170. [Google Scholar] [CrossRef] [PubMed]
- Magnadóttir, B.; Bragason, B.T.; Bricknell, I.R.; Bowden, T.; Nicholas, A.P.; Hristova, M.; Guðmundsdóttir, S.; Dodds, A.W.; Lange, S. Peptidylarginine deiminase and deiminated proteins are detected throughout early halibut ontogeny—Complement components C3 and C4 are post-translationally deiminated in halibut (Hippoglossus hippoglossus L.). Dev. Comp. Immunol. 2019, 92, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Criscitiello, M.F.; Kraev, I.; Lange, S. Deiminated proteins in extracellular vesicles and plasma of nurse shark (Ginglymostoma cirratum)—Novel insights into shark immunity. Fish Shellfish. Immunol. 2019, 92, 249–255. [Google Scholar] [CrossRef] [PubMed]
- Lange, S.; Gögel, S.; Leung, K.Y.; Vernay, B.; Nicholas, A.P.; Causey, C.P.; Thompson, P.R.; Greene, N.D.; Ferretti, P. Protein deiminases: New players in the developmentally regulated loss of neural regenerative ability. Dev. Biol. 2011, 355, 205–214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Criscitiello, M.F.; Kraev, I.; Petersen, L.H.; Lange, S. Deimination Protein Profiles in Alligator mississippiensis Reveal Plasma and Extracellular Vesicle-Specific Signatures Relating to Immunity, Metabolic Function, and Gene Regulation. Front. Immunol. 2020, 11, 651. [Google Scholar] [CrossRef] [PubMed]
- Novák, L.; Zubáčová, Z.; Karnkowska, A.; Kolisko, M.; Hroudová, M.; Stairs, C.W.; Simpson, A.G.; Keeling, P.J.; Roger, A.J.; Čepička, I.; et al. Arginine deiminase pathway enzymes: Evolutionary history in metamonads and other eukaryotes. BMC Evol. Biol. 2016, 16, 197. [Google Scholar] [CrossRef] [Green Version]
- Bielecka, E.; Scavenius, C.; Kantyka, T.; Jusko, M.; Mizgalska, D.; Szmigielski, B.; Potempa, B.; Enghild, J.J.; Prossnitz, E.R.; Blom, A.M.; et al. Peptidyl arginine deiminase from Porphyromonas gingivalis abolishes anaphylatoxin C5a activity. J. Biol. Chem. 2014, 289, 32481–32487. [Google Scholar] [CrossRef] [Green Version]
- Kosgodage, U.S.; Matewele, P.; Mastroianni, G.; Kraev, I.; Brotherton, D.; Awamaria, B.; Nicholas, A.P.; Lange, S.; Inal, J.M. Peptidylarginine deiminase inhibitors reduce bacterial membrane vesicle release and sensitize bacteria to antibiotic treatment. Front. Cell. Infect. Microbiol. 2019, 9, 227. [Google Scholar] [CrossRef] [Green Version]
- El-Sayed, A.S.A.; Shindia, A.A.; AbouZaid, A.A.; Yassin, A.M.; Ali, G.S.; Sitohy, M.Z. Biochemical characterization of peptidylarginine deiminase-like orthologs from thermotolerant Emericella dentata and Aspergillus nidulans. Enzyme Microb. Technol. 2019, 124, 41–53. [Google Scholar] [CrossRef]
- Gavinho, B.; Sabatke, B.; Feijoli, V.; Rossi, I.V.; da Silva, J.M.; Evans-Osses, I.; Palmisano, G.; Lange, S.; Ramirez, M.I. Peptidylarginine deiminase inhibition abolishes the production of large extracellular vesicles from Giardia intestinalis, affecting host-pathogen interactions by hindering adhesion to host cells. Front. Cell. Infect. Microbiol. 2020, 10, 417. [Google Scholar] [CrossRef]
- Bowden, T.J.; Kraev, I.; Lange, S. Extracellular vesicles and post-translational protein deimination signatures in haemolymph of the American lobster (Homarus americanus). Fish Shellfish Immunol. 2020, 106, 79–102. [Google Scholar] [CrossRef] [PubMed]
- Bowden, T.J.; Kraev, I.; Lange, S. Post-translational protein deimination signatures and extracellular vesicles (EVs) in the Atlantic horseshoe crab (Limulus polyphemus). Dev. Comp. Immunol. 2020, 110, 103714. [Google Scholar] [CrossRef] [PubMed]
- Bowden, T.J.; Kraev, I.; Lange, S. Extracellular Vesicles and Post-Translational Protein Deimination Signatures in Mollusca—The Blue Mussel (Mytilus edulis), Soft Shell Clam (Mya arenaria), Eastern Oyster (Crassostrea virginica) and Atlantic Jacknife Clam (Ensis leei). Biology 2020, 9, 416. [Google Scholar] [CrossRef] [PubMed]
- Inal, J.M.; Ansa-Addo, E.A.; Lange, S. Interplay of host-pathogen microvesicles and their role in infectious disease. Biochem. Soc. Trans. 2013, 41, 258–262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Colombo, M.; Raposo, G.; Théry, C. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu. Rev. Cell Dev. Biol. 2014, 30, 255–289. [Google Scholar] [CrossRef]
- Kholia, S.; Jorfi, S.; Thompson, P.R.; Causey, C.P.; Nicholas, A.P.; Inal, J.M.; Lange, S. A novel role for peptidylarginine deiminases in microvesicle release reveals therapeutic potential of PAD inhibition in sensitizing prostate cancer cells to chemotherapy. J. Extracell. Vesicles 2015, 4, 26192. [Google Scholar] [CrossRef] [Green Version]
- Kosgodage, U.S.; Uysal-Onganer, P.; MacLatchy, A.; Kraev, I.; Chatterton, N.P.; Nicholas, A.P.; Inal, J.M.; Lange, S. Peptidylarginine Deiminases Post-Translationally Deiminate Prohibitin and Modulate Extracellular Vesicle Release and MicroRNAs in Glioblastoma Multiforme. Int. J. Mol. Sci. 2018, 20, 103. [Google Scholar] [CrossRef] [Green Version]
- Uysal-Onganer, P.; MacLatchy, A.; Mahmoud, R.; Kraev, I.; Thompson, P.R.; Inal, J.M.; Lange, S. Peptidylarginine Deiminase Isozyme-Specific PAD2, PAD3 and PAD4 Inhibitors Differentially Modulate Extracellular Vesicle Signatures and Cell Invasion in Two Glioblastoma Multiforme Cell Lines. Int. J. Mol. Sci. 2020, 21, 1495. [Google Scholar] [CrossRef] [Green Version]
- Turchinovich, A.; Drapkina, O.; Tonevitsky, A. Transcriptome of extracellular vesicles: State-of-the-art. Front. Immunol. 2018, 10, 202. [Google Scholar] [CrossRef] [Green Version]
- Vagner, T.; Chin, A.; Mariscal, J.; Bannykh, S.; Engman, D.M.; di Vizio, D. Protein composition reflects extracellular vesicle heterogeneity. Proteomics 2019, 19, e1800167. [Google Scholar] [CrossRef]
- Magnadottir, B.; Kraev, I.; Guđmundsdóttir, S.; Dodds, A.W.; Lange, S. Extracellular vesicles from cod (Gadus morhua L.) mucus contain innate immune factors and deiminated protein cargo. Dev. Comp. Immunol. 2019, 99, 103397. [Google Scholar] [CrossRef] [PubMed]
- Magnadottir, B.; Uysal-Onganer, P.; Kraev, I.; Dodds, A.W.; Gudmundsdottir, S.; Lange, S. Extracellular vesicles, deiminated protein cargo and microRNAs are novel serum biomarkers for environmental rearing temperature in Atlantic cod (Gadus morhua L.). Aquac. Rep. 2020, 16, 100245. [Google Scholar] [CrossRef]
- Criscitiello, M.F.; Kraev, I.; Lange, S. Deiminated proteins in extracellular vesicles and serum of llama (Lama glama)-Novel insights into camelid immunity. Mol. Immunol. 2020, 117, 37–53. [Google Scholar] [CrossRef] [PubMed]
- Criscitiello, M.F.; Kraev, I.; Lange, S. Post-translational protein deimination signatures in serum and serum-extracellular vesicles of Bos taurus reveal immune, anti-pathogenic, anti-viral, metabolic and cancer-related pathways for deimination. Int. J. Mol. Sci. 2020, 21, 2861. [Google Scholar] [CrossRef] [PubMed]
- Pamenter, M.E.; Uysal-Onganer, P.; Huynh, K.W.; Kraev, I.; Lange, S. Post-translational deimination of immunological and metabolic protein markers in plasma and extracellular vesicles of naked mole-rat (Heterocephalus glaber). Int. J. Mol. Sci. 2019, 20, 5378. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Phillips, R.A.; Kraev, I.; Lange, S. Protein deimination and extracellular vesicle profiles in Antarctic seabirds. Biology 2020, 9, 15. [Google Scholar] [CrossRef] [Green Version]
- Lange, S.; Kraev, I.; Magnadóttir, B.; Dodds, A.W. Complement component C4-like protein in Atlantic cod (Gadus morhua L.)—Detection in ontogeny and identification of post-translational deimination in serum and extracellular vesicles. Dev. Comp. Immunol. 2019, 101, 103437. [Google Scholar] [CrossRef]
- Magnadottir, B.; Uysal-Onganer, P.; Kraev, I.; Svansson, V.; Hayes, P.; Lange, S. Deiminated proteins and extracellular vesicles—Novel serum biomarkers in whales and orca. Comp. Biochem. Physiol. Part D Genom. Proteom. 2020, 34, 100676. [Google Scholar] [CrossRef]
- Magnadottir, B.; Uysal-Onganer, P.; Kraev, I.; Svansson, V.; Skírnisson, K.; Lange, S. Deiminated proteins and extracellular vesicles as novel biomarkers in pinnipeds: Grey seal (Halichoerus gryptus) and harbour seal (Phoca vitulina). Biochimie 2020, 171–172, 79–90. [Google Scholar] [CrossRef]
- Iliev, D.; Strandskog, G.; Nepal, A.; Aspar, A.; Olsen, R.; Jørgensen, J.; Wolfson, D.; Ahluwalia, B.S.; Handzhiyski, J.; Mironova, R. Stimulation of exosome release by extracellular DNA is conserved across multiple cell types. FEBS J. 2018, 285, 3114–3133. [Google Scholar] [CrossRef]
- Lange, S.; Dodds, A.W.; Magnadottir, B. Isolation and characterization of complement component C3 from Atlantic cod (Gadus morhua L.) and Atlantic halibut (Hippoglossus hippoglossus L.). Fish Shellfish Immunol. 2004, 16, 227–239. [Google Scholar] [CrossRef]
- Sancandi, M.; Uysal-Onganer, P.; Kraev, I.; Mercer, A.; Lange, S. Protein Deimination Signatures in Plasma and Plasma-EVs and Protein Deimination in the Brain Vasculature in a Rat Model of Pre-Motor Parkinson’s Disease. Int. J. Mol. Sci. 2020, 21, 2743. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kosgodage, U.S.; Matewele, P.; Awamaria, B.; Kraev, I.; Warde, P.; Mastroianni, G.; Nunn, A.V.; Guy, G.W.; Bell, J.D.; Inal, J.M.; et al. Cannabidiol Is a Novel Modulator of Bacterial Membrane Vesicles. Front. Cell. Infect. Microbiol. 2019, 9, 324. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Hubal, M.J.; Kraus, V.B. Immune cell extracellular vesicles and their mitochondrial content decline with ageing. Immun. Ageing 2020, 17, 1. [Google Scholar] [CrossRef]
- Faught, E.; Henrickson, L.; Vijayan, M.M. Plasma exosomes are enriched in Hsp70 and modulated by stress and cortisol in rainbow trout. J. Endocrinol. 2017, 232, 237–246. [Google Scholar] [CrossRef]
- Cadonic, I.G.; Ikert, H.; Craig, P.M. Acute air exposure modulates the microRNA abundance in stress responsive tissues and circulating extracellular vesicles in rainbow trout (Oncorhynchus mykiss). Comp. Biochem. Physiol. Part D Genom. Proteom. 2020, 34, 100661. [Google Scholar] [CrossRef]
- Antwi-Baffour, S.; Malibha-Pinchbeck, M.; Stratton, D.; Jorfi, S.; Lange, S.; Inal, J. Plasma mEV levels in Ghanain malaria patients with low parasitaemia are higher than those of healthy controls, raising the potential for parasite markers in mEVs as diagnostic targets. J. Extracell. Vesicles 2019, 9, 1697124. [Google Scholar] [CrossRef]
- Théry, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles 2018, 7, 1535750. [Google Scholar] [CrossRef] [Green Version]
- Nicholas, A.P.; Whitaker, J.N. Preparation of a monoclonal antibody to citrullinated epitopes: Its characterization and some applications to immunohistochemistry in human brain. Glia 2002, 37, 328–336. [Google Scholar] [CrossRef]









| Protein ID | Species Name | Matches | Total Score |
|---|---|---|---|
| Protein Name | Common Name | (Sequences) | (p < 0.05) ⱡ |
| A0A6J2W3P0_CHACN | Chanos chanos | 16 (13) | 538 |
| Uncharacterised protein (histone H3-like) | Milkfish | ||
| A0A672ZYE0_9TELE | Sphaeramia orbicularis | 12 (9) | 451 |
| Uncharacterised protein | Orbiculate cardinalfish | ||
| A0A0A1G3Q1_9TELE | Oxyeleotris marmorata | 10 (10) | 427 |
| Beta-actin | Marble goby | ||
| A0A3P8Y5X6_ESOLU | Esox Lucius | 26 (8) | 356 |
| IF rod domain-containing protein | Northern pike | ||
| * W5ZLY1_9TELE | Campylomormyrus compressirostris | 8 (8) | 336 |
| Cytoplasmic 2 actin | Elephantfish | ||
| A0A3B4ZTX8_9TELE | Stegastes partitus | 8 (7) | 324 |
| Uncharacterized protein (NTR domain-containing protein; Complement component C3) | Bicolour damselfish | ||
| A0A3B4THR8_SERDU | Seriola dumerili | 9 (8) | 299 |
| Uncharacterized protein (NTR domain-containing protein; anaphylatoxin-like, Complement component C3) | Greater amberjack | ||
| A0A6G0HQ07_LARCR | Larimichthys crocea | 8 (6) | 281 |
| Histone H4 | Yellow croaker | ||
| A0A3Q3IVX9_MONAL | Monopterus albus | 8 (7) | 276 |
| Uncharacterized protein (Complement C3) | Asian swamp eel | ||
| A0A3P9BEG5_9CICH | Maylandia zebra | 6 (6) | 273 |
| Uncharacterized protein (Anaphylatoxin-like, complement C3) | Zebra mbuna | ||
| A0A484CCU5_PERFV | Perca flavescens | 8 (7) | 271 |
| Uncharacterized protein (complement C3) | Yellow perch | ||
| A5JV31_HIPHI | Hippoglossus hippoglossus | 7 (7) | 261 |
| Phosvitin | Atlantic halibut | ||
| * A0A087XQB5_POEFO | Poecilia formosa | 6 (5) | 256 |
| Tubulin alpha chain | Amazon molly | ||
| A0A6F9CZC7_9TELE | Coregonus sp. ‘balchen’ | 5 (4) | 251 |
| Uncharacterized protein (tubulin alpha-chain) | Whitefish, salmonidae | ||
| * Q1RLR3_DANRE | Danio rerio | 8 (5) | 237 |
| Keratin 93 | Zebrafish | ||
| A0A1S5XZE7_9TELE | Lipogramma levinsoni | 7 (7) | 231 |
| Histone H3 | Hourglass basslet | ||
| A3F5V1_ORENI | Oreochromis niloticus | 7 (7) | 222 |
| Beta actin (Fragment) | Nile tilapia | ||
| A0A5N5KJN7_PANHP | Pangasianodon hypophthalmus | 6 (4) | 185 |
| IF rod domain-containing protein | Iridescent shark | ||
| A0A4W6CP97_LATCA | Lates calcarifer | 5 (4) | 179 |
| Uncharacterized protein (Alpha-2-macroglobulin) | Barramundi/Asian sea bass | ||
| * H2MSJ5_ORYLA | Oryzias latipes | 5 (4) | 159 |
| Uncharacterized protein | Medaka/Japanese rice fish | ||
| A0A060WDP8_ONCMY | Oncorhynchus mykiss | 3 (3) | 136 |
| Elongation factor 1-alpha | Rainbow trout | ||
| A0A671UYU7_SPAAU | Sparus aurata | 3 (1) | 117 |
| Uncharacterized protein (A2M_recep domain-containing protein) | Gilt-head bream | ||
| G3Q4A0_GASAC | Gasterosteus aculeatus | 2 (2) | 116 |
| Fibrinogen beta chain | Three-spined stickleback | ||
| A0A0F8AH88_LARCR | Larimichthys crocea | 2 (2) | 107 |
| Ig heavy chain V region 5A | Yellow croaker | ||
| A0A4W6FLR7_LATCA | Lates calcarifer | 3 (3) | 104 |
| Uncharacterized protein (NTR domain-containing protein; anaphylatoxin like; A2M_N_2 domain-containing; complement C5) | Barramundi/Asian sea bass | ||
| A0A4Z2B138_9TELE | Takifugu bimaculatus | 3 (3) | 99 |
| Anaphylatoxin-like domain-containing protein | Pufferfish | ||
| Q4KVK3_HIPHI | Hippoglossus hippoglossus | 2 (2) | 94 |
| Complement component c3 (Fragment) | Atlantic halibut | ||
| * A0A5J5C7F1_9PERO | Etheostoma spectabile | 2 (2) | 94 |
| Uncharacterized protein (Fragment) | Orangethroat darter | ||
| * A0A0P7WL38_SCLFO | Scleropages formosus | 4 (2) | 93 |
| Trypsin-3-like | Asian arowana | ||
| Q5DVG8_PLAFE | Platichthys flesus | 3 (2) | 84 |
| Apolipoprotein AI | European flounder | ||
| A0A0F8ABH4_LARCR | Larimichthys crocea | 3 (1) | 82 |
| Granzyme B(G,H) | Yellow croaker | ||
| A0A484D989_PERFV | Perca flavescens | 3 (2) | 71 |
| Peptidase S1 domain-containing protein | Yellow perch | ||
| * A0A5N5Q536_PANHP | Pangasianodon hypophthalmus | 2 (2) | 70 |
| Centrosomal protein of 162 kDa | Iridescent shark | ||
| * A0A0P7UEW6_SCLFO | Scleropages formosus | 1 (1) | 69 |
| 2-phospho-D-glycerate hydro-lyase | Asian arowana | ||
| A0A060YWU0_ONCMY | Oncorhynchus mykiss | 4 (2) | 68 |
| Peptidase S1 domain-containing protein | Rainbow trout | ||
| * A0A1A7WRH6_9TELE | Iconisemion striatum | 2 (2) | 64 |
| Integrin beta | Killifish | ||
| A0A3B5M528_9TELE | Xiphophorus couchianus | 1 (1) | 64 |
| Serotransferrin | Monterrey platyfish | ||
| A0A060Z3N3_ONCMY | Oncorhynchus mykiss | 2 (2) | 63 |
| Ig-like domain-containing protein | Rainbow trout | ||
| A0A060W543_ONCMY | Oncorhynchus mykiss | 2 (2) | 62 |
| Histone H2A | Rainbow trout | ||
| A0A0R4IU44_DANRE | Danio rerio | 1 (1) | 61 |
| Inter-alpha-trypsin inhibitor heavy chain 3b | Zebrafish | ||
| HV05_CARAU | Carassius auratus | 2 (1) | 60 |
| Ig heavy chain V region 5A | Goldfish | ||
| * A0A060XD44_ONCMY | Oncorhynchus mykiss | 4 (2) | 60 |
| Uncharacterized protein | Rainbow trout | ||
| A0A4W5L5T6_9TELE | Hucho hucho | 1 (1) | 57 |
| Thioredoxin | Danube salmon | ||
| * A0A3Q3LZB0_9TELE | Mastacembelus armatus | 1 (1) | 57 |
| Uncharacterized protein | Zig-zag eel/Spiny eel | ||
| * A0A5J5DS23_9PERO | Etheostoma spectabile | 1 (1) | 57 |
| Uncharacterized protein | Orangethroat darter | ||
| * A0A3B3QST7_9TELE | Paramormyrops kingsleyae | 1 (1) | 55 |
| Uncharacterized protein | Elephantfish | ||
| * A0A0E9RVI6_ANGAN | Anguilla Anguilla | 1 (1) | 53 |
| Uncharacterized protein | European eel | ||
| * A0A3Q3SSB4_9TELE | Mastacembelus armatus | 1 (1) | 53 |
| * Myosin_tail_1 domain-containing protein | Zig-zag eel/Spiny eel |
| Protein ID | Species Name | Matches | Total Score |
|---|---|---|---|
| Protein Name | Common Name | (Sequences) | (p < 0.05) ⱡ |
| A5JV31_HIPHI | Hippoglossus hippoglossus | 145 (56) | 3616 |
| Phosvitin | Atlantic halibut | ||
| A5JV30_HIPHI | Hippoglossus hippoglossus | 90 (52) | 3303 |
| Phosvitin | Atlantic halibut | ||
| Q4KVK3_HIPHI | Hippoglossus hippoglossus | 69 (25) | 1690 |
| Complement component c3 (fragment) | Atlantic halibut | ||
| A0A2U9BPE5_SCOMX | Scophthalmus maximus | 89 (24) | 1426 |
| Complement component C3 isoform 2 | Turbot | ||
| A0A3B4THR8_SERDU | Seriola dumerili | 79 (22) | 1269 |
| Uncharacterized protein (NTR domain-containing protein, Complement C3-like, A2M_recep domain-containing protein) | Greater amberjack | ||
| A0A3B4TYC3_SERDU | Seriola dumerili | 65 (21) | 1250 |
| NTR domain-containing protein | Greater amberjack | ||
| Q9PTY1_PAROL | Paralichthys olivaceus | 70 (22) | 1176 |
| Complement component C3 | Olive flounder | ||
| G4WAB7_EPICO | Epinephelus coioides | 57 (20) | 1145 |
| Complement component c3 | Orange-spotted grouper | ||
| A0A3P9BEG5_9CICH | Maylandia zebra | 66 (19) | 1120 |
| Uncharacterized protein (Anaphylatoxin-like domain-containing protein; C3a) | Zebra mbuna | ||
| * A0A669BPJ4_ORENI | Oreochromis niloticus | 71 (18) | 1097 |
| Uncharacterized protein | Nile tilapia | ||
| A0A671YHA0_SPAAU | Sparus aurata | 45 (17) | 904 |
| Uncharacterized protein (C3) | Gilt-head bream | ||
| * A0A6A5FQW4_PERFL | Perca fluviatilis | 57 (16) | 885 |
| Uncharacterized protein | European perch | ||
| A0A484CCU5_PERFV | Perca flavescens | 56 (17) | 879 |
| Uncharacterized protein (Anaphylatoxin-like domain-containing protein) | Yellow perch | ||
| F8R8R1_DICLA | Dicentrarchus labrax | 59 (15) | 871 |
| Complement component c3-2 | European bass | ||
| A0A484DL37_PERFV | Perca flavescens | 42 (15) | 784 |
| Anaphylatoxin-like domain-containing protein | Yellow perch | ||
| A0A4W6E087_LATCA | Lates calcarifer | 43 (15) | 744 |
| Complement component c3a, duplicate 5 | Barramundi/Asian sea bass | ||
| A0A6A5FJW4_PERFL | Perca fluviatilis | 14 (12) | 600 |
| Uncharacterized protein (Integrase catalytic domain-containing protein, Alpha-2-macroglobulin-like) | European perch | ||
| A0A2P9DTV2_SOLSE | Solea senegalensis | 16 (10) | 594 |
| Phosvitin | Senegalese sole | ||
| Q6QZI2_PSEAM | Pseudopleuronectes americanus | 37 (9) | 574 |
| Complement component C3 (Fragment) | Winter flounder | ||
| A0A3Q1ID66_ANATE | Anabas testudineus | 25 (9) | 569 |
| Phosvitin | Climbing perch | ||
| * A0A4W6F6V9_LATCA | Lates calcarifer | 15 (9) | 549 |
| Apolipoprotein Bb, tandem duplicate 2 | Barramundi/Asian sea bass | ||
| A0A6G1PAV1_9TELE | Channa argus | 38 (10) | 540 |
| Complement C3 Complement C3 beta chain Complement C3 alpha chain | Northern snakehead | ||
| * A0A4P8JD10_9TELE | Lateolabrax maculatus | 13 (9) | 532 |
