New Frontiers in Peripheral Nerve Regeneration: Concerns and Remedies
Abstract
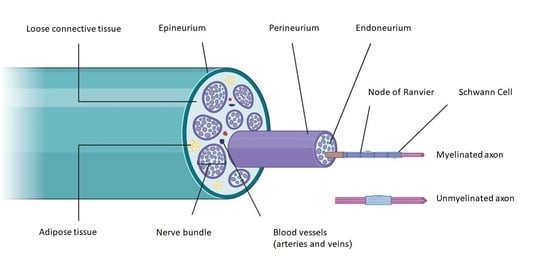
:1. Introduction
2. Molecular Basis for Nerve Regeneration
2.1. Cell Adhesion Molecules and Extracellular Matrix Proteins in Peripheral Nerve Regeneration
2.2. Guidance Molecules in Nerve Regeneration
2.3. Neurotrophins and Cytokines in Peripheral Nerve Regeneration
2.4. The Role of Exosomes in Regeneration of Peripheral Nerves
2.5. The Role of miRNAs in the Regeneration of Peripheral Nerves
2.6. The Role of Exosomes Derived from Mesenchymal Stem Cells (MSCs) in Peripheral Nerve Regeneration
3. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| 3′UTR | 3′ untranslated Region |
| AAV | adeno-associated vectors |
| ATF-3 | activating transcription factor 3 |
| bFGF | basic fibroblast growth factor |
| CMAK2 | calcium/calmodulin-dependent kinase 2 |
| CNTF | ciliary neurotrophic factor |
| CREB | cAMP responsive element binding protein |
| DCC | deleted in Colorectal Cancer |
| DRG | dorsal root ganglia |
| DSCAM | DS cell adhesion molecule |
| ECM | extracellular matrix |
| FAK | focal adhesion kinase |
| FPRL1 | formyl peptide receptor 1 |
| GAP43 protein | growth-associated protein-43 |
| GDNF | glial cell line-derived neurotrophic factor |
| GMF-β | glia Maturation factor-β |
| GMSC | gingiva mesenchymal stem cells |
| GPI | glycosylphosphatidylinositol |
| hAMSCs | amniotic MSCs |
| HGF | hepatocyte growth factor |
| HIF-1α | hypoxia inducible factor 1 subunit alpha |
| ILK | integrin-linked kinase |
| JNK | c-jun N-terminal kinase |
| LASS2 | longevity assurance homolog 2 |
| LIF | leukemia inhibitory factor |
| MAPK | mitogen-activated protein kinase |
| MMPs | matrix metalloproteinases |
| MSCs | mesenchymal stem cells |
| NCAM | neural cell adhesion molecule |
| NGF | nerve growth factor |
| NRP-1 | neuropilin-1 receptor |
| NT-3 | neurotrophin 3 |
| OSM | oncostatin M |
| PAs | plasminogen activators |
| PKA | protein kinase A |
| PKC | protein kinase C |
| PNS | peripheral nervous system |
| PTEN | phosphatase and tensin homolog |
| RAG | regeneration associated gene |
| STAT3 | signal transducer and activator of transcription 3 |
| suPAR | soluble urokinase receptor |
| TGF-β | transforming growