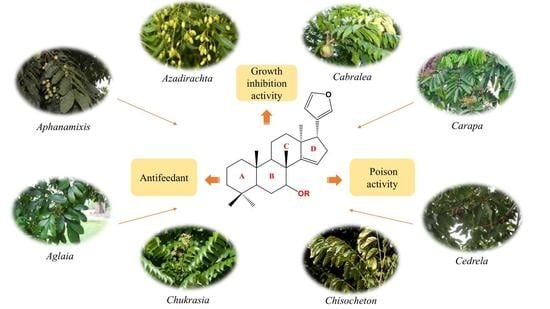
Insecticidal Triterpenes in Meliaceae: Plant Species, Molecules and Activities: Part Ⅰ (Aphanamixis-Chukrasia)
Abstract
:1. Introduction
2. Structures of Triterpenes
3. Plant Species and Their Insecticidal Chemicals
3.1. Aglaia
3.2. Aphanamixis
3.2.1. Rings A,B-seco Limonoids
3.2.2. Ring A-seco Limonoids
3.3. Azadirachta
3.3.1. Ring C-seco Chemicals
3.3.2. Ring D-seco Chemicals
3.3.3. Rings Intact Limonoids: Azadirones, Azadiraindin A and Meliatetraolenone
3.3.4. Pentanortriterpenoids
3.3.5. Octanortriterpenoids
3.3.6. Protolimonoids
3.4. Cabralea
3.5. Carapa
3.6. Cedrela
3.6.1. The Ring Intact Limonoid: Cedrelone
3.6.2. Ring D-seco Limonoids
3.6.3. Rings A,D-seco Limonoids and Rings B,D-seco Limonoids
3.6.4. The Rearranged Limonoids
3.6.5. Pentacyclic Triterpenes
3.7. Chisocheton
3.8. Chukrasia
4. Structures and Structure–Activity Relationship (SAR) of the Insecticidal Chemicals
4.1. Structures of the Insecticidal Chemicals
4.2. Structure–Activity Relationship (SAR) of the Insecticidal Chemicals
5. Insecticidal Mechanism of Action
6. Future Outlook
Funding
Conflicts of Interest
References
- Dalvi, R.R.; Salunkhe, D.K. Toxicological implications of pesticides: Their toxic effects on seeds of food plants. Toxicology 1975, 3, 269–285. [Google Scholar] [CrossRef]
- Hubbard, M.; Hynes, R.K.; Erlandson, M.; Bailey, K.L. The biochemistry behind biopesticide efficacy. Sustain. Chem. Process. 2014, 2, 18. [Google Scholar] [CrossRef] [Green Version]
- Christenhusz, M.J.M.; Byng, J.W. The number of known plants species in the world and its annual increase. Phytotaxa 2016, 261, 201–217. [Google Scholar] [CrossRef] [Green Version]
- González-Coloma, A.; López-Balboa, C.; Reina, M.; Fraga, B.M. Triterpene-based plant defenses. Phytochem. Rev. 2011, 10, 245–260. [Google Scholar] [CrossRef] [Green Version]
- Alvarenga, N.; Ferro, E.A. Bioactive Triterpenes and Related Compounds from Celastraceae. Stud. Nat. Prod. Chem. 2005, 36, 635–702. [Google Scholar]
- Geng, S.S. Triterpene Chemistry; Chemical Industry Press: Beijing, China, 2008. [Google Scholar]
- Champagne, D.E.; Koul, O.; Isman, M.B.; Scudder, G.G.E.; Towers, G.H.N. Biological activity of limonoids from the rutales. Phytochemistry 1992, 31, 377–394. [Google Scholar] [CrossRef]
- Nakatani, M.; Iwashita, T.; Mizukawa, K. Trichilinin, a new hexacyclic limonoid from Trichilia roka. Heterocycles 1987, 26, 43–46. [Google Scholar] [CrossRef]
- Tan, Q.G.; Luo, X.D. Meliaceous limonoids: Chemistry and biological activities. Chem. Rev. 2011, 111, 7437–7522. [Google Scholar] [CrossRef] [PubMed]
- Carpinella, M.C.; Defago, M.T.; Valladares, G.; Palacios, S.M. Antifeedant and insecticide properties of a limonoid from Melia azedarach (Meliaceae) with potential use for pest management. J. Agric. Food Chem. 2003, 51, 369–374. [Google Scholar] [CrossRef] [PubMed]
- Koul, O.; Isman, M.B. Effects of azadirachtin on the dietary utilization and development of the variegated cutworm Peridroma saucia. J. Insect Physiol. 1991, 37, 591–598. [Google Scholar] [CrossRef]
- Kubo, I.; Klocke, J.A. Azadirachtin, insect ecdysis inhibitor. Agric. Biol. Chem. 1982, 46, 1951–1953. [Google Scholar]
- Garcia, E.S.; Azambuja, P.D.; Forster, H.; Rembold, H. Feeding and molt inhibition by azadirachtins A, B, and 7-acetyl-azadirachtin A in Rhodnius prolixus nymphs. Z. Naturforsch. C 1984, 39, 1155–1158. [Google Scholar] [CrossRef]
- Mordue, A.J.; Nasiruddin, M. Azadirachtin treatment and host-plant selection. In Proceedings of the 8th International Symposium on Insect-Plant Relationships, Wageningen, The Netherlands, 9–13 March 1992; Springer: Dordrecht, The Netherlands, 1992; Volume 49, pp. 176–178. [Google Scholar]
- Bohnenstengel, F.I.; Wray, V.; Witte, L.; Srivastavad, R.P.; Proksch, P. Insecticidal meliacarpins (C-seco limonoids) from Melia azedarach. Phytochemistry 1999, 50, 977–982. [Google Scholar] [CrossRef]
- Cui, C.; He, S.J. Application and development of azadirachtin. Technol. Dev. Chem. Ind. 2014, 5, 40–42. (In Chinese) [Google Scholar]
- Hofer, M.; Greger, H.; Mereiter, K. 6α-acetoxygedunin. Acta Crystallogr. Sect. E Struct. Rep. Online 2009, 65, 1942–1943. [Google Scholar] [CrossRef] [PubMed]
- Jie, Y.C.; Duo, Z.C.; Hong, P.H.; Kong, N.C.; Zhang, Y.; Di, Y.T.; Zhang, Q.; Hua, J.; Jing, S.X.; Li, S.L.; et al. Limonoids from Aphanamixis polystachya and their antifeedant activity. Nat. Prod. 2014, 77, 472–482. [Google Scholar]
- Zhang, Y.; Wang, J.S.; Wang, X.B.; Gu, Y.C.; Wei, D.D.; Guo, C.; Yang, M.H.; Kong, L. Limonoids from the fruits of Aphanamixis polystachya, (Meliaceae) and their biological activities. J. Agric. Food Chem. 2013, 61, 2171–2182. [Google Scholar] [CrossRef] [PubMed]
- Abdelgaleil, S.; Doe, M.; Nakatani, M. Rings B,D-seco limonoid antifeedants from Swietenia mahogani. Phytochemistry 2013, 96, 312–317. [Google Scholar] [CrossRef] [PubMed]
- Singh, G.; Rup, P.; Koul, O. Acute, sublethal and combination effects of azadirachtin and Bacillus thuringiensis toxins on Helicoverpa armigera (Lepidoptera: Noctuidae) larvae. Bull. Entomol. Res. 2007, 97, 351–357. [Google Scholar] [CrossRef]
- Koul, O.; Singh, G.; Singh, R.; Singh, J.; Daniewski, W.M.; Berlozecki, S. Bioefficacy and mode-of-action of some limonoids of salannin group from Azadirachta indica A. Juss and their role in a multicomponent system against lepidopteran larvae. J. Biosci. 2004, 29, 409–416. [Google Scholar] [CrossRef] [PubMed]
- Ishida, M.; Serit, M.; Nakata, K.; Juneja, L.R.; Kim, M.; Takahashi, S. Several antifeedants from neem oil, Azadirachta indica A. Juss., against Reticulitermes speratus Kolbe (Isoptera: Rhinotermitidae). Biosci. Biotechnol. Biochem. 2014, 56, 1835–1838. [Google Scholar] [CrossRef] [Green Version]
- Yan, Y.X.; Liu, J.Q.; Wang, H.W.; Chen, J.X.; Chen, J.C.; Chen, L.; Zhou, L.; Qiu, M.H. Identification and antifeedant activities of limonoids from Azadirachta indica. Chem. Biodivers. 2015, 12, 1040–1046. [Google Scholar] [CrossRef]
- Siddiqui, B.S.; Afshan, F.; Ghiasuddin; Faizi, S.; Naqvi, S.N.H.; Tariq, R.M. Two insecticidal tetranortriterpenoids from Azadirachta indica. Phytochemistry 2000, 53, 371–376. [Google Scholar] [CrossRef]
- Bina, S.S.; Farhana, F.A.; Naeem, H.S.; Naqvi, S.N.H.; Tariq, R.M. Two new triterpenoids from Azadirachta indica and their insecticidal activity. J. Nat. Prod. 2002, 65, 1216–1218. [Google Scholar]
- Siddiqui, S.; Faizi, S.; Mahmood, T.; Siddiqui, B.S. Two new insect growth regulator meliacins from Azadirachta indica A. Juss (Meliaceae). JACS 1986, 1, 1021–1025. [Google Scholar] [CrossRef]
- Ambrozin, A.R.P.; Leite, A.C.; Bueno, F.C.; Vieira, P.C.; Fernandes, J.B.; Bueno, O.C.; da Silva, M.F.G.F.; Pagnocca, F.C.; Hebling, M.J.A.; Bacci, M. Limonoids from andiroba oil and Cedrela fissilis and their insecticidal activity. J. Brazil. Chem. Soc. 2006, 17, 542–547. [Google Scholar] [CrossRef] [Green Version]
- Kipassa, N.T.; Iwagawa, T.; Okamura, H.; Doe, M.; Morimoto, Y.; Nakatani, M. Limonoids from the stem bark of Cedrela odorata. Phytochemistry 2008, 69, 1782–1787. [Google Scholar] [CrossRef]
- Lee, S.M.; Olsen, J.I.; Schweizer, M.P. 7-deacetyl-17β-hydroxyazadiradione, a new limonoid insect growth inhibitor from Azadirachta indica. Phytochemistry 1988, 27, 2773–2775. [Google Scholar] [CrossRef]
- Cai, J.Y.; Zhang, Y.; Luo, S.; Chen, D.Z.; He, H.P. Aphanamixoid A, a potent defensive limonoid, with a new carbon skeleton from Aphanamixis polystachya. Org Lett. 2012, 14, 2524–2527. [Google Scholar] [CrossRef]
- Kraus, W.; Maile, R. 1-tigloyl-3-acetylazadirachtol, a new limonoid from the marrango tree, Azadirachta excelsa Jack (Meliaceae). Indian Chem. Soc. 1997, 74, 870–873. [Google Scholar] [CrossRef]
- Kanokmedhakul, S.; Kanokmedhakul, K.; Prajuabsuk, T.; Panichajakul, S.; Panyamee, P.; Prabpai, S.; Kongsaeree, P. Azadirachtin derivatives from seed kernels of Azadirachta excelsa. J. Nat. Prod. 2005, 68, 1047–1050. [Google Scholar] [CrossRef] [PubMed]
- Koul, O.; Daniewski, W.M.; Multani, J.S.; Gumulka, M.; Singh, G. Antifeedant effects of the limonoids from Entandrophragma candolei (Meliaceae) on the gram pod borer, Helicoverpa armigera (Lepidoptera: Noctuidae). J. Agric. Food Chem. 2003, 51, 7271–7275. [Google Scholar] [CrossRef] [PubMed]
- Koul, O.; Multani, J.S.; Singh, G.; Daniewski, M.; Berlozecki, S. 6β-hydroxygedunin from Azadirachta indica. Its potentiation effects with some non-azadirachtin limonoids in neem against lepidopteran larvae. J. Agric. Food Chem. 2003, 51, 2937–2942. [Google Scholar] [CrossRef] [PubMed]
- Morgan, E.D.; Thornton, M.D. Azadirachtin in the fruit of Melia azedarach. Phytochemistry 1973, 12, 391–392. [Google Scholar] [CrossRef]
- Rodríguez, B.; Caballero, C.; Ortego, F.; Castaera, P. A new tetranortriterpenoid from Trichilia havanensis. J. Nat. Prod. 2003, 66, 452–454. [Google Scholar] [CrossRef] [PubMed]
- Shilpi, J.A.; Saha, S.; Lim, C.S.; Nahar, L.; Sarker, S.D.; Awang, K. Advances in Chemistry and Bioactivity of the Genus Chisocheton Blume. Chem. Biodivers. 2016, 13, 483–503. [Google Scholar] [CrossRef] [PubMed]
- Jennifer, M.A.; Simmonds, M.S.J.; Ley, S.V. Actions of azadirachtin, a plant allelochemical, against insects. Pestic. Sci. 1998, 54, 277–284. [Google Scholar] [CrossRef]
- Suresh, G.; Gopalakrishnan, G.; Wesley, D.; Singh, N.D.P.; Malathi, R.; Rajan, S.S. Insect antifeedant activity of the tetranortriterpenoids from the Rutales. A perusal of structural relations. J. Agric. Food Chem. 2002, 50, 4484–4490. [Google Scholar] [CrossRef]
- Govindachari, T.R.; Narsimhan, N.S.; Suresh, G.; Partho, P.D.; Gopalakrishnan, G.; Kumari, G.N.K. Structure related insect antifeedant and growth regulating activities of some limonoids. Chem. Ecol. 1995, 21, 1585–1600. [Google Scholar] [CrossRef] [PubMed]
- Nakatani, M.; Abdelgaleil, S.A.M.; Saad, M.M.G.; Huang, R.C.; Doe, M.; Iwagawa, T. Phragmalin limonoids from Chukrasia tabularis. Phytochemistry 2004, 65, 2833–2841. [Google Scholar] [CrossRef]
- Koul, O.; Isman, M.B. Toxicity of the limonoid allelochemical cedrelone to noctuid larvae. Entomol. Exp. Appl. 2011, 64, 281–287. [Google Scholar] [CrossRef]
- Siddiqui, B.S.; Afshan, F.; Gulzar, T.; Sultana, R.; Naqvi, S.N.H.; Tariq, R.M. Tetracyclic triterpenoids from the leaves of Azadirachta indica and their insecticidal activities. Chem. Pharm. Bull. 2003, 51, 415–417. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siddiqui, B.S.; Afshan, F.; Faizi, S.; Naqvi, S.N.H.; Tariq, R.M. New insect-growth-regulator meliacin butenolides from the leaves of Azadirachta indica A. Juss. J. Am. Chem. Soc. 1999, 16, 2367–2370. [Google Scholar] [CrossRef]
- Chong, S.L.; Hematpoor, A.; Hazni, H.; Azirun, M.S.; Litaudon, M.; Supratman, U.; Murata, M.; Awang, K. Mosquito larvicidal limonoids from the fruits of Chisocheton erythrocarpus Hiern. Phytochem. Lett. 2019, 30, 69–73. [Google Scholar] [CrossRef]
- Cespedes, C.L.; Calderon, J.S.; Lina, L.; Aranda, E. Agric growth inhibitory effects onfall armyworm Spodoptera frugiperda of some limonoids isolated from Cedrela spp. (Meliaceae). J. Agric. Food Chem. 2000, 48, 1903–1908. [Google Scholar] [CrossRef] [PubMed]
- Sarria, A.L.F.; Soares, M.S.; Matos, A.P.; Fernandes, J.B.; Vieira, P.C.; Silva, M.F.G.F. Effect of triterpenoids and limonoids isolated from Cabralea canjerana and Carapa guianensis (Meliaceae) against Spodoptera frugiperda (JE Smith). Z. Natur. C 2011, 66, 245–250. [Google Scholar] [CrossRef] [PubMed]
- Cespedes, C.L.; Carlos, L.C.A.; Avila, J.G.; Marin, J.C.; Domínguez, M.; Torres, P.; Aranda, E. Natural compounds as antioxidant and molting inhibitors can play a role as a model for search of new botanical pesticides. Adv. Phytomed. 2006, 3, 1–27. [Google Scholar] [CrossRef]