| Apolipoprotein Bb.1 | Spotted sea bass | ||
| * A0A6A5DT05_PERFL | Perca fluviatilis | 16 (9) | 529 |
| Vitellogenin domain-containing protein | European perch | ||
| A0A673IJP2_9TELE | Sinocyclocheilus rhinocerous | 48 (12) | 528 |
| IF rod domain-containing protein | Sinocyclocheilus cavefish (Cyprinid) | ||
| A0A4W6CMC4_LATCA | Lates calcarifer | 14 (11) | 525 |
| Uncharacterized protein (Alpha-2-macroglobulin) | Barramundi/Asian sea bass | ||
| A0A3P8Y5X6_ESOLU | Esox Lucius | 51 (11) | 499 |
| IF rod domain-containing protein | Northern pike | ||
| A0A6G1PQL3_9TELE | Channa argus | 12 (10) | 497 |
| Alpha-2-macroglobulin | Northern snakehead | ||
| A0A6A4SX26_SCOMX | Scophthalmus maximus | 51 (11) | 463 |
| IF rod domain-containing protein | Turbot | ||
| Q5DVG8_PLAFE | Platichthys flesus | 26 (9) | 453 |
| Apolipoprotein AI | European flounder | ||
| * A0A3B4T6U1_SERDU | Seriola dumerili | 12 (9) | 440 |
| Vitellogenin domain-containing protein | Greater amberjack | ||
| A0A665VQL3_ECHNA | Echeneis naucrates | 9 (8) | 409 |
| Uncharacterized protein (A2M_N_2 domain-containing protein) | Live sharksucker | ||
| * A0A2U9D044_SCOMX | Scophthalmus maximus | 14 (8) | 407 |
| Putative apolipoprotein B-100-like isoform 2 | Turbot | ||
| * Q9PVW6_PAROL | Paralichthys olivaceus | 14 (7) | 403 |
| Complement component C9 | Olive flounder | ||
| A0A4W6FLR7_LATCA | Lates calcarifer | 10 (8) | 386 |
| Uncharacterized protein (Anaphylatoxin-like domain-containing, A2M_N_2 domain containing protein, NTR domain containing protein, Complement C5) | Barramundi/Asian sea bass | ||
| A0A4W6CP97_LATCA | Lates calcarifer | 17 (7) | 362 |
| Uncharacterized protein (A2M_recep domain-containing protein, TED_complement domain-containing protein) | Barramundi/Asian sea bass | ||
| * A0A3P8RR96_AMPPE | Amphiprion percula | 12 (5) | 353 |
| Complement component C9 | Orange clownfish | ||
| A0A3Q1HZ43_ANATE | Anabas testudineus | 13 (9) | 336 |
| Uncharacterized protein (Inter-alpha-trypsin inhibitor, VIT domain-containing protein) | Climbing perch | ||
| * A0A3Q1H6Y9_ANATE | Anabas testudineus | 8 (8) | 336 |
| Complement component 8 subunit beta | Climbing perch | ||
| A0A6G1PI27_9TELE | Channa argus | 13 (7) | 324 |
| Inter-alpha-trypsin inhibitor heavy chain H3 | Northern snakehead | ||
| A0A6A5FLM2_PERFL | Perca fluviatilis | 10 (6) | 323 |
| Uncharacterized protein (alpha-2-macroglobulin-like, A2M_recep domain-containing protein) | European perch | ||
| A0A6A5FFR2_PERFL | Perca fluviatilis | 15 (7) | 323 |
| Anaphylatoxin-like domain-containing protein | European perch | ||
| A0A484DIJ5_PERFV | Perca flavescens | 11 (7) | 321 |
| Uncharacterized protein (Alpha-2-macroglobulin) | Yellow perch | ||
| A0A6A5FE70_PERFL | Perca fluviatilis | 11 (7) | 318 |
| Uncharacterized protein (A2M_recep domain-containing, MG2 domain-containing protein) | European perch | ||
| A0A6J2W3P0_CHACN | Chanos chanos | 8 (7) | 312 |
| uncharacterized protein LOC115819396 (Histone H4, Histone H3, Histone H2B) | Milkfish | ||
| * A0A665V532_ECHNA | Echeneis naucrates | 8 (6) | 310 |
| Plasminogen | Live sharksucker | ||
| A0A3Q3L7G2_9TELE | Mastacembelus armatus | 6 (6) | 308 |
| Complement component c3b, tandem duplicate 2 | Zig-zag eel/Spiny eel | ||
| * CO8B_PAROL | Paralichthys olivaceus | 5 (5) | 304 |
| Complement component C8 beta chain | Olive flounder | ||
| A0A671PIL3_9TELE | Sinocyclocheilus anshuiensis | 17 (6) | 301 |
| IF rod domain-containing protein | Sinocyclocheilus cavefish (Cyprinoid) | ||
| * A0A3Q3E5X5_9LABR | Labrus bergylta | 7 (4) | 298 |
| Uncharacterized protein (C1q domain-containing protein) | Ballan wrasse | ||
| * A0A3Q0S0V4_AMPCI | Amphilophus citrinellus | 18 (5) | 292 |
| Uncharacterized protein | Midas cichlid | ||
| A0A6A4SU52_SCOMX | Scophthalmus maximus | 7 (7) | 291 |
| Uncharacterized protein (Complement component c3b) | Turbot | ||
| * A0A3P8TA20_AMPPE | Amphiprion percula | 11 (7) | 290 |
| Zgc:112265 | Orange clownfish | ||
| A0A096MDQ7_POEFO | Poecilia formosa | 11 (6) | 288 |
| Phosvitin | Amazon molly | ||
| Q5XVQ2_FUNHE | Fundulus heteroclitus | 17 (5) | 288 |
| Apolipoprotein A1 (Fragment) | Atlantic killifish, mud minnow | ||
| * Q6QZI9_PSEAM | Pseudopleuronectes americanus | 12 (5) | 284 |
| Complement component C9 (Fragment) | Winter flounder | ||
| * A0A4U5UPP9_COLLU | Collichthys lucidus | 7 (5) | 283 |
| Apolipoprotein B-100 | (Big head croaker) | ||
| A0A3Q1EMN2_9TELE | Acanthochromis polyacanthus | 8 (5) | 280 |
| Uncharacterized protein (beta actin, actin cytoplasmic-1) | Spiny chromis damselfish | ||
| * A0A6J2P874_COTGO | Cottoperca gobio | 7 (5) | 280 |
| plasminogen | Channel bull blenny | ||
| A0A3B4VID4_SERDU | Seriola dumerili | 9 (5) | 279 |
| Uncharacterized protein (MG2 domain-containing protein) | Greater amberjack | ||
| * A0A3Q3M9S2_9TELE | Mastacembelus armatus | 12 (4) | 278 |
| Uncharacterized protein | Zig-zag eel/Spiny eel | ||
| W5ZMG9_9TELE | Campylomormyrus compressirostris | 7 (4) | 267 |
| Cytoplasmic 1 actin | Elephantfish | ||
| A0A553Q7M4_9TELE | Danionella translucida | 6 (6) | 262 |
| Uncharacterized protein (Histone H2A, H2B putative, H3) | Micro glassfish (Cyprinid) | ||
| A0A3Q1H0X2_ANATE | Anabas testudineus | 5 (5) | 260 |
| Complement component c3b, tandem duplicate 2 | Climbing perch | ||
| * A0A6A4SHP5_SCOMX | Scophthalmus maximus | 12 (5) | 258 |
| Uncharacterized protein | Turbot | ||
| * G3NNM8_GASAC | Gasterosteus aculeatus | 6 (6) | 256 |
| Uncharacterized protein | Three-spined stickleback | ||
| * A0A0P7YVM9_SCLFO | Scleropages formosus | 10 (5) | 251 |
| Keratin, type I cytoskeletal 13-like | Asian arowana | ||
| * A0A6A4SWR2_SCOMX | Scophthalmus maximus | 7 (6) | 251 |
| EGF-like domain-containing protein | Turbot | ||
| A0A2U9B3I5_SCOMX | Scophthalmus maximus | 13 (6) | 247 |
| Alpha-2-macroglobulin | Turbot | ||
| A0A4Z2BCD9_9TELE | Takifugu bimaculatus | 6 (5) | 242 |
| Uncharacterized protein | Pufferfish | ||
| (Complement C5 C3 and PZP-like alpha-2-macroglobulin domain-containing protein) | |||
| A0A671TD78_SPAAU | Sparus aurata | 5 (5) | 238 |
| Complement component c3b, tandem duplicate 2 | Gilt-head bream | ||
| * A0A0A0QKL5_OPLFA | Oplegnathus fasciatus | 6 (5) | 234 |
| Complement component 4 | Striped beakfish | ||
| * A0A6A4RUD7_SCOMX | Scophthalmus maximus | 6 (6) | 233 |
| Vitellogenin domain-containing protein | Turbot | ||
