Regulation of the Fructose Transporter Gene Slc2a5 Expression by Glucose in Cultured Microglial Cells
Abstract
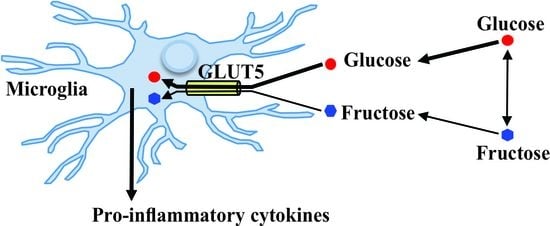
:1. Introduction
2. Results
2.1. Expression of Glucose Transporter Genes in Mouse Primary Microglia and Mouse Microglial Cell Line SIM-A9 Cells
2.2. Effect of Glucose on the Activity of Mouse Primary Microglia
2.3. Effect of Glucose on the Expression of Genes Encoding Glucose Transporters and Pro-Inflammatory Cytokines in Mouse Primary Microglia
2.4. Effect of Glucose on the Activity of SIM-A9 Cells
2.5. Effect of Glucose on the Expression of Genes Encoding Glucose Transporters and Pro-Inflammatory Cytokines in SIM-A9 Cells
2.6. Effect of Fructose on the Expression of Genes Encoding Glucose Transporters and Pro-Inflammatory Cytokines in SIM-A9 Cells
2.7. Expression of Slc2a5 mRNA in Activated SIM-A9 Cells
2.8. Effect of GLUT5 Inhibition on the Expression of Pro-Inflammatory Cytokine Genes in SIM-A9 Cells
3. Discussion
4. Materials and Methods
4.1. Primary Microglial Culture
4.2. Culture of SIM-A9 Microglia Cell Line
4.3. Western Blot Analysis
4.4. RNA Analysis
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mittelbronn, M.; Dietz, K.; Schluesener, H.J.; Meyermann, R. Local distribution of microglia in the normal adult human central nervous system differs by up to one order of magnitude. Acta Neuropathol. 2001, 101, 249–255. [Google Scholar] [CrossRef]
- Douglass, J.D.; Dorfman, M.D.; Thaler, J.P. Glia: Silent partners in energy homeostasis and obesity pathogenesis. Diabetologia 2017, 60, 226–236. [Google Scholar] [CrossRef] [PubMed]
- De Souza, C.T.; Araujo, E.P.; Bordin, S.; Ashimine, R.; Zollner, R.L.; Boschero, A.C.; Saad, M.J.; Velloso, L.A. Consumption of a fat-rich diet activates a proinflammatory response and induces insulin resistance in the hypothalamus. Endocrinology 2005, 146, 4192–4199. [Google Scholar] [CrossRef] [Green Version]
- Thaler, J.P.; Yi, C.X.; Schur, E.A.; Guyenet, S.J.; Hwang, B.H.; Dietrich, M.O.; Zhao, X.; Sarruf, D.A.; Izgur, V.; Maravilla, K.R.; et al. Obesity is associated with hypothalamic injury in rodents and humans. J. Clin. Investig. 2012, 122, 153–162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.; Zhang, G.; Zhang, H.; Karin, M.; Bai, H.; Cai, D. Hypothalamic IKKbeta/NF-kappaB and ER stress link overnutrition to energy imbalance and obesity. Cell 2008, 135, 61–73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valdearcos, M.; Robblee, M.M.; Benjamin, D.I.; Nomura, D.K.; Xu, A.W.; Koliwad, S.K. Microglia dictate the impact of saturated fat consumption on hypothalamic inflammation and neuronal function. Cell Rep. 2014, 9, 2124–2138. [Google Scholar] [CrossRef] [Green Version]