- Koul, O. Feeding deterrence induced by plant limonoids in the larvae of Spodoptera litura (F.) (Lepidoptera, Noctuidae). Z. Angew. Entomol. 1983, 95, 166–171. [Google Scholar] [CrossRef]
- Jimenez, A.; Villarreal, C.; Toscano, R.A.; Cook, M.; Mata, R. Limonoids from Swietenia humilis and Guarea grandiflora (Meliaceae). Phytochemistry 1998, 49, 1981–1988. [Google Scholar] [CrossRef]
- Abhijit, J. Process development of pesticide production from Azadirachta Indica A. Juss. Int. J. Agric. 2012, 23, 2848–2854. [Google Scholar]
- Ngo, N.T.N.; Lai, N.T.D.; Le, H.C.; Nguyen, L.T.T.; Nguyen, L.H.D. Chemical constituents of Aglaia elaeagnoidea and Aglaia odorata and their cytotoxicity. Nat. Prod. Res. 2021, 11, 1893723. [Google Scholar] [CrossRef] [PubMed]
- Nugroho, B.W.; Edrada, R.A.; Wray, V.; Wittec, L.; Bringmannd, G.; Gehlinge, M.; Proksch, P. An insecticidal rocaglamide derivatives and related compounds from Aglaia odorata (Meliaceae). Phytochemistry 1999, 51, 367–376. [Google Scholar] [CrossRef]
- Yang, S.H.; Zeng, S.Y.; Zheng, L.S. Insecticidal active constituents from twig of Aglaia odorata. Chin. J. Zhong Cao Yao 2004, 11, 1207–1211. [Google Scholar]
- Liu, B.; Yang, L.; Xu, Y.K.; Liao, S.G.; Luo, H.R.; Na, Z. Two new triterpenoids from Gelsemium elegans and Aglaia odorata. Nat. Prod. Commun. 2013, 8, 1373–1376. [Google Scholar] [CrossRef] [PubMed]
- Robin, B.B.; Kathleen, D. Triterpenoids of Aglaia odorata. Configuration of trisubstituted epoxides. J. Chem. Soc. 1977, 5, 510–512. [Google Scholar]
- Cai, X.H.; Luo, X.D.; Zhou, J.; Hao, X.J. Compound representatives of a new type of triterpenoid from Aglaia odorata. Org. Lett. 2005, 7, 2877–2879. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.W.; Jin, H.Z.; Zhang, W.D. Constituents from Aphanamixis species and their biological activities. Phytochem. Rev. 2013, 12, 915–942. [Google Scholar] [CrossRef]
- Wu, S.L.; Zou, Q.P.; Xie, X.Y.; Ren, J.J.; Zhang, F.; OuYang, J.R.; Yin, P.C.; Dong, F.W.; He, H.P. Two new triterpenoids from the fruits of Aphanamixis polystachya. J. Asian Nat. Prod. Res. 2021, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Camero, C.M.; Vassallo, A.; De, L.M.; Temraz, A.; Tommas, N.D. Limonoids from Aphanamixis polystachya leaves and their interaction with Hsp90. Planta Med. 2018, 84, 964–970. [Google Scholar] [CrossRef] [Green Version]
- Chen, S.K.; Chen, B.Y.; Li, H. Flora of China; Science Press: Beijing, China, 1997; Volume 43, pp. 239–240. [Google Scholar]
- Zhang, Y.; Wang, J.S.; Gu, Y.C.; Wang, X.B.; Kong, L.Y. Diverse prieurianin-type limonoid derivatives from the fruits of Aphanamixis grandifolia and their absolute configuration determination. Tetrahedron 2014, 37, 6594–6606. [Google Scholar] [CrossRef]
- Wang, H.; Yang, Z.K.; Fan, Z.J.; Wu, Q.J.; Zhang, Y.J.; Mi, N.; Wang, S.X.; Zhang, Z.C.; Song, H.B.; Liu, F. Synthesis and insecticidal activity of N-tert-butyl-N, N′-diacylhydrazines containing 1,2,3-thiadiazoles. J. Agric. Food Chem. 2011, 59, 628–634. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.D.; Wu, S.H.; Wu, D.G.; Ma, Y.B.; Qi, S.H. Novel antifeeding limonoids from Dysoxylum hainanense. Tetrahedron 2002, 58, 7797–7804. [Google Scholar] [CrossRef]
- Luo, S.H.; Luo, Q.; Niu, X.M.; Xie, M.J.; Zhao, X.; Schneider, B.; Gershenzon, J.; Li, S.H. Glandular trichomes of Leucosceptrum canum harbor defensive sesterterpenoids. Angew. Chem. 2010, 122, 4573–4577. [Google Scholar] [CrossRef]
- Jarvis, A.P.; Morgan, E.D.; Edwards, C. Rapid separation of triterpenoids from Neem seed extracts. Phytochem. Anal. 1999, 10, 39–43. [Google Scholar] [CrossRef]
- Nat, J.M.; Sluis, W.G.; De Silva, K.T.D.; Labadiea, R.P. Ethnopharmacognostical survey of Azadirachta indica A. Juss (Meliaceae). J. Ethnopharmacol. 1991, 35, 1–24. [Google Scholar] [PubMed]
- Biswas, K.; Chattopadhyay, I.; Banerjee, R.K. Biological activities and medicinal properties of neem (Azadirachta indica). Curr. Sci. Bangalore 2002, 82, 1336–1345. [Google Scholar]
- Xu, H.H.; Lai, D.; Zhang, Z.X. Research and application of botanical pesticide azadirachtin. J. South China Agric. Univ. 2017, 38, 1–11. [Google Scholar]
- Bezzar, B.R.; Kilani, M.S.; Maroua, F.; Aribi, N. Azadirachtin induced larval avoidance and antifeeding by disruption of food intake and digestive enzymes in Drosophila melanogaster (Diptera: Drosophilidae). Pestic. Biochem. Physiol. 2017, 143, 135–140. [Google Scholar] [CrossRef] [PubMed]
- Radhika, S.; Sahayaraj, K.; Senthil-Nathan, S.; Hunter, W.B. Individual and synergist activities of monocrotophos with neem based pesticide, Vijayneem against Spodoptera litura Fab. Physiol. Mol. Plant P. 2017, 101, 54–68. [Google Scholar] [CrossRef]
- Lucantoni, L.; Giusti, F.; Cristofaro, M.; Pasqualini, L.; Esposito, F.; Lupetti, P.; Habluetzel, A. Effects of a neem extract on blood feeding, oviposition and oocyte ultrastructure in Anopheles stephensi Liston (Diptera: Culicidae). Tissue Cell 2006, 38, 361–371. [Google Scholar] [CrossRef]
- Lynn, O.M.; Song, W.G.; Shim, J.K.; Kim, J.E.; Lee, K.Y. Effects of azadirachtin and neem-based formulations for the control of sweetpotato whitefly and root-knot nematode. J. Korean Soc. Appl. Biol. 2010, 53, 598–604. [Google Scholar] [CrossRef]
- Flores, C.F.; Martínez, D.G.P.; Villafranca, S.M.; Pérez, M.F. Preparation and characterization of azadirachtin alginate-biosorbent based formulations: Water release kinetics and photodegradation study. J. Agric. Food Chem. 2015, 63, 8391–8398. [Google Scholar] [CrossRef] [PubMed]
- Mordue, A.J.; Blackwell, A. Azadirachtin: An update. J. Insect Physiol. 1993, 39, 903–924. [Google Scholar] [CrossRef]
- Devakumar, C.; Kumar, R. Total synthesis of azadirachtin: A chemical odyssey. Curr. Sci. 2009, 95, 573–575. [Google Scholar]
- Ley, S.V. Synthesis and chemistry of the insect antifeedant azadirachtin. Pure. Appl. Chem. 1994, 66, 2099–2102. [Google Scholar] [CrossRef]
- Kraus, W.; Bokel, M.; Schwinger, M. The chemistry of azadirachtin and other insecticidal constituents of Meliaceae. Proceedings 1993, 34, 18. [Google Scholar]