| A0A672YA60_9TELE | Sphaeramia orbicularis | 7 (6) | 232 |
| Uncharacterized protein (inter-alpha-trypsin inhibitor heavy chain) | Orbiculate cardinalfish | ||
| * A0A672JL95_SALFA | Salarias fasciatus | 5 (5) | 232 |
| Uncharacterized protein (complement C7) | Lawnmower blenny | ||
| * A0A3B4Y8X6_SERLL | Seriola lalandi | 10 (4) | 231 |
| Uncharacterized protein (Hephaestin-like protein 1, Desmoglein-2) | Yellowtail amberjack | ||
| * A0A3B4UHS2_SERDU | Seriola dumerili | 4 (4) | 229 |
| Uncharacterized protein | Greater amberjack | ||
| * A0A087YMZ0_POEFO | Poecilia formosa | 11 (6) | 229 |
| Uncharacterized protein (Ceruloplasmin) | Amazon molly | ||
| A0A3Q4G4S3_NEOBR | Neolamprologus brichardi | 11 (5) | 229 |
| Uncharacterized protein (NTR domain-containing protein) | Lyretail cichlid | ||
| * A0A3Q1EBE7_9TELE | Acanthochromis polyacanthus | 4 (4) | 228 |
| Vitellogenin domain-containing protein | Spiny chromis damselfish | ||
| A0A3P9A8D3_ESOLU | Esox Lucius | 8 (4) | 218 |
| Uncharacterized protein (Alpha-2-macroglobulin, A2M_recep domain-containing) | Northern pike | ||
| * A0A3P8WZ01_CYNSE | Cynoglossus semilaevis | 7 (4) | 217 |
| Vitellogenin domain-containing protein | Tongue sole | ||
| * A0A3B4F9T0_9CICH | Pundamilia nyererei | 3 (3) | 215 |
| Carboxypeptidase Q | Cichlid | ||
| * A0A6J2QSS9_COTGO | Cottoperca gobio | 3 (2) | 209 |
| complement component C9 | Channel bull blenny | ||
| * A0A672GNQ4_SALFA | Salarias fasciatus | 7 (4) | 208 |
| Vitellogenin domain-containing protein | Lawnmower blenny | ||
| * A0A3B4ULR2_SERDU | Seriola dumerili | 9 (5) | 207 |
| Zgc:112265 | Greater amberjack | ||
| A0A3B4THN2_SERDU | Seriola dumerili | 4 (4) | 205 |
| Fibrinogen beta chain | Greater amberjack | ||
| * A0A2U9BK85_SCOMX | Scophthalmus maximus | 3 (3) | 203 |
| Putative complement component C8 alpha chain | Turbot | ||
| * G8DP14_PLAFE | Platichthys flesus | 4 (4) | 201 |
| Beta 1-globin | European flounder | ||
| * A0A0F8C5A6_LARCR | Larimichthys crocea | 6 (5) | 200 |
| Antithrombin-III | Yellow croaker | ||
| * A0A2U9CEJ2_SCOMX | Scophthalmus maximus | 4 (4) | 200 |
| Complement component 7 | Turbot | ||
| A0A5C6MX12_9TELE | Takifugu flavidus | 17 (5) | 196 |
| Complement C3 | Yellowbelly pufferfish | ||
| * Q6QZI5_PSEAM | Pseudopleuronectes americanus | 4 (4) | 194 |
| Complement component C8 beta chain | Winter flounder | ||
| * A0A3B3BJ38_ORYME | Oryzias melastigma | 7 (4) | 192 |
| Vitellogenin domain-containing protein | Marine medaka | ||
| * A0A6J2S534_COTGO | Cottoperca gobio | 5 (5) | 190 |
| apolipoprotein B-100 | Channel bull blenny | ||
| * A0A3Q1JFY5_ANATE | Anabas testudineus | 5 (3) | 187 |
| Uncharacterized protein (ceruloplasmin) | Climbing perch | ||
| A0A672I1M9_SALFA | Salarias fasciatus | 6 (4) | 186 |
| Uncharacterized protein (Inter-alpha-trypsin inhibitor heavy chain, VIT domain-containing protein) | Lawnmower blenny | ||
| A0A3B5AT07_9TELE | Stegastes partitus | 7 (4) | 185 |
| IF rod domain-containing protein | Bicolour damselfish | ||
| * A0A4Z2CEC7_9TELE | Takifugu bimaculatus | 4 (4) | 183 |
| Uncharacterized protein (complement C4) | Pufferfish | ||
| * A0A3Q3II57_MONAL | Monopterus albus | 5 (4) | 183 |
| Uncharacterized protein | Asian swamp eel | ||
| * A0A3Q3FIH8_KRYMA | Kryptolebias marmoratus | 7 (4) | 180 |
| Uncharacterized protein | Mangrove rivulus(killilfish) | ||
| * A0A2U9AYP3_SCOMX | Scophthalmus maximus | 5 (3) | 177 |
| Complement component 4 | Turbot | ||
| * A0A6J2RDF1_COTGO | Cottoperca gobio | 4 (4) | 176 |
| complement C4-B-like | Channel bull blenny | ||
| A0A4W6ERJ2_LATCA | Lates calcarifer | 5 (4) | 173 |
| Fibrinogen gamma chain | Barramundi/Asian sea bass | ||
| * A0A2I4C034_9TELE | Austrofundulus limnaeus | 3 (3) | 167 |
| collagen alpha-1(XII) chain | Killifish | ||
| * A0A6I9PPD4_9TELE | Notothenia coriiceps | 4 (4) | 167 |
| complement C4-like | Black rockcod/Antarctic yellowbelly rockcod | ||
| * H3BWT7_TETNG | Tetraodon nigroviridis | 5 (3) | 163 |
| Ceruloplasmin | Green spotted puffer | ||
| * Q4SXM5_TETNG | Tetraodon nigroviridis | 5 (4) | 160 |
| Chromosome 12 SCAF12357, whole genome shotgun sequence | Green spotted puffer | ||
| A0A1A8F2V0_9TELE | Nothobranchius korthausae | 5 (2) | 160 |
| Uncharacterized protein (Alpha2-macroglobulin) | Killifish | ||
| * A0A3B5BD88_9TELE | Stegastes partitus | 4 (4) | 159 |
| Vitellogenin domain-containing protein | Bicolour damselfish | ||
| A0A6G1QB31_9TELE | Channa argus | 9 (2) | 159 |
| Serotransferrin | Northern snakehead | ||
| * A0A060WU48_ONCMY | Oncorhynchus mykiss | 2 (2) | 157 |
| Uncharacterized protein (Desmoplakin) | Rainbow trout | ||
| *A0A3Q3FAE5_9LABR | Labrus bergylta | 4 (3) | 155 |
| Complement component 8 subunit beta | Ballan wrasse | ||
| A0A6J2Q526_COTGO | Cottoperca gobio | 4 (3) | 155 |
| fibrinogen gamma chain | Channel bull blenny | ||
| * A0A3B4UV22_SERDU | Seriola dumerili | 6 (4) | 154 |
| Antithrombin-III | Greater amberjack | ||
| * A0A3Q2QAA5_FUNHE | Fundulus heteroclitus | 4 (3) | 154 |
| Uncharacterized protein | Atlantic killifish, mud minnow | ||
| * A0A6J2P7B9_COTGO | Cottoperca gobio | 3 (2) | 153 |
| apolipoprotein B-100-like | Channel bull blenny | ||
| * A0A484D0P7_PERFV | Perca flavescens | 6 (5) | 153 |
| Uncharacterized protein (ceruloplasmin) | Yellow perch | ||
| * A0A3B4TA89_SERDU | Seriola dumerili | 3 (3) | 149 |
| Uncharacterized protein | Greater amberjack | ||
| * A0A673XMC1_SALTR | Salmo trutta | 3 (3) | 148 |
| Uncharacterized protein (complement C4, C4-B) | Brown trout | ||
| F8U8N8_CHELB | Chelon labrosus | 4 (3) | 146 |
| Alpha 2 macroglobulin (fragment) | Thicklip grey mullet | ||
| F2Y9S5_MORSA | Morone saxatilis | 3 (3) | 145 |
| Phosvitin | Striped bass | ||
| * A0A3P9Q7U6_POERE | Poecilia reticulate | 4 (4) | 144 |
| Complement component C9 | Guppy | ||
| A0A0F8AH88_LARCR | Larimichthys crocea | 9 (3) | 143 |
| Ig heavy chain V region 5A | Yellow croaker | ||
| * A0A667Y3E0_9TELE | Myripristis murdjan | 6 (3) | 142 |
| Vitellogenin domain-containing protein | Blacktipped soldierfish | ||
| * A0A672QEF7_SINGR | Sinocyclocheilus graham | 8 (4) | 141 |
| Uncharacterized protein | Golden-line barbell | ||
| * A0A3B5B7I8_9TELE | Stegastes partitus | 5 (4) | 141 |
| Antithrombin-III | Bicolour damselfish | ||
| * A0A0B6VKQ1_ORYCL | Oryzias celebensis | 3 (3) | 139 |
| B5 protein | Celebes medaka | ||