- Buckman, L.B.; Hasty, A.H.; Flaherty, D.K.; Buckman, C.T.; Thompson, M.M.; Matlock, B.K.; Weller, K.; Ellacott, K.L. Obesity induced by a high-fat diet is associated with increased immune cell entry into the central nervous system. Brain Behav. Immun. 2014, 35, 33–42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valdearcos, M.; Douglass, J.D.; Robblee, M.M.; Dorfman, M.D.; Stifler, D.R.; Bennett, M.L.; Gerritse, I.; Fasnacht, R.; Barres, B.A.; Thaler, J.P.; et al. Microglial inflammatory signaling orchestrates the hypothalamic immune response to dietary excess and mediates obesity susceptibility. Cell Metab. 2017, 26, 185–197 e3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, Y.; Ottaway, N.; Schriever, S.C.; Legutko, B.; Garcia-Caceres, C.; de la Fuente, E.; Mergen, C.; Bour, S.; Thaler, J.P.; Seeley, R.J.; et al. Hormones and diet, but not body weight, control hypothalamic microglial activity. Glia 2014, 62, 17–25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yenari, M.A.; Giffard, R.G. Ischemic vulnerability of primary murine microglial cultures. Neurosci. Lett. 2001, 298, 5–8. [Google Scholar] [CrossRef]
- Routh, V.H.; Hao, L.; Santiago, A.M.; Sheng, Z.; Zhou, C. Hypothalamic glucose sensing: Making ends meet. Front. Syst. Neurosci. 2014, 8, 236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Camandola, S. Astrocytes, emerging stars of energy homeostasis. Cell Stress 2018, 2, 246–252. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Plata-Salaman, C.R.; Oomura, Y.; Kai, Y. Tumor necrosis factor and interleukin-1 beta: Suppression of food intake by direct action in the central nervous system. Brain Res. 1988, 448, 106–114. [Google Scholar] [CrossRef]
- Chance, W.T.; Fischer, J.E. Aphagic and adipsic effects of interleukin-1. Brain Res. 1991, 568, 261–264. [Google Scholar] [CrossRef]
- Mizuno, T.M.; Lew, P.S.; Spirkina, A.; Xu, Y. Mediation of glucose-induced anorexia by central nervous system interleukin 1 signaling. Behav. Brain Res. 2013, 256, 512–519. [Google Scholar] [CrossRef]
- Quan, Y.; Jiang, C.T.; Xue, B.; Zhu, S.G.; Wang, X. High glucose stimulates TNFalpha and MCP-1 expression in rat microglia via ROS and NF-kappaB pathways. Acta Pharmacol. Sin. 2011, 32, 188–193. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Dong, H.; Zhang, S.; Lu, S.; Sun, J.; Qian, Y. Enhancement of LPS-induced microglial inflammation response via TLR4 under high glucose conditions. Cell Physiol. Biochem. 2015, 35, 1571–1581. [Google Scholar] [CrossRef]
- Vuong, B.; Odero, G.; Rozbacher, S.; Stevenson, M.; Kereliuk, S.M.; Pereira, T.J.; Dolinsky, V.W.; Kauppinen, T.M. Exposure to gestational diabetes mellitus induces neuroinflammation, derangement of hippocampal neurons, and cognitive changes in rat offspring. J. Neuroinflamm. 2017, 14, 80. [Google Scholar] [CrossRef] [Green Version]
- Huang, Y.; Liao, Z.; Lin, X.; Wu, X.; Chen, X.; Bai, X.; Zhuang, Y.; Yang, Y.; Zhang, J. Overexpression of miR-146a might regulate polarization transitions of BV-2 cells induced by high glucose and glucose fluctuations. Front. Endocrinol. 2019, 10, 719. [Google Scholar] [CrossRef] [Green Version]
- Hsieh, C.F.; Liu, C.K.; Lee, C.T.; Yu, L.E.; Wang, J.Y. Acute glucose fluctuation impacts microglial activity, leading to inflammatory activation or self-degradation. Sci. Rep. 2019, 9, 840. [Google Scholar] [CrossRef] [Green Version]