- Govindachari, T.R.; Sandhya, G.; Raj, S.P.G. Azadirachtins H and I: Two new tetranortriterpenoids from Azadirachta indica. J. Nat. Prod. 1992, 55, 596–601. [Google Scholar] [CrossRef]
- Rembold, H. The azadirachtins—Their potential for insect control. Econ. Med. Plant Res. 1989, 3, 57–72. [Google Scholar]
- Hummel, H.E.; Hein, D.F.; Schmutterer, H. The coming of age of azadirachtins and related tetranortriterpenoids. J. Biopest 2012, 5, 82. [Google Scholar]
- Rembold, H. The Azadirachtins: Potent insect growth inhibitors. Mem. Inst. Oswaldo Cruz 1987, 82, 61–66. [Google Scholar] [CrossRef] [Green Version]
- Ley, S.V.; Anderson, J.C.; Blaney, W.M.; Morgan, E.D.; Sheppard, R.N.; Sunmonds, M.S.J.; Slawm, A.M.Z.; Snuth, S.C.; Wllhamsa, D.J.; Wood, A. Chemistry of insect antifeedants from Azadirachta indica (part 11): Characterisation and structure activity relationships of some novel rearranged azadirachtins. Tetrahedron 1991, 47, 9231–9246. [Google Scholar] [CrossRef]
- Thompson, D.G.; Tonon, A.; Beltran, E.; Felix, H. Inhibition of larval growth and adult fecundity in Asian long-horned beetle (Anoplophora glabripennis) exposed to azadirachtins under quarantine laboratory conditions. Pest Manag. Sci. 2018, 74, 1351–1361. [Google Scholar] [CrossRef] [PubMed]
- Sinha, S.; Walia, S.; Kumar, J.; Panwar, V.P.S.; Parmar, B.S. Egg hatching inhibitory activity of Azadirachtins A, B and H against maize stem borer Chilo Partellus (Swinhoe). Pestic. Res. J. 2005, 17, 6–9. [Google Scholar]
- Govindachari, T.R.; Suresh, G.; Geetha, G.; Wesley, S.D. Insect antifeedant and growth regulating activities of neem seed oil-the role of major tetranortriterpenoids. J. Appl. Entomol. 2000, 124, 287–291. [Google Scholar] [CrossRef]
- Cui, B.; Chai, H.; Constant, H.L.; Santisuk, T.; Reutrakul, V.; Beecher, C.W.W.; Farnsworth, N.R.; Cordell, G.A.; Pezzuto, J.M.; Kinghorn, A.D. Limonoids from Azadirachta excelsa. Phytochemistry 1998, 47, 1283–1287. [Google Scholar] [CrossRef]
- Madhusudana, R.M.; Meshulam, H.; Zelnik, R.; Lavie, D. Structure and stereochemistry of limonoids of Cabralea eichleriana. Phytochemistry 1975, 14, 1071–1075. [Google Scholar] [CrossRef]
- Braga, P.; Soares, M.S.M.; Fátima, G.F.; Vieira, P.C.; Fernandes, J.B.; Pinheiro, A.L. Dammarane triterpenes from Cabralea canjerana (Vell.) Mart. (Meliaceae): Their chemosystematic significance. Biochem. Syst. Ecol. 2006, 34, 282–290. [Google Scholar] [CrossRef]
- Adesida, G.A.; Adesogan, E.K.; Okorie, D.A.; Taylor, D.A.H.; Styles, B.T. The limonoid chemistry of the genus Khaya (Meliaceae). Phytochemistry 1971, 10, 1845–1853. [Google Scholar] [CrossRef]
- Adesogan, E.K.; Taylor, D.A.H. Extractives from Khaya senegalensis (Desr.) A. Juss. J. Chem. Soc. C Org. 1968, 16, 790–791. [Google Scholar] [CrossRef]
- Luco, J.M.; Sosa, M.C.; Cesco, J.C.; Tonn, C.E.; Giordano, O.S. Molecular connectivity and hydrophobicity in the study of antifeedant activity of clerodane diterpenoids. Pestic. Sci. 1994, 41, 1–6. [Google Scholar] [CrossRef]
- Magrini, F.E.; Specht, A.; Gaio, J.; Girelli, C.P.; Migues, I.; Heinzen, H.; Saldana, J.; Sartori, V.C. Antifeedant activity and effects of fruits and seeds extracts of Cabralea canjerana canjerana (Vell.) Mart. (Meliaceae) on the immature stages of the fall armyworm Spodoptera frugiperda (JE Smith) (Lepidoptera: Noctuidae). Ind. Crops. Prod. 2015, 65, 150–158. [Google Scholar] [CrossRef]
- Alvarez, D.; Zuleta, D.; Saldamando, C.; Lobo-Echeverri, T. Selective activity of Carapa guianensis and Swietenia macrophylla (Meliaceae) against the corn and rice strains of Spodoptera frugiperda (Lepidoptera, Noctuidae). Int. J. Pest Manag. 2021. [Google Scholar] [CrossRef]
- Sarquis, I.R.; Sarquis, R.S.F.R.; Marinho, V.H.S.; Neves, F.B.; Araújo, I.F.; Damasceno, L.F.; Ferreira, R.M.A.; Souto, R.N.P.; Cavalho, I.C.T.; Ferreira, I.M. Carapa guianensis Aubl (Meliaceae) oil associated with silk fibroin, as alternative to traditional surfactants, and active against larvae of the vector Aedes aegypti. Ind. Crops. Prod. 2020, 157, 112931. [Google Scholar] [CrossRef]
- Isman, M.B.; Matsuura, H.; MacKinnon, S.; Durst, T.; Towers, G.H.N.; Arnason, J.T. Phytochemistry of the Meliaceae: So many terpenoids, so few insecticides. Phytochem. Redund. Ecol. Interact. 1996, 30, 155–178. [Google Scholar]
- Siddiqui, S.; Fuchs, S.; Lucke, J.; Voelter, W. Structure of a new natural compound in Melia azadirachta Linn. [Azadirachta indica]: 17-hydroxyazadiradion in the fruit. Tetrahedron Lett. 1978, 7, 611–612. [Google Scholar] [CrossRef]
- Kraus, W.; Cramer, R. 17-EPI-azadiradion uno 17-β-hydroxy-azadiradion, zwei neue inhaltsstoffe aus Azadirachta indica A. Juss. Tetrahedron Lett. 1978, 19, 2395–2398. [Google Scholar] [CrossRef]
- Nogueira, T.S.R.; Passos, M.S.; Nascimento, L.P.S.; Arantes, M.B.D.; Monteiro, N.O.; Boeno, S.I.D.; de Carvalho, A.; Azevedo, O.D.; Terra, W.D.; Vieira, M.G.C. Chemical Compounds and Biologic Activities: A Review of Cedrela Genus. Molecules 2020, 22, 5401. [Google Scholar] [CrossRef]
- Leo, M.D.; Milella, L.; Braca, A.; Tommasi, N.D. Cedrela and Toona genera: A rich source of bioactive limonoids and triterpenoids. Phytochem. Rev. 2018, 4, 751–783. [Google Scholar] [CrossRef]
- Chatterjee, A.; Chakrabortty, T.; Chandrasekharan, S. Chemical investigation of Cedrela toona. Phytochemistry 1971, 10, 2533–2535. [Google Scholar] [CrossRef]
- Leite, A.C.; Bueno, F.C.; Oliveira, C.G.; Fernandes, J.B.; Vieira, P.C.; Silva, M.F.G.F.; Bueno, O.C.; Pagnocca, F.C.; Hebling, M.J.A.; Bacci, M. Limonoids from Cipadessa fruticosa and Cedrela fissilis and their insecticidal activity. J. Brazil. Chem. Soc. 2005, 16, 1391–1395. [Google Scholar] [CrossRef] [Green Version]
- Green, M.B.; Hedin, P.A. Natural Resistance of Plants to Pests; American Chemical Society: Washington, DC, USA, 1986. [Google Scholar]