| * A0A671TKG8_SPAAU | Sparus aurata | 4 (2) | 138 |
| Uncharacterized protein | Gilt-head bream | ||
| * A0A4P8JCG0_9TELE | Lateolabrax maculatus | 3 (2) | 136 |
| Apolipoprotein Bb.2 | Spotted sea bass | ||
| * A0A3B4FS46_9CICH | Pundamilia nyererei | 4 (3) | 132 |
| IGv domain-containing protein | Cichlid | ||
| A0A3P9H4Z3_ORYLA | Oryzias latipes | 9 (3) | 132 |
| Uncharacterized protein (A2M_N_2 domain-containing protein, anaphylatoxin-like domain) | Medaka/Japanese rice fish | ||
| A0A0F8AKQ4_LARCR | Larimichthys crocea | 5 (3) | 131 |
| Alpha-2-macroglobulin | Yellow croaker | ||
| A0A3B4TIN1_SERDU | Seriola dumerili | 3 (3) | 130 |
| Phosvitin | Greater amberjack | ||
| B6RUP0_ORYDN | Oryzias dancena | 4 (3) | 129 |
| Beta-actin (Fragment) | Indian ricefish | ||
| * A0A484CD54_PERFV | Perca flavescens | 3 (3) | 129 |
| Uncharacterized protein (Complement C7) | Yellow perch | ||
| A0A3Q4FXR7_NEOBR | Neolamprologus brichardi | 4 (3) | 128 |
| Ig-like domain-containing protein | Lyretail cichlid | ||
| Q5SET8_9TELE | Bembras japonica | 3 (3) | 128 |
| Histone H3 (Fragment) | Red flathead | ||
| A0A3Q1IXI9_ANATE | Anabas testudineus | 3 (3) | 128 |
| Uncharacterized protein (A2M_recep domain-containing protein) | Climbing perch | ||
| * A0A4Z2H8W0_9TELE | Liparis tanakae | 2 (2) | 126 |
| Biotinidase | Tanaka’s snailfish | ||
| A0A6G1PSN0_9TELE | Channa argus | 6 (5) | 126 |
| Alpha-2-macroglobulin | Northern snakehead | ||
| A0A669DKF1_ORENI | Oreochromis niloticus | 4 (3) | 125 |
| Uncharacterized protein (Ig-like domain-containing protein) | Nile tilapia | ||
| A0A3B1JCF6_ASTMX | Astyanax mexicanus | 6 (3) | 123 |
| IF rod domain-containing protein | Mexican tetra/blind cave fish | ||
| * A0A3Q2YHX2_HIPCM | Hippocampus comes | 3 (3) | 122 |
| Complement component 8 subunit beta | Tiger tail seahorse | ||
| A0A3Q3IC70_MONAL | Monopterus albus | 1 (1) | 121 |
| Ig-like domain-containing protein | Asian swamp eel | ||
| * A0A0F8AI97_LARCR | Larimichthys crocea | 2 (2) | 121 |
| Collagenase 3 | Yellow croaker | ||
| A0A6J2PEG5_COTGO | Cottoperca gobio | 2 (2) | 120 |
| complement C5-like | Channel bull blenny | ||
| A0A6A4TFM7_SCOMX | Scophthalmus maximus | 3 (2) | 119 |
| Ig-like domain-containing protein | Turbot | ||
| * A0A3Q0R4Z0_AMPCI | Amphilophus citrinellus | 5 (3) | 119 |
| Complement component C9 | Midas cichlid | ||
| A0A6I9NNH1_9TELE | Notothenia coriiceps | 3 (2) | 118 |
| inter-alpha-trypsin inhibitor heavy chain H2 | Black rockcod/Antarctic yellowbelly rockcod | ||
| A0A437D6V7_ORYJA | Oryzias javanicus | 2 (2) | 114 |
| Chitinase | Javanese ricefish | ||
| A0A3Q3EEY5_9LABR | Labrus bergylta | 3 (3) | 113 |
| Fibrinogen C-terminal domain-containing protein | Ballan wrasse | ||
| * A0A1S3SMN1_SALSA | Salmo salar | 1 (1) | 111 |
| cathepsin L1-like | Atlantic salmon | ||
| * A0A3Q3IZL2_MONAL | Monopterus albus | 4 (3) | 111 |
| Uncharacterized protein | Asian swamp eel | ||
| * A0A3P8YF02_ESOLU | Esox Lucius | 6 (3) | 109 |
| Vitellogenin domain-containing protein | Northern pike | ||
| * A0A3B3CJZ7_ORYME | Oryzias melastigma | 3 (2) | 109 |
| Complement 4B (Chido blood group) | Marine medaka | ||
| * A0A2U9CVZ8_SCOMX | Scophthalmus maximus | 2 (2) | 108 |
| Putative complement component C8 gamma chain | Turbot | ||
| A0A3Q3RJX0_9TELE | Mastacembelus armatus | 2 (1) | 108 |
| Ig-like domain-containing protein | Zig-zag eel/Spiny eel | ||
| * A0A3Q4HZS4_NEOBR | Neolamprologus brichardi | 3 (3) | 108 |
| Uncharacterized protein (Ceruloplasmin) | Lyretail cichlid | ||
| A0A6G1PYT4_9TELE | Channa argus | 4 (3) | 108 |
| Complement C5 C3 and PZP-like alpha-2-macroglobulin domain-containing protein 4 | Northern snakehead | ||
| * A0A2U9AV20_SCOMX | Scophthalmus maximus | 2 (2) | 107 |
| Prothrombin | Turbot | ||
| A0A4W6EWH0_LATCA | Lates calcarifer | 3 (3) | 107 |
| Peptidase S1 domain-containing protein | Barramundi/Asian sea bass | ||
| * H3C6P0_TETNG | Tetraodon nigroviridis | 2 (2) | 106 |
| Plasminogen | Green spotted puffer | ||
| A0A3P8SDE5_AMPPE | Amphiprion percula | 14 (2) | 105 |
| Serotransferrin | Orange clownfish | ||
| A0A3B4BP10_PYGNA | Pygocentrus nattereri | 10 (2) | 105 |
| Uncharacterized protein | Red-bellied piranha | ||
| * A0A3B4TQB5_SERDU | Seriola dumerili | 1 (1) | 105 |
| SERPIN domain-containing protein | Greater amberjack | ||
| * D5A7I1_DICLA | Dicentrarchus labrax | 4 (2) | 104 |
| Hemopexin | European bass | ||
| * A0A2U9CU10_SCOMX | Scophthalmus maximus | 3 (3) | 103 |
| Putative insulin-like growth factor-binding protein complex acid labile subunit | Turbot | ||
| * A0A6J2PA80_COTGO | Cottoperca gobio | 3 (3) | 103 |
| histone H2B 1/2-like | Channel bull blenny | ||
| * A0A3P8SSL4_AMPPE | Amphiprion percula | 2 (2) | 102 |
| Uncharacterized protein (Ig-like domain-containing protein, Nattectin) | Orange clownfish | ||
| * G3NN36_GASAC | Gasterosteus aculeatus | 4 (3) | 99 |
| Uncharacterized protein | Three-spined stickleback | ||
| A0A4W4FLR8_ELEEL | Electrophorus electricus | 3 (2) | 99 |
| Fibrinogen beta chain | Electric eel | ||
| A0A671TDU8_SPAAU | Sparus aurata | 2 (2) | 97 |
| Ig-like domain-containing protein | Gilt-head bream | ||
| A0A6I9PPY0_9TELE | Notothenia coriiceps | 2 (2) | 96 |
| fibrinogen gamma chain | Black rockcod/Antarctic yellowbelly rockcod | ||
| A0A671TNW0_SPAAU | Sparus aurata | 4 (3) | 96 |
| Histone H3 | Gilt-head bream | ||
| * A0A3B4XVK3_SERLL | Seriola lalandi dorsalis | 2 (2) | 96 |
| Vitellogenin domain-containing protein | Yellowtail amberjack | ||
| * A0A3Q3L1F9_9TELE | Mastacembelus armatus | 1 (1) | 95 |
| Complement component 1, r subcomponent | Zig-zag eel/Spiny eel | ||
| A0A1A8AN27_NOTFU | Nothobranchius furzeri | 3 (3) | 95 |
| Fibrinogen, gamma polypeptide | turquoise killifish | ||
| * A0A2D0QC28_ICTPU | Ictalurus punctatus | 2 (2) | 93 |
| Ig heavy chain Mem5-like | Channel catfish | ||
| A0A3P8R4C1_ASTCA | Astatotilapia calliptera | 6 (2) | 96 |
| Uncharacterized protein (Ig-like domain-containing protein) | Eastern happy/eastern river bream | ||
| A0A3B4H9E9_9CICH | Pundamilia nyererei | 3 (2) | 93 |
| Ig-like domain-containing protein | Cichlid | ||
| A0A3B4UNU3_SERDU | Seriola dumerili | 4 (2) | 93 |
| Ig-like domain-containing protein | Greater amberjack | ||
| * A0A060XWP2_ONCMY | Oncorhynchus mykiss | 92 | |
| SERPIN domain-containing protein | Rainbow trout | ||
| * A0A1A8CRV1_9TELE | Nothobranchius kadleci | 8 (2) | 91 |