- Sonneville, R.; den Hertog, H.M.; Guiza, F.; Gunst, J.; Derese, I.; Wouters, P.J.; Brouland, J.P.; Polito, A.; Gray, F.; Chretien, F.; et al. Impact of hyperglycemia on neuropathological alterations during critical illness. J. Clin. Endocrinol. Metab. 2012, 97, 2113–2123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burant, C.F.; Takeda, J.; Brot-Laroche, E.; Bell, G.I.; Davidson, N.O. Fructose transporter in human spermatozoa and small intestine is GLUT5. J. Biol. Chem. 1992, 267, 14523–14526. [Google Scholar] [CrossRef]
- Payne, J.; Maher, F.; Simpson, I.; Mattice, L.; Davies, P. Glucose transporter Glut 5 expression in microglial cells. Glia 1997, 21, 327–331. [Google Scholar] [CrossRef]
- Shu, H.J.; Isenberg, K.; Cormier, R.J.; Benz, A.; Zorumski, C.F. Expression of fructose sensitive glucose transporter in the brains of fructose-fed rats. Neuroscience 2006, 140, 889–895. [Google Scholar] [CrossRef]
- Ho, C.Y.; Lin, Y.T.; Chen, H.H.; Ho, W.Y.; Sun, G.C.; Hsiao, M.; Lu, P.J.; Cheng, P.W.; Tseng, C.J. CX3CR1-microglia mediates neuroinflammation and blood pressure regulation in the nucleus tractus solitarii of fructose-induced hypertensive rats. J Neuroinflamm. 2020, 17, 185. [Google Scholar] [CrossRef]
- Xu, M.X.; Yu, R.; Shao, L.F.; Zhang, Y.X.; Ge, C.X.; Liu, X.M.; Wu, W.Y.; Li, J.M.; Kong, L.D. Up-regulated fractalkine (FKN) and its receptor CX3CR1 are involved in fructose-induced neuroinflammation: Suppression by curcumin. Brain Behav. Immun. 2016, 58, 69–81. [Google Scholar] [CrossRef] [PubMed]
- Cigliano, L.; Spagnuolo, M.S.; Crescenzo, R.; Cancelliere, R.; Iannotta, L.; Mazzoli, A.; Liverini, G.; Iossa, S. Short-term fructose feeding induces inflammation and oxidative stress in the hippocampus of young and adult rats. Mol. Neurobiol. 2018, 55, 2869–2883. [Google Scholar] [CrossRef]
- Stansley, B.; Post, J.; Hensley, K. A comparative review of cell culture systems for the study of microglial biology in Alzheimer’s disease. J. Neuroinflamm. 2012, 9, 115. [Google Scholar] [CrossRef] [Green Version]
- Nagamoto-Combs, K.; Kulas, J.; Combs, C.K. A novel cell line from spontaneously immortalized murine microglia. J. Neurosci. Methods 2014, 233, 187–198. [Google Scholar] [CrossRef] [Green Version]
- DeMars, K.M.; Yang, C.; Castro-Rivera, C.I.; Candelario-Jalil, E. Selective degradation of BET proteins with dBET1, a proteolysis-targeting chimera, potently reduces pro-inflammatory responses in lipopolysaccharide-activated microglia. Biochem. Biophys. Res. Commun. 2018, 497, 410–415. [Google Scholar] [CrossRef]
- Milanski, M.; Degasperi, G.; Coope, A.; Morari, J.; Denis, R.; Cintra, D.E.; Tsukumo, D.M.; Anhe, G.; Amaral, M.E.; Takahashi, H.K.; et al. Saturated fatty acids produce an inflammatory response predominantly through the activation of TLR4 signaling in hypothalamus: Implications for the pathogenesis of obesity. J. Neurosci. 2009, 29, 359–370. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, Y.; Lai, K.; Zhong, Q.; Demin, K.A.; Kalueff, A.V.; Song, C. High-glucose/high-cholesterol diet in zebrafish evokes diabetic and affective pathogenesis: The role of peripheral and central inflammation, microglia and apoptosis. Prog. Neuropsychopharm. Biol. Psychiatry 2020, 96, 109752. [Google Scholar] [CrossRef]