- Arnason, J.T.; Philogene, B.J.R.; Donskov, N.; Kubo, I. Limonoids from the Meliaceae and Rutaceae reduce feeding, growth and development of Ostrinia nubilalis. Entomol. Exp. Appl. 1987, 43, 221–226. [Google Scholar] [CrossRef]
- Bueno, F.C.; Godoy, M.P.; Leite, A.C.; Bueno, O.C.; Pagnocca, F.C.; Fernandes, J.B.; Hebling, M.J.A.; Bacci, M., Jr.; Vieira, P.C.; Silva, G.F. Toxicity of Cedrela fissilis to Atta Sexdens rubropilosa (Hymenoptera: Formicidae) and its symbiotic fungus. Sociobiology 2005, 45, 389–399. [Google Scholar]
- Laphookhieo, S.; Maneerat, W.; Koysomboon, S.; Kiattansakul, R.; Chantrapromma, K.; Syers, J.K. A novel limonoid from the seeds of Chisocheton siamensis. Can. J. Chem. 2008, 39, 205–208. [Google Scholar] [CrossRef]
- Connolly, J.D.; Labbe, C.; Rycroft, D.S.; Taylor, D.A.H. Tetranortriterpenoids and related compounds. Part 22. New apotirucailol derivatives and tetranortriterpenoids from the wood and seeds of Chisocheton paniculatus (Meliaceae). Chem. Soc. 1980, 11, 2959–2964. [Google Scholar] [CrossRef]
- Lavie, D.; Levy, E.C.; Jain, M.K. Limonoids of biogenetic interest from Melia azadirachta L. Tetrahedron 1971, 27, 3927–3939. [Google Scholar] [CrossRef]
- Luo, J.; Wang, J.S.; Wang, X.B.; Huang, X.F.; Luo, J.G.; Kong, L.Y. Chukvelutilides A–F, phragmalin limonoids from the stem barks of Chukrasia tabularis var. velutina. Tetrahedron 2009, 65, 3425–3431. [Google Scholar] [CrossRef]
- Fan, C.Q.; Wang, X.N.; Yin, S.; Zhang, C.R.; Wang, F.D.; Yue, J.M. Tabularisins A–D, phragmalin orthoesters with new skeleton isolated from the seeds of Chukrasia tabularis. Tetrahedron 2007, 63, 6741–6747. [Google Scholar] [CrossRef]
- Yin, J.L.; Fang, X.; Liu, E.D.; Yuan, C.M.; Li, S.F.; Zhang, Y.; He, H.P.; Li, S.L.; Di, Y.T.; Hao, X.J. Phragmalin limonoids from the stem barks of Chukrasia tabularis var. velutina. Planta Med. 2014, 80, 1304–1309. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.L.; Liu, H.L.; Guo, Y.W. Phragmalin limonoids from Chukrasia tabularis var. velutina. Planta Med. 2012, 78, 286–290. [Google Scholar] [CrossRef]
- Truong, V.L.; Jeong, W.S. Cellular defensive mechanisms of tea polyphenols: Structure-activity relationship. Int. J. Mol. Sci. 2021, 17, 9109. [Google Scholar] [CrossRef] [PubMed]
- Kilani, M.S.; Morakchi, G.H.; Sifi, K. Azadirachtin-based insecticide: Overview, risk assessments, and future directions. Front. Agron. 2021, 3, 32. [Google Scholar] [CrossRef]
- Hein, D.F.; Hummel, H.E.; Ley, S.V. Structure activity relationships in azadirachtin a derivatives: Feeding activity and degree of efficiency tested on Epilachna varivestis larvae. Meded. Fac. Landbouwkd. En Toegep. Biol. Wet. Univ. Gent 1999, 64, 197–204. [Google Scholar]
- Lai, D.; Jin, X.; Wang, H.; Xu, H. Gene expression profile change and growth inhibition in Drosophila larvae treated with azadirachtin. J. Biotechnol. 2014, 185, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Dawkar, V.V.; Barage, S.H.; Barbole, R.S.; Fatangare, A.; Grimalt, S.; Haldar, S.; Heckel, D.G.; Gupta, V.S.; Thulasiram, H.V.; Svatos, A. Azadirachtin-A from Azadirachta indica impacts multiple biological targets in cotton bollworm Helicoverpa armigera. ACS Omega 2019, 4, 9531–9541. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Kim, S.H.; Montell, C. Avoiding DEET through insect gustatory receptors. Neuron 2010, 67, 555–561. [Google Scholar] [CrossRef] [Green Version]
- Weiss, L.A.; Dahanukar, A.; Kwon, J.Y.; Banerjee, D.; Carlson, J.R. The molecular and cellular basis of bitter taste in Drosophila. Neuron 2011, 69, 258–272. [Google Scholar] [CrossRef] [Green Version]
- Delventhal, R.; Carlson, J.R. Bitter taste receptors confer diverse functions to neurons. eLife 2016, 5, 220. [Google Scholar] [CrossRef] [PubMed]
- Luntz, A.J.M.; Morgan, E.D.; Nisbet, A.J. Azadirachtin, a Natural Product in Insect Control. Compr. Mol. Insect Sci. 2005, 6, 117–135. [Google Scholar]
- Khosravi, R.; Sendi, J.J. Effect of neem pesticide (Achook) on midgut enzymatic activities and selected biological compounds in the hemolymph of lesser mulberry pyralid, Glyphodes pyloalis walker (Lepidoptera: Pyralidae). J. Plant Prot. Res. 2013, 53, 238–247. [Google Scholar] [CrossRef]
- Shannag, H.K.; Capinera, J.L.; Freihat, N.M. Effects of neem-based insecticides on consumption and utilization of food in larvae of Spodoptera eridania (Lepidoptera: Noctuidae). J. Insect Sci. 2015, 15, 152. [Google Scholar] [CrossRef] [Green Version]
- Yan, Y.; Gu, H.; Xu, H.; Zhang, Z. Induction of aversive taste memory by azadirachtin and its effects on dopaminergic neurons of Drosophila. J. South China Agric. Univ. 2017, 38, 12–18. [Google Scholar]
- Qiao, J.; Zou, X.; Lai, D.; Yan, Y.; Wang, Q.; Li, W.; Deng, S.; Xu, H.; Gu, H. Azadirachtin blocks the calcium channel and modulates the cholinergic miniature synaptic current in the central nervous system of Drosophila. Pest Manag. Sci. 2014, 70, 1041–1047. [Google Scholar] [CrossRef] [PubMed]
- Boulahbel, B.; Aribi, N.; Kilani, M.S.; Soltani, N. Insecticidal activity of azadirachtin on Drosophila melanogaster and recovery of normal status by exogenous 20-hydroxyecdysone. Afr. Entomol. 2015, 23, 224–233. [Google Scholar] [CrossRef]
- Oulhaci, M.C.; Denis, B.; Kilani, M.S.; Sandoz, J.C.; Kaiser, L.; Joly, D.; Aribi, N. Azadirachtin effects on mating success, gametic abnormalities and progeny survival in Drosophila melanogaster (Diptera). Pest Manag. Sci. 2018, 74, 174–180. [Google Scholar] [CrossRef] [PubMed]
- Vivekananthan, T.; Selvisabhanayakam, S.N. Histopathological observations on testes of adult blister beetle, Mylabris indica (thunberg) (Coleoptera: Meloidae) treated with neem. J. Entomol. Res. 2014, 38, 45–52. [Google Scholar]
- Ghazawi, N.A.; El-Shranoubi, E.D.; El-Shazly, M.M.; Abdel Rahman, K.M. Effects of azadirachtin on mortality rate and reproductive system of the grasshopper Heteracris littoralis Ramb (Orthoptera: Acrididae). J. Orthoptera Res. 2007, 16, 57–65. [Google Scholar] [CrossRef] [Green Version]