| Uncharacterized protein | Killifish | ||
| * A0A2U9CFI3_SCOMX | Scophthalmus maximus | 2 (2) | 90 |
| Putative sushi domain-containing protein 2 isoform 2 | Turbot | ||
| A0A5C6NS08_9TELE | Takifugu flavidus | 5 (2) | 90 |
| Ig heavy chain V region VH558 A1/A4 | Yellowbelly pufferfish | ||
| * A0A4W4DXU4_ELEEL | Electrophorus electricus | 3 (3) | 89 |
| 14_3_3 domain-containing protein | Electric eel | ||
| * A0A0F8B5M5_LARCR | Larimichthys crocea | 1 (1) | 88 |
| Catechol O-methyltransferase domain-containing protein 1 | Yellow croaker | ||
| * A0A5N5KRW8_PANHP | Pangasianodon hypophthalmus | 3 (3) | 88 |
| Uncharacterized protein (pleckstrin homology domain-containing family) | Iridescent shark | ||
| * A0A5C6NRB2_9TELE | Takifugu flavidus | 2 (2) | 87 |
| Apolipoprotein B-100 | Yellowbelly pufferfish | ||
| A0A2D0RGG9_ICTPU | Ictalurus punctatus | 3 (2) | 87 |
| catenin beta-1 isoform X3 | Channel catfish | ||
| * A0A6I9P4Q9_9TELE | Notothenia coriiceps | 1 (1) | 86 |
| apolipoprotein B-100-like | Black rockcod/Antarctic yellowbelly rockcod | ||
| * A0A087XVJ8_POEFO | Poecilia formosa | 2 (1) | 86 |
| Uncharacterized protein (IGv domain-containing protein) | Amazon molly | ||
| * H1AB41_PLASA | Platichthys stellatus | 4 (2) | 85 |
| Lysozyme | Starry flounder | ||
| * A0A4P8JEC9_9TELE | Lateolabrax maculatus | 2 (2) | 84 |
| Apolipoprotein Ba | Spotted sea bass | ||
| A0A3Q4ACH4_MOLML | Mola mola | 2 (2) | 84 |
| Inter-alpha-trypsin inhibitor heavy chain 3 | Ocean sunfish | ||
| * A0A484CC61_PERFV | Perca flavescens | 3 (1) | 84 |
| Uncharacterized protein (Hyaluronan-binding protein 2) | Yellow perch | ||
| A0A060Z3N3_ONCMY | Oncorhynchus mykiss | 3 (2) | 86 |
| Ig-like domain-containing protein | Rainbow trout | ||
| * A0A3B4ZU87_9TELE | Stegastes partitus | 3 (2) | 83 |
| Uncharacterized protein (complement factor H-like) | Bicolour damselfish | ||
| A0A3B3QDE5_9TELE | Paramormyrops kingsleyae | 2 (1) | 83 |
| Ig-like domain-containing protein | Elephantfish | ||
| A0A3B3CFL8_ORYME | Oryzias melastigma | 5 (2) | 83 |
| Ig-like domain-containing protein | Marine medaka | ||
| * A0A3Q3W6Q7_MOLML | Mola mola | 2 (2) | 82 |
| Sushi domain containing 2 | Ocean sunfish | ||
| A0A4W5L5T6_9TELE | Hucho hucho | 3 (2) | 82 |
| Thioredoxin | Danube salmon | ||
| * G1DHP8_GOBRA | Gobiocypris rarus | 2 (2) | 81 |
| Vitellogenin (Fragment) | Rare gudgeon/rare minnow | ||
| * A0A3B3QP35_9TELE | Paramormyrops kingsleyae | 3 (2) | 80 |
| Uncharacterized protein | Elephantfish | ||
| * A0A3B4Z082_9TELE | Stegastes partitus | 2 (2) | 80 |
| Uncharacterized protein (complement C6) | Bicolour damselfish | ||
| A0A669CCK4_ORENI | Oreochromis niloticus | 6 (2) | 80 |
| Uncharacterized protein (Ig-like domain-containing protein) | Nile tilapia | ||
| * A0A484C6M0_PERFV | Perca flavescens | 1 (1) | 80 |
| Uncharacterized protein | Yellow perch | ||
| * A0A3P8U2B4_AMPPE | Amphiprion percula | 4 (2) | 80 |
| Keratin 98 | Orange clownfish | ||
| * A0A060WHH8_ONCMY | Oncorhynchus mykiss | 2 (2) | 79 |
| Junction plakoglobin | Rainbow trout | ||
| A0A3B4ULY5_SERDU | Seriola dumerili | 4 (2) | 78 |
| Ig-like domain-containing protein | Greater amberjack | ||
| H3C0U1_TETNG | Tetraodon nigroviridis | 3 (2) | 77 |
| Ig-like domain-containing protein | Green spotted puffer | ||
| A0A087X4F8_POEFO | Poecilia formosa | 1 (1) | 77 |
| Uncharacterized protein (Ig-like domain-containing protein) | Amazon molly | ||
| A0A3P9IRN4_ORYLA | Oryzias latipes | 2 (2) | 77 |
| Ig-like domain-containing protein | Medaka/Japanese rice fish | ||
| A0A060W543_ONCMY | Oncorhynchus mykiss | 2 (2) | 77 |
| Histone H2A | Rainbow trout | ||
| A0A3B4UFJ1_SERDU | Seriola dumerili | 2 (1) | 75 |
| Ig-like domain-containing protein | Greater amberjack | ||
| A0A0F8ABH4_LARCR | Larimichthys crocea | 5 (1) | 75 |
| Granzyme B(G,H) | Yellow croaker | ||
| * A0A3B4UPX8_SERDU | Seriola dumerili | 1 (1) | 74 |
| Zona pellucida sperm-binding protein 3 | Greater amberjack | ||
| * A0A3P8U813_AMPPE | Amphiprion percula | 2 (2) | 73 |
| Si:ch1073-416d2.3 | Orange clownfish | ||
| * A0A3Q1KAD2_ANATE | Anabas testudineus | 2 (2) | 72 |
| SERPIN domain-containing protein | Climbing perch | ||
| * A0A4W5RID4_9TELE | Hucho hucho | 2 (1) | 71 |
| RRM domain-containing protein | Danube salmon | ||
| * A0A3Q2QNZ9_FUNHE | Fundulus heteroclitus | 2 (2) | 71 |
| Uncharacterized protein (Sushi domain containing 2) | Atlantic killifish, mud minnow | ||
| * A0A4W5LQ29_9TELE | Hucho hucho | 8 (2) | 70 |
| ATP-synt ab_N domain-containing protein | Danube salmon | ||
| A0A3Q2PS35_FUNHE | Fundulus heteroclitus | 5 (2) | 70 |
| Ig-like domain-containing protein | Atlantic killifish, mud minnow | ||
| * A0A6G1QID3_9TELE | Channa argus | 2 (2) | 70 |
| Complement component C6 | Northern snakehead | ||
| * A0A3B3X986_9TELE | Poecilia Mexicana | 1 (1) | 70 |
| Uncharacterized protein (F-BAR domain-containing protein) | Atlantic (shortfin) molly | ||
| * A0A498LNY2_LABRO | Labeo rohita | 6 (2) | 70 |
| Retrotransposon-derived PEG10 | Rohu | ||
| * A0A6G1PD67_9TELE | Channa argus | 2 (2) | 70 |
| Apoptosis-stimulating of p53 protein 2 Bcl2-binding protein | Northern snakehead | ||
| * A0A1S3L2W1_SALSA | Salmo salar | 5 (2) | 70 |
| FH2 domain-containing protein 1-like | Atlantic salmon | ||
| A0A3B3HM39_ORYLA | Oryzias latipes | 1 (1) | 69 |
| Ig-like domain-containing protein | Medaka/Japanese rice fish | ||
| * A0A3Q1HK94_ANATE | Anabas testudineus | 6 (2) | 69 |
| Protein-tyrosine-phosphatase | Climbing perch | ||
| A0A3Q3JUN7_MONAL | Monopterus albus | 2 (2) | 68 |
| IF rod domain-containing protein | Asian swamp eel | ||
| * A0A671X983_SPAAU | Sparus aurata | 3 (2) | 68 |
| Uncharacterized protein (Early endosome antigen 1, FYVE-type domain-containing protein) | Gilt-head bream | ||
| * A0A3B3DTR8_ORYME | Oryzias melastigma | 3 (2) | 68 |
| Uncharacterized protein | Marine medaka | ||
| * A0A3Q3XI23_MOLML | Mola mola | 3 (2) | 67 |
| Zgc:112265 | Ocean sunfish | ||
| A0A671YT10_SPAAU | Sparus aurata | 2 (2) | 67 |
| Uncharacterized protein (Immunoglobulin like and fibronectin type III domain containing 1, tandem duplicate 2) | Gilt-head bream | ||
| * A0A3B5ACM2_9TELE | Stegastes partitus | 6 (2) | 66 |
| Uncharacterized protein | Bicolour damselfish | ||
| A0A3P9H0Y9_ORYLA | Oryzias latipes | 2 (2) | 65 |
| Ig-like domain-containing protein | Medaka/Japanese rice fish | ||