- Wang, L.Q.; Zhou, H.J. LncRNA MALAT1 promotes high glucose-induced inflammatory response of microglial cells via provoking MyD88/IRAK1/TRAF6 signaling. Sci. Rep. 2018, 8, 8346. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Wu, S.; Hong, Z.; Chen, X.; Shan, X.; Fischbach, S.; Xiao, X. Chronic hyperglycemia regulates microglia polarization through ERK5. Aging 2019, 11, 697–706. [Google Scholar] [CrossRef]
- Yoshifuji, H.; Umehara, H.; Maruyama, H.; Itoh, M.; Tanaka, M.; Kawabata, D.; Fujii, T.; Mimori, T. Amelioration of experimental arthritis by a calpain-inhibitory compound: Regulation of cytokine production by E-64-d in vivo and in vitro. Int. Immunol. 2005, 17, 1327–1336. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taib, T.; Leconte, C.; van Steenwinckel, J.; Cho, A.H.; Palmier, B.; Torsello, E.; Lai Kuen, R.; Onyeomah, S.; Ecomard, K.; Benedetto, C.; et al. Neuroinflammation, myelin and behavior: Temporal patterns following mild traumatic brain injury in mice. PLoS ONE 2017, 12, e0184811. [Google Scholar] [CrossRef] [Green Version]
- Silver, I.A.; Erecinska, M. Extracellular glucose concentration in mammalian brain: Continuous monitoring of changes during increased neuronal activity and upon limitation in oxygen supply in normo-, hypo-, and hyperglycemic animals. J. Neurosci. 1994, 14, 5068–5076. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Routh, V.H. Glucose-sensing neurons: Are they physiologically relevant? Physiol. Behav. 2002, 76, 403–413. [Google Scholar] [CrossRef]
- Minokoshi, Y.; Alquier, T.; Furukawa, N.; Kim, Y.B.; Lee, A.; Xue, B.; Mu, J.; Foufelle, F.; Ferre, P.; Birnbaum, M.J.; et al. AMP-kinase regulates food intake by responding to hormonal and nutrient signals in the hypothalamus. Nature 2004, 428, 569–574. [Google Scholar] [CrossRef]
- Cha, S.H.; Wolfgang, M.; Tokutake, Y.; Chohnan, S.; Lane, M.D. Differential effects of central fructose and glucose on hypothalamic malonyl-CoA and food intake. Proc. Natl. Acad. Sci. USA 2008, 105, 16871–16875. [Google Scholar] [CrossRef] [Green Version]
- Oshio, K.; Watanabe, H.; Song, Y.; Verkman, A.S.; Manley, G.T. Reduced cerebrospinal fluid production and intracranial pressure in mice lacking choroid plexus water channel Aquaporin-1. FASEB J. 2005, 19, 76–78. [Google Scholar] [CrossRef] [PubMed]
- Pardridge, W.M. CSF, blood-brain barrier, and brain drug delivery. Expert Opin. Drug Deliv. 2016, 13, 963–975. [Google Scholar] [CrossRef] [PubMed]
- Winkler, Z.; Kuti, D.; Polyak, A.; Juhasz, B.; Gulyas, K.; Lenart, N.; Denes, A.; Ferenczi, S.; Kovacs, K.J. Hypoglycemia-activated hypothalamic microglia impairs glucose counterregulatory responses. Sci. Rep. 2019, 9, 6224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bernier, L.P.; York, E.M.; Kamyabi, A.; Choi, H.B.; Weilinger, N.L.; MacVicar, B.A. Microglial metabolic flexibility supports immune surveillance of the brain parenchyma. Nat. Commun. 2020, 11, 1559. [Google Scholar] [CrossRef] [PubMed]