- Aribi, N.; Oulhaci, M.C.; Kilani, M.S.; Sandoz, J.C.; Kaiser, L.; Denis, B.; Joly, D. Azadirachtin impact on mate choice, female sexual receptivity and male activity in Drosophila melanogaster (Diptera: Drosophilidae). Pestic. Biochem. Physiol. 2017, 143, 95–101. [Google Scholar] [CrossRef]
- Liu, P.F.; Wang, W.; Ling, X.; Lu, Q.; Zhang, J.; He, R.; Chen, H. Regulation hormone-related genes in Ericerus pela (Hemiptera: Coccidae) for dimorphic metamorphosis. J. Insect Sci. 2019, 19, 16–25. [Google Scholar]
- Zhao, J.; Zhou, Y.; Li, X.; Cai, W.; Hua, H. Silencing of juvenile hormone epoxide hydrolase gene (Nljheh) enhances short wing formation in a macropterous strain of the brown planthopper, Nilaparvata lugens. J. Insect Physiol. 2017, 102, 18–26. [Google Scholar] [CrossRef]
- Shu, B.; Wang, W.; Hu, Q.; Huang, J.; Hu, M.; Zhong, G. A comprehensive study on apoptosis induction by azadirachtin in Spodoptera frugiperda cultured cell line Sf9. Arch. Insect Biochem. Physiol. 2015, 89, 153–168. [Google Scholar] [CrossRef]
- Roberston, S.L.; Ni, W.; Dhadialla, T.S.; Nisbet, A.J.; McCusker, C.; Ley, S.V.; Mordue, W. Identification of a putative azadirachtin-binding complex from Drosophila Kc167 cells. Arch. Insect Biochem. Physiol. 2007, 64, 200–208. [Google Scholar]



























| Family | Genus | Species |
|---|---|---|
| Meliaceae | Aglaia | Aglaia elaeagnoidea (A. Juss.) Benth. |
| Aphanamixis | Aphanamixis grandifolia Bl. | |
| Aphanamixis polystachya (Wall.) R. Parker | ||
| Azadirachta | Azadirachta excelsa (Jack) Jacobs | |
| Azadirachta indica A. Juss | ||
| Azadirachta siamensis Val. | ||
| Cabralea | Cabralea canjerana (Vell.) Mart | |
| Carapa | Carapa guianensis Aubl. | |
| Cedrela | Cedrela dugessi (S. Watson) | |
| Cedrela fissilis Vell. | ||
| Cedrela odorata L. | ||
| Cedrela salvadorensis L. | ||
| Cedrela sinensis Juss. | ||
| Cedrela toona Roxb. Ex Rottler et Willd. | ||
| Chisocheton ceramicus (Miq.) C.DC. | ||
| Chisocheton | Chisocheton paniculatus (Roxb.) Hiern | |
| Chisocheton siamensis Craib | ||
| Chisocheton erythrocarpus Hiern | ||
| Chukrasia | Chukrasia tabularis A. Juss. |
| Compound | Plant Source | Insect | Activity | Ref. |
|---|---|---|---|---|
| Aphanamixoid A | Aphanamixis polystachya | Helicoverpa armigera | AFD *, EC50 = 0.015 μmol/cm2 (24 h) | [31] |
| Aphanamixoid C | Aphanamixis polystachya | Helicoverpa armigera | AFD, EC50 = 0.017 μmol/cm2 (24 h) | [18] |
| Aphanamixoid F | Aphanamixis polystachya | Helicoverpa armigera | AFD, EC50 = 0.008 μmol/cm2 (24 h) | |
| Aphanamixoid G | Aphanamixis polystachya | Helicoverpa armigera | AFD, EC50 = 0.012 μmol/cm2 (24 h) | |
| Prieurianin | Aphanamixis polystachya | Helicoverpa armigera | AFD, EC50 = 18.8 μg/mL (7 d) | [34] |
| Epoxyprieurianin | Aphanamixis polystachya | Helicoverpa armigera | AFD, EC50 = 3.2 μg/mL (7 d) | [34] |
| Azadirachtin | Azadirachta indica Azadirachta excelsa | Epilachna varivesti | AFD, EC50 = 13 μg/mL (24 h) | [9,10,11,12,13,15,16,33,35,36] |
| Epilachna paenulata | AFD, LD50 = 1.24 μg/cm2 (96 h) | |||
| Helicoverpa armigera | AFD, EC50 = 0.26 μg/mL (6 h) | |||
| Locusta migratoria | AFD, MIC = 25 μg/mL | |||
| Locusta migratoria | AFD, ED50 = 3 μg/mL (48 h) | |||
| Ostrinia nubilalis | AFD, PC50 = 3.5 μg/mL (48 h) | |||
| Peridroma saucia | AFD, EC50 = 0.26 μg/mL (72 h) | |||
| Pieris rapae | AFD, AR = 100(1000 μg/mL) (24 h) | |||
| Phyllotreta striolata | AFD, MIC = 10 μg/mL | |||
| Reticulitermes speratus | AFD, PC95 = 65.293 (25 d) | |||
| Rhodnius prolixus | AFD, ED50 = 25.0 μg/mL (25 d) | |||
| Schistocerca gregaria | AFD, ED50 = 0.001 μg/mL | |||
| Spodoptera littoralis | AFD, AI = 98.8 ± 1.11 (1 μg/mL) (8 h) | |||
| Azadirone | Azadirachta indica | Leptinotarsa decemlineata | AI = 11.6–26.9(100–500 μg/mL) (20 h) | [37] |
| 7-deacetylgedunin | Azadirachta indica | Reticulitermes speratus | AFD, PC95 = 113.7 μg/disc (30 d) | [23] |
| Cedrela fissilis | ||||
| Cedrela sinensis | ||||
| Chisocheton compound F | Chisocheton paniculatus | Pieris brassicae | Antifeedant activity | [38] |
| Salannin | Azadirachta indica | Reticulitermes speratus | AFD, PC95 = 203.3 μg/disc (30 d) | [23] |
| Spodoptera litura | FRA50 # = 2.8 µg/cm2 (7 d) | [22] | ||
| Gedunin | Azadirachta indica | Reticulitermes speratus | AFD, PC95 = 218.4 μg/disc (30 d) | [23] |
| Cedrela dugessi | ||||
| Cedrela fissilis | ||||
| Cedrela sinensis | ||||
| Cedrela salvadorensis | ||||
| Cabralea eichleriana | ||||
| Carapa guianensis | ||||
| Chisocheton paniculatus | ||||
| 17β-hydroxy- azadiradione | Azadirachta indica | Reticulitermes speratus | AFD, PC95 = 235.6 μg/disc (30 d) | [23] |
| Carapa guianensis | ||||
| nimbandiol | Azadirachta indica | Reticulitermes speratus | AFD, PC95 = 254.4 μg/disc (30 d) | [23] |
| 3-deacetylsalannin | Azadirachta indica | Reticulitermes speratus | AFD, PC95 = 1373.1 μg/disc (30 d) | [23] |
| 6-deacetylnimbin | Azadirachta indica | Reticulitermes speratus | AFD, PC95 = 1581.2 μg/disc (30 d) | [23] |
| Azadirachtin B | Azadirachta indica | Locusta migratoria | AFD, EC50 = 12 μg/mL | [39] |
| Azadirachta excelsa | Epilachna varivesti | AFD, EC50 = 30 μg/mL | [9] | |
| Nimbolide | Azadirachta indica | Epilachna varivesti | AFD, EC50 = 90 μg/mL | [9] |
| Azadirachta excelsa | ||||
| Azadirachtin L | Azadirachta indica | Epilachna varivesti | AFD, EC50 = 6 μg/mL | [9] |
| Azadirachta excelsa | ||||
| 1-tigloyl-3-acetyl- azadirachtol | Azadirachta excelsa | Epilachna varivesti | AFD, EC50 = 6 μg/mL | [9] |
| Azadirachta siamensis | ||||
| Salannol | Azadirachta indica | Spodoptera litura | FRA50 = 2.3 µg/cm2 (7 d) | [22] |
| Azadiraindin A | Azadirachta indica | Plutella xylostella | AR = 28% at 2000 μg/mL (48 h) | [24] |
| Epoxyazadiradione | Azadirachta indica | Plutella xylostella | AR = 37.2% at 2000 μg/mL (48 h) | [24] |