| * A0A5C6N3H2_9TELE | Takifugu flavidus | 4 (2) | 65 |
| Keratin, type I cytoskeletal 18 | Yellowbelly pufferfish | ||
| * A0A3B5L5A5_9TELE | Xiphophorus couchianus | 3 (2) | 65 |
| Thyroid hormone receptor interactor 11 | Monterrey platyfish | ||
| * Q2PZ29_SOLSE | Solea senegalensis | 2 (1) | 65 |
| Lysozyme | Senegalese sole | ||
| A0A667YBU1_9TELE | Myripristis murdjan | 5 (2) | 65 |
| Ig-like domain-containing protein | Blacktipped soldierfish | ||
| * A0A672GWK0_SALFA | Salarias fasciatus | 2 (2) | 64 |
| Uncharacterized protein (Complement factor B-like) | Lawnmower blenny | ||
| * A0A3B4CEW8_PYGNA | Pygocentrus nattereri | 2 (2) | 64 |
| Uncharacterized protein (Roundabout-like axon guidance receptor protein 2) | Red-bellied piranha | ||
| * A0A3B4EX20_9CICH | Pundamilia nyererei | 6 (2) | 64 |
| Uncharacterized protein (Apolipoprotein M) | Cichlid | ||
| * A0A2I4BMF1_9TELE | Austrofundulus limnaeus | 1 (1) | 63 |
| protein Z-dependent protease inhibitor-like | Killifish | ||
| * A0A3Q3EPX4_9LABR | Labrus bergylta | 2 (2) | 62 |
| Vitellogenin domain-containing protein | Ballan wrasse | ||
| * A0A3B4T5U4_SERDU | Seriola dumerili | 3 (2) | 62 |
| Uncharacterized protein (Myosin phosphatase Rho interacting protein) | Greater amberjack | ||
| A0A3B3T2D8_9TELE | Paramormyrops kingsleyae | 1 (1) | 62 |
| Ig-like domain-containing protein | Elephantfish | ||
| * A0A3Q1FWV1_9TELE | Acanthochromis polyacanthus | 2 (2) | 62 |
| Multidrug and toxin extrusion protein | Spiny chromis damselfish | ||
| A0A3B4YHZ5_SERLL | Seriola lalandi dorsalis | 1 (1) | 61 |
| IGv domain-containing protein | Yellowtail amberjack | ||
| * R4I5B0_EPICO | Epinephelus coioides | 3 (2) | 61 |
| Immmunoglobulin light chain | Orange-spotted grouper | ||
| * A0A3Q0R568_AMPCI | Amphilophus citrinellus | 3 (2) | 61 |
| FH2 domain containing 4 | Midas cichlid | ||
| A0A3B4WXW5_SERLL | Seriola lalandi dorsalis | 2 (2) | 60 |
| Ig-like domain-containing protein | Yellowtail amberjack | ||
| G3PK20_GASAC | Gasterosteus aculeatus | 3 (2) | 60 |
| Serotransferrin | Three-spined stickleback | ||
| * A0A484DB45_PERFV | Perca flavescens | 1 (1) | 60 |
| Uncharacterized protein (Pentaxin) | Yellow perch | ||
| * A0A671SV95_9TELE | Sinocyclocheilus anshuiensis | 2 (2) | 60 |
| FERM domain-containing protein | Sinocyclocheilus cavefish (Cyprinoid) | ||
| A0A023REA6_9TELE | Menidia estor | 1 (1) | 60 |
| Elongation factor 1-alpha | Pike silverside | ||
| * A0A6J2PC09_COTGO | Cottoperca gobio | 2 (2) | 60 |
| nesprin-2 | Channel bull blenny | ||
| * A0A0S7MGP3_9TELE | Poeciliopsis prolifica | 3 (2) | 59 |
| ZN287 (Fragment) | Blackstripe livebearer | ||
| * A0A3Q3VSX4_MOLML | Mola mola | 1 (1) | 59 |
| Uncharacterized protein | Ocean sunfish | ||
| * A0A553Q8B1_9TELE | Danionella translucida | 3 (2) | 58 |
| Uncharacterized protein | Micro glassfish (Cyprinid) | ||
| * A0A0P7TM62_SCLFO | Scleropages formosus | 1 (1) | 58 |
| Keratin, type I cytoskeletal 18-like | Asian arowana | ||
| * A0A060XKV1_ONCMY | Oncorhynchus mykiss | 3 (2) | 58 |
| [Histone H3]-trimethyl-L-lysine(9) demethylase | Rainbow trout | ||
| * E7F6Y7_DANRE | Danio rerio | 4 (2) | 58 |
| DNA polymerase kappa | Zebrafish | ||
| * F8W5U5_DANRE | Danio rerio | 2 (2) | 58 |
| Centrosomal protein of 290 kDa | Zebrafish | ||
| * A0A2U9CTT6_SCOMX | Scophthalmus maximus | 7 (2) | 57 |
| Putative utrophin | Turbot | ||
| * A0A3B3BVC4_ORYME | Oryzias melastigma | 2 (2) | 57 |
| Uncharacterized protein | Marine medaka | ||
| * A0A3B4UZF1_SERDU | Seriola dumerili | 1 (1) | 57 |
| [Histone H3]-lysine(4) N-trimethyltransferase | Greater amberjack | ||
| A0A060VW86_ONCMY | Oncorhynchus mykiss | 1 (1) | 56 |
| Uncharacterized protein (Tubulin alpha, tubulin domain containing) | Rainbow trout | ||
| * A0A671TLU7_SPAAU | Sparus aurata | 3 (2) | 56 |
| Reverse transcriptase | Gilt-head bream | ||
| A0A3Q4H8B0_NEOBR | Neolamprologus brichardi | 1 (1) | 56 |
| Ig-like domain-containing protein | Lyretail cichlid | ||
| * A0A0U2ERZ3_CORCL | Coregonus clupeaformis | 6 (1) | 56 |
| Glyceraldehyde 3-phosphate dehydrogenase | Lake whitefish | ||
| * A0A0R4IVM1_DANRE | Danio rerio | 11 (2) | 55 |
| LSM14A mRNA-processing body assembly factor b | Zebrafish | ||
| * A0A3P8VC95_CYNSE | Cynoglossus semilaevis | 1 (1) | 54 |
| Uncharacterized protein | Tongue sole | ||
| * Q9DFN6_GILMI | Gillichthys mirabilis | 1 (1) | 54 |
| Glyceraldehyde-3-phosphate dehydrogenase | |||
| * A0A3B3BWJ2_ORYME | Oryzias melastigma | 2 (2) | 54 |
| Uncharacterized protein | Marine medaka | ||
| * A0A6A4SGZ4_SCOMX | Scophthalmus maximus | 1 (1) | 54 |
| C1q domain-containing protein | Turbot | ||
| * A0A3B4EJ56_PYGNA | Pygocentrus nattereri | 2 (2) | 54 |
| von Willebrand factor | Red-bellied piranha | ||
| * A0A1S3RE28_SALSA | Salmo salar | 1 (1) | 53 |
| uncharacterized protein LOC106602330 isoform X1 | Atlantic salmon | ||
| * A0A2I4CMN8_9TELE | Austrofundulus limnaeus | 2 (2) | 53 |
| titin-like | Killifish |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Magnadóttir, B.; Kraev, I.; Dodds, A.W.; Lange, S. The Proteome and Citrullinome of Hippoglossus hippoglossus Extracellular Vesicles—Novel Insights into Roles of the Serum Secretome in Immune, Gene Regulatory and Metabolic Pathways. Int. J. Mol. Sci. 2021, 22, 875. https://doi.org/10.3390/ijms22020875
Magnadóttir B, Kraev I, Dodds AW, Lange S. The Proteome and Citrullinome of Hippoglossus hippoglossus Extracellular Vesicles—Novel Insights into Roles of the Serum Secretome in Immune, Gene Regulatory and Metabolic Pathways. International Journal of Molecular Sciences. 2021; 22(2):875. https://doi.org/10.3390/ijms22020875
Chicago/Turabian StyleMagnadóttir, Bergljót, Igor Kraev, Alister W. Dodds, and Sigrun Lange. 2021. "The Proteome and Citrullinome of Hippoglossus hippoglossus Extracellular Vesicles—Novel Insights into Roles of the Serum Secretome in Immune, Gene Regulatory and Metabolic Pathways" International Journal of Molecular Sciences 22, no. 2: 875. https://doi.org/10.3390/ijms22020875
APA StyleMagnadóttir, B., Kraev, I., Dodds, A. W., & Lange, S. (2021). The Proteome and Citrullinome of Hippoglossus hippoglossus Extracellular Vesicles—Novel Insights into Roles of the Serum Secretome in Immune, Gene Regulatory and Metabolic Pathways. International Journal of Molecular Sciences, 22(2), 875. https://doi.org/10.3390/ijms22020875