- Churchward, M.A.; Tchir, D.R.; Todd, K.G. Microglial function during glucose deprivation: Inflammatory and neuropsychiatric implications. Mol. Neurobiol. 2018, 55, 1477–1487. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Pavlou, S.; Du, X.; Bhuckory, M.; Xu, H.; Chen, M. Glucose transporter 1 critically controls microglial activation through facilitating glycolysis. Mol Neurodegener. 2019, 14, 2. [Google Scholar] [CrossRef] [Green Version]
- Bady, I.; Marty, N.; Dallaporta, M.; Emery, M.; Gyger, J.; Tarussio, D.; Foretz, M.; Thorens, B. Evidence from glut2-null mice that glucose is a critical physiological regulator of feeding. Diabetes 2006, 55, 988–995. [Google Scholar] [CrossRef]
- Mesonero, J.; Matosin, M.; Cambier, D.; Rodriguez-Yoldi, M.J.; Brot-Laroche, E. Sugar-dependent expression of the fructose transporter GLUT5 in Caco-2 cells. Biochem. J. 1995, 312, 757–762. [Google Scholar] [CrossRef] [Green Version]
- Matosin-Matekalo, M.; Mesonero, J.E.; Laroche, T.J.; Lacasa, M.; Brot-Laroche, E. Glucose and thyroid hormone co-regulate the expression of the intestinal fructose transporter GLUT5. Biochem. J. 1999, 339, 233–239. [Google Scholar] [CrossRef]
- Gonzalez, R.G.; Barnett, P.; Aguayo, J.; Cheng, H.M.; Chylack, L.T., Jr. Direct measurement of polyol pathway activity in the ocular lens. Diabetes 1984, 33, 196–199. [Google Scholar] [CrossRef] [Green Version]
- Hwang, J.J.; Jiang, L.; Hamza, M.; Dai, F.; Belfort-DeAguiar, R.; Cline, G.; Rothman, D.L.; Mason, G.; Sherwin, R.S. The human brain produces fructose from glucose. JCI Insight 2017, 2, e90508. [Google Scholar] [CrossRef]
- Miller, C.C.; Martin, R.J.; Whitney, M.L.; Edwards, G.L. Intracerebroventricular injection of fructose stimulates feeding in rats. Nutr. Neurosci. 2002, 5, 359–362. [Google Scholar] [CrossRef]
- Jang, C.; Hui, S.; Lu, W.; Cowan, A.J.; Morscher, R.J.; Lee, G.; Liu, W.; Tesz, G.J.; Birnbaum, M.J.; Rabinowitz, J.D. The small intestine converts dietary fructose into glucose and organic acids. Cell Metab. 2018, 27, 351–361 e3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wolfgang, M.J.; Cha, S.H.; Sidhaye, A.; Chohnan, S.; Cline, G.; Shulman, G.I.; Lane, M.D. Regulation of hypothalamic malonyl-CoA by central glucose and leptin. Proc. Natl. Acad. Sci. USA 2007, 104, 19285–19290. [Google Scholar] [CrossRef] [Green Version]
- Dave, K.M.; Ali, L.; Manickam, D.S. Characterization of the SIM-A9 cell line as a model of activated microglia in the context of neuropathic pain. PLoS ONE 2020, 15, e0231597. [Google Scholar] [CrossRef] [Green Version]
- Chen, W.L.; Wang, Y.Y.; Zhao, A.; Xia, L.; Xie, G.; Su, M.; Zhao, L.; Liu, J.; Qu, C.; Wei, R.; et al. Enhanced fructose utilization mediated by SLC2A5 is a unique metabolic feature of acute myeloid leukemia with therapeutic potential. Cancer Cell 2016, 30, 779–791. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weng, Y.; Fan, X.; Bai, Y.; Wang, S.; Huang, H.; Yang, H.; Zhu, J.; Zhang, F. SLC2A5 promotes lung adenocarcinoma cell growth and metastasis by enhancing fructose utilization. Cell Death Discov. 2018, 4, 38. [Google Scholar] [CrossRef] [PubMed]