| Desfuranoazadiradione | Azadirachta indica | Plutella xylostella | AR = 39.6% at 2000 μg/mL (48 h) | [24] |
| Azadiradione | Azadirachta indica | Plutella xylostella | AR = 90.6% at 2000 μg/mL (48 h) | [24] |
| Chisocheton siamensis | ||||
| 7-deacetoxy-7-oxo- gedunin | Cedrela fissilis | Spodoptera littoralis | AFD at 1000 μg/mL (3–10 h) | [20] |
| Cabralea eichleriana | ||||
| Carapa guianensis | ||||
| Methyl angolensate | Cedrela fissilis | Spodoptera litura | AFD, PFI = 65.3 at 1 μg/cm2 (24 h) | [40] |
| Cabralea canjerana | ||||
| 11β-acetoxyobacunyl acetate | Cedrela odorata | Spodoptera littoralis | AFD at 1000 μg/mL | [29] |
| 11β,19-diacetoxy-l-de- acetyl-l-epidihy- dronomilin | Cedrela odorata | Spodoptera littoralis | AFD at 1000 μg/mL | [29] |
| 11β-acetoxyobacunol | Cedrela odorata | Spodoptera littoralis | AFD at 1000 μg/mL | [29] |
| Odoralide | Cedrela odorata | Spodoptera littoralis | AFD at 1000 μg/mL | [29] |
| Swietenolide | Cedrela odorata | Spodoptera littoralis | AFD at 1000 μg/mL | [29] |
| 8β,14α-dihydro- swietenolide | Cedrela odorata | Spodoptera littoralis | AFD at 500 μg/mL | [29] |
| 3β,6-dihydroxydihydro -carapin | Cedrela odorata | Spodoptera littoralis | AFD at 1000 μg/mL | [29] |
| 3β-hydroxydihydro- carapin | Cedrela odorata | Spodoptera littoralis | AFD at 1000 μg/mL | [29] |
| Xyloccensin K | Cedrela odorata | Spodoptera littoralis | AFD at 1000 μg/mL | [29] |
| Cedrodorin | Cedrela odorata | Spodoptera littoralis | AFD at 1000 μg/mL | [29] |
| Ocotillone | Cabralea canjerana | Spodoptera litura | AFD, PFI = 44.5 at 1 μg/cm2 (24) | [41] |
| Tabulalin | Chukrasia tabularis | Spodoptera littoralis | AFD at 500 μg/mL (2–12 h) | [42] |
| Tabulalide D | Chukrasia tabularis | Spodoptera littoralis | AFD at 500 μg/mL (2–12 h) | [42] |
| TabulalideA | Chukrasia tabularis | Spodoptera littoralis | AFD at 1000 μg/mL (2–12 h) | [42] |
| Tabulalide B | Chukrasia tabularis | Spodoptera littoralis | AFD at 1000 μg/mL (2–12 h) | [42] |
| Tabulalide E | Chukrasia tabularis | Spodoptera littoralis | AFD at 1000 μg/mL (2–12 h) | [42] |
| Compound | Plant Source | Insect | Activity | Ref. |
|---|---|---|---|---|
| Aphapolynin D | Aphanamixis polystachya | Diabrotica balteata | MS: 66 5–9 d) | [19] |
| Aphanalide F | Aphanamixis polystachya | Diabrotica balteata | MS: 66 (5–9 d) | |
| Aphapolynin F | Aphanamixis polystachya | Diabrotica balteata | MS: 33 (5–9 d) | |
| Dregenana-1 | Aphanamixis polystachya | Diabrotica balteata | MS: 33 (5–9 d) | |
| Aphanalide E | Aphanamixis polystachya | Diabrotica balteata | MS: 33 (5–9 d) | |
| Aphanalide G | Aphanamixis polystachya | Diabrotica balteata | MS: 33 (5–9 d) | |
| Aphanalide H | Aphanamixis polystachya | Diabrotica balteata | MS: 99 (5–9 d) | |
| Aphapolynin C | Aphanamixis polystachya | Diabrotica balteata | MS: 99 (5–9 d) | |
| Aphanamixis polystachya | Caenorhabditis elegans | MS: 66 (5–9 d) | ||
| Aphapolynin A | Aphanamixis polystachya | Plutella xylostella | MS: 66 (5–9 d) | |
| Zaphaprinin I | Aphanamixis polystachya | Plutella xylostella | MS: 99 (5–9 d) | |
| Zaphaprinin R | Aphanamixis polystachya | Plutella xylostella | MS: 99 (5–9 d) | |
| Azadirachtin | Azadirachta indica Azadirachta excelsa | Spodoptera littoralis | LC50 = 0.32 μg/mL (12 d) | [9,10,11,12,13,15,16,33,35,36] |
| Anopheles gambiae | LD50 = 57.1 μg/mL (24 h) | |||
| Plutella xylostella | LD50 = 7.04–0.87 (24–96 h) | |||
| 7-deacetylgedunin | Azadirachta indica | Atta sexdens rubropilosa | S50 = 9 d at 100 μg/mL | [28] |
| Cedrela fissilis | ||||
| Cedrela sinensis | ||||
| Gedunin | Azadirachta indica | Spodoptera frugiperda | LC50 = 39 μg/mL (7 d) | [43] |
| Cedrela dugessi | ||||
| Cedrela fissilis | ||||
| Cedrela sinensis | ||||
| Cedrela salvadorensis | ||||
| Cabralea eichleriana | ||||
| Carapa guianensis | ||||
| Chisocheton paniculatus | ||||
| Nimocinol | Azadirachta indica | Aedes aegypti | LC50 = 21 μg/mL (24 h) | [25] |
| 6α-O-acetyl-7-deacetyl- nimocinol | Azadirachta indica | Aedes aegypti | LC50 = 83 μg/mL (24 h) | [25] |
| 22,23-dihydronimocinol | Azadirachta indica | Anopheles stephensi | LC50 = 60 μg/mL (24 h) | [26] |
| desfurano-6α-hydroxy- azadiradione | Azadirachta indica | Anopheles stephensi | LC50 = 43 μg/mL (24 h) | [26] |
| Meliatetraolenone | Azadirachta indica | Anopheles stephensi | LC50 = 16 μg/mL (24 h) | [26] |
| Odoratone | Azadirachta indica | Anopheles stephensi | LC50 = 154 μg/mL (24 h) | [44] |
| Azadirachtin O | Azadirachta excelsa | Plutella xylostella | LD50 = 3.92 μg/g (24 h) | [33] |
| Azadirachtin P | Azadirachta excelsa | Plutella xylostella | LD50 = 2.19 μg/g (24 h) | [33] |
| Azadirachtin Q | Azadirachta excelsa | Plutella xylostella | LD50 = 1.10 μg/g (96 h) | [33] |
| Azadirachtin B | Azadirachta excelsa | Plutella xylostella | LD50 = 1.06 μg/g (96 h) | [33] |
| Azadirachtin L | Azadirachta excelsa | Plutella xylostella | LD50 = 1.92 μg/g (96 h) | [33] |
| Azadirachtin M | Azadirachta excelsa | Plutella xylostella | LD50 = 1.30 μg/g (96 h) | [33] |
| 11α-azadirachtin H | Azadirachta excelsa | Plutella xylostella | LD50 = 0.75 μg/g (96 h) | [33] |
| Azadirachtol | Azadirachta excelsa | Plutella xylostella | LD50 = 1.78 μg/g (96 h) | [33] |
| 23-O-methylnimocinolide | Azadirachta indica | Aedes aegypti | LC50 = 53 μg/mL (24 h) | [45] |
| 7-O-deacetyl-23-O-methyl- 7α-O-senecioyl-nimocinolide | Azadirachta indica | Aedes aegypti | LC50 = 14 μg/mL (24 h) | [45] |
| 6α-acetoxygedunin | Aglaia elaeagnoidea | Atta sexdens rubropilosa | S50 = 8 d at 100 μg/mL | [28] |
| Carapa guianensis | ||||
| Cedrela fissilis | ||||
| Chisochetonpaniculatus | ||||
| 14-deoxy-Δ14,15-xyloccensin K | Chisocheton erythrocarpus Hiern | Aedes aegypti, Aedes albopictus Culex Quinquefasciatus | LC50 = 10.2 μg/mL (24 h) LC50 = 12.16 μg/mL (24 h) LC50 = 16.82 μg/mL (24 h) | [46] |