- Dunn-Meynell, A.A.; Govek, E.; Levin, B.E. Intracarotid glucose selectively increases Fos-like immunoreactivity in paraventricular, ventromedial and dorsomedial nuclei neurons. Brain Res. 1997, 748, 100–106. [Google Scholar] [CrossRef]
- Becskei, C.; Lutz, T.A.; Riediger, T. Glucose reverses fasting-induced activation in the arcuate nucleus of mice. Neuroreport 2008, 19, 105–109. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Zhang, X.; Chen, M.; Chen, J.; Gao, T.; Yao, S. Dexmedetomidine inhibits inflammation in microglia cells under stimulation of LPS and ATP by c-Fos/NLRP3/caspase-1 cascades. EXCLI J. 2018, 17, 302–311. [Google Scholar]
- Poritsanos, N.J.; Wong, D.; Vrontakis, M.E.; Mizuno, T.M. Regulation of hepatic PPARgamma2 and lipogenic gene expression by melanocortin. Biochem. Biophys. Res. Commun. 2008, 376, 384–388. [Google Scholar] [CrossRef] [PubMed]







| Gene | Control | LPS (2.5 ng/mL) | p Value * |
|---|---|---|---|
| Il1a | 100.0 ± 2.2% | 308.9 ± 14.9% | <0.0001 |
| Il1b | 100.0 ± 5.9% | 247,149.5 ± 12,442.2% | <0.0001 |
| Slc2a5 | 100.0 ± 2.3% | 154.1 ± 17.2% | <0.05 |
| Gene | Accession No. | Direction | Sequences | Exon |
|---|---|---|---|---|
| Slc2a1 | NM_011400 | Forward | 5′-GGATCACTGCAGTTCGGCTAT-3′ | 2 |
| Reverse | 5′-CGTAGCGGTGGTTCCATGTT-3′ | 3 | ||
| Slc2a2 | NM_031197 | Forward | 5′-CATTGCTGGACGAAGTGTATCAG-3′ | 4 |
| Reverse | 5′-GGAGCGATCTCTCCAATGTACAT-3′ | 5 | ||
| Slc2a5 | NM_019741 | Forward | 5′-AAGCGACGACGTCCAGTATGT-3′ | 10 |
| Reverse | 5′-GAATCGCCGTCCCCAAAG-3′ | 11 | ||
| Il1α | NM_010554 | Forward | 5′-GCCCGTGTTGCTGAAGGA-3′ | 6–7 |
| Reverse | 5′-TGGATAAGCAGCTGATGTGAAGTAGT-3′ | 7 | ||
| Il1β | NM_008361 | Forward | 5′-TTGACGGACCCCAAAAGATG-3′ | 3 |
| Reverse | 5′-TGCTGCTGCGAGATTTGAAG-3′ | 4 | ||
| Cyclophilin | X52803 | Forward | 5′-AAGCATACAGGTCCTGGCATCT-3′ | 4 |
| Reverse | 5′-TGCCATCCAGCCATTCAGT-3′ | 4–5 | ||
| Hprt | NM_013556 | Forward | 5′-AGTCCCAGCGTCGTGATTAG-3′ | 1–2 |
| Reverse | 5′-TGATGGCCTCCCATCTCCTT-3′ | 3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mizuno, T.M.; Lew, P.S.; Jhanji, G. Regulation of the Fructose Transporter Gene Slc2a5 Expression by Glucose in Cultured Microglial Cells. Int. J. Mol. Sci. 2021, 22, 12668. https://doi.org/10.3390/ijms222312668
Mizuno TM, Lew PS, Jhanji G. Regulation of the Fructose Transporter Gene Slc2a5 Expression by Glucose in Cultured Microglial Cells. International Journal of Molecular Sciences. 2021; 22(23):12668. https://doi.org/10.3390/ijms222312668
Chicago/Turabian StyleMizuno, Tooru M., Pei San Lew, and Gursagar Jhanji. 2021. "Regulation of the Fructose Transporter Gene Slc2a5 Expression by Glucose in Cultured Microglial Cells" International Journal of Molecular Sciences 22, no. 23: 12668. https://doi.org/10.3390/ijms222312668
APA StyleMizuno, T. M., Lew, P. S., & Jhanji, G. (2021). Regulation of the Fructose Transporter Gene Slc2a5 Expression by Glucose in Cultured Microglial Cells. International Journal of Molecular Sciences, 22(23), 12668. https://doi.org/10.3390/ijms222312668