| 14-deoxyxyloccensin K | Chisocheton erythrocarpus Hiern Chisocheton ceramicus | Aedes aegypti, Aedes albopictus Culex Quinquefasciatus | LC50 = 3.19 μg/mL (24 h) LC50 = 3.01 μg/mL (24 h) LC50 = 3.64 μg/mL (24 h) | [46] |
| Photogedunin epimer mixture | Cedrela dugessi | Spodoptera frugiperda | LC50 = 10 μg/mL (7 d) | [47] |
| Photoacetic acid acetate mixture | Cedrela dugessi | Spodoptera frugiperda | LC50 = 8 μg/mL (7 d) | [47] |
| 7-deacetoxy-7-oxo-gedunin | Cedrela fissilis | Atta sexdens rubropilosa | S50 = 11 d at 100 μg/mL | [28] |
| Cabralea eichleriana | ||||
| Carapa guianensis | ||||
| Photogedunin | Cedrela fissilis | Atta sexdens rubropilosa | S50 = 9 d at 100 μg/mL | [28] |
| 1,2-dihydro-3β-hydroxy-7- deacetoxy-7-oxogedunin | Cedrela fissilis | Atta sexdens rubropilosa | S50 = 9 d at 100 μg/mL | |
| Cipadesin B | Cedrela fissilis | Atta sexdens rubropilosa | S50 = 9 d at 100 μg/mL | [28] |
| Swietemahonolide | Cedrela fissilis | Atta sexdens rubropilosa | S50 = 8 d at 100 μg/mL | |
| 3β-acetoxycarapin | Cedrela fissilis | Atta sexdens rubropilosa | S50 = 8 d at 100 μg/mL | |
| Oleanolic acid | Cedrela fissilis | Atta sexdens rubropilosa | S50 = 6 d at 100 μg/mL | |
| Oleanonic acid | Cedrela fissilis | Atta sexdens rubropilosa | S50 = 8 d at 100 μg/mL | |
| Methyl angolensate | Cedrela fissilis | Spodoptera frugiperda | MR: 40% at 50 mg/kg (7 d) | [48] |
| Cabralea canjerana | ||||
| Photogeduninepimeric acetate mixture | Cedrela salvadorensis | Spodoptera frugiperda | SR 50% at 10 μg/mL (24 h) | [49] |
| Photogeduninepimeric mixture | Cedrela salvadorensis | Spodoptera frugiperda | SR 17% at 10 μg/mL (24 h) | |
| Ocotillone | Cabralea canjerana | Spodoptera frugiperda | MR: 40% at 50 mg/kg (7 d) | [48] |
| β-photogedunin | Carapa guianensis | Spodoptera frugiperda | LM 53.3% at 50 μg/mL (7 d) | [48] |
| PM 20.0% at 50 μg/mL (7 d) |
| Compound | Plant Source | Insect | Activity | Ref. |
|---|---|---|---|---|
| Azadirachtin | Azadirachta indica Azadirachta excelsa | Helicoverpa armigera | IGR, EC50 = 0.26 μg/mL (7 d) | [9,10,11,12,13,15,16,33,35,36] |
| Rhodnius prolixus | IGR, ED50 = 0.40 μg/mL (7 d) | |||
| Heliothis zea Heliothis virescens | IGR, ED50 = 0.70 μg/mL (10 d) | |||
| Spodoptera frugiperda, Pectinophora gossypiella | IGR, ED50 = 0.40 μg/mL (10 d) | |||
| Spodoptera litura | IGR, EC50 = 0.21 μg/mL (7 d) | |||
| Spodoptera littoralis | EC50 = 0.11 μg/mL (6 d) | |||
| Nimocinolide | Azadirachta indica | Musca domestica | FI at 100 μg/mL | [27] |
| Isonimocinolide | Azadirachta indica | Musca domestica | FI at 100 μg/mL | [27] |
| Aedes uegypti | mutagenic properties | |||
| 7-deacetylazadiradione | Azadirachta indica | Heliothis virescens | IGR, EC50 = 1600 μg/mL | [30] |
| Chisocheton paniculatus | ||||
| Salannin | Azadirachta indica | Helicoverpa armigera | IGR EC50 = 86.5 μg/mL (7 d) | [22] |
| Azadirachta indica | Spodoptera litura | IGR EC50 = 87.7 μg/mL (7 d) | ||
| 3-O-acetyl salannol | Azadirachta indica | Helicoverpa armigera | IGR EC50 = 64.2 μg/mL (7 d) | [22] |
| Azadirachta indica | Spodoptera litura | IGR EC50 = 65.6 μg/mL; RF50 at 2.0 µg/cm2 (7 d) | ||
| Salannol | Azadirachta indica | Helicoverpa armigera | IGR, EC50 was 79.7 μg/mL (7 d) | [22] |
| Azadirachta indica | Spodoptera litura | IGR, EC50 = 77.4 μg/mL (7 d) | ||
| 6β-hydroxygedunin | Azadirachta indica | Helicoverpa armigera | IGR EC50 = 24.2 μg/mL (7 d) | [35] |
| Azadirachta indica | Spodoptera litura | IGR EC50= 391.4 μg/mL (7 d) | ||
| Nimbinene | Azadirachta indica | Helicoverpa armigera | IGR EC50 was 21.5 μg/mL (7 d) | [35] |
| Azadirachta indica | Spodoptera litura | IGR EC50 = 404.5 μg/mL (7 d) | ||
| Azadiradione | Azadirachta indica | Heliothis virescens | IGR, EC50= 560 μg/mL | [30] |
| Chisocheton siamensis | ||||
| Azadirachta indica | Heliothis virescens | IGR, EC50 = 560 μg/mL | [30] | |
| Chisocheton siamensis | ||||
| 6α-acetoxygedunin | Aglaia elaeagnoidea | Ostrinia nubilalis | reduced growth at 50 μg/mL | [17] |
| Carapa guianensis | ||||
| Cedrela fissilis | ||||
| Chisocheton paniculatus | ||||
| Cedrelanolide I | Cedrela salvadorensis | Ostrinia nubilalis | reduced weight at 50 μg/mL | [51] |
| Cedrelone | Cedrela odorata | Peridroma saucia | IGR, EC50 = 53.1 μg/mL (9 d) | [29] |
| Cedrela toona | ||||
| Cabraleadiol | Cabralea canjerana | Spodoptera frugiperda | LPE, 1.2 d | [48] |
| 3β-deacetylfissinolide | Cabralea canjerana | Spodoptera frugiperda | LPE, 1.2 d | [48] |
| β-photogedunin | Carapa guianensis | Spodoptera frugiperda | PWI at 50 mg/kg (7 d) | [48] |
| Cedrelanolide I | Cedrela salvadorensis | Ostrinia nubilalis | reduced weight at 50 μg/mL | [51] |
| Meliantriol | Azadirachta indica | Locusts | chewing prevention | [52] |
| 7-deacetyl-17β-hydroxy-azadiradione | Azadirachta indica | Heliothis virescens | IGR, EC50 = 240 μg/mL | [30] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, M.; Yang, S.; Huang, J.; Zhou, L. Insecticidal Triterpenes in Meliaceae: Plant Species, Molecules and Activities: Part Ⅰ (Aphanamixis-Chukrasia). Int. J. Mol. Sci. 2021, 22, 13262. https://doi.org/10.3390/ijms222413262
Lin M, Yang S, Huang J, Zhou L. Insecticidal Triterpenes in Meliaceae: Plant Species, Molecules and Activities: Part Ⅰ (Aphanamixis-Chukrasia). International Journal of Molecular Sciences. 2021; 22(24):13262. https://doi.org/10.3390/ijms222413262
Chicago/Turabian StyleLin, Meihong, Sifan Yang, Jiguang Huang, and Lijuan Zhou. 2021. "Insecticidal Triterpenes in Meliaceae: Plant Species, Molecules and Activities: Part Ⅰ (Aphanamixis-Chukrasia)" International Journal of Molecular Sciences 22, no. 24: 13262. https://doi.org/10.3390/ijms222413262
APA StyleLin, M., Yang, S., Huang, J., & Zhou, L. (2021). Insecticidal Triterpenes in Meliaceae: Plant Species, Molecules and Activities: Part Ⅰ (Aphanamixis-Chukrasia). International Journal of Molecular Sciences, 22(24), 13262. https://doi.org/10.3390/ijms222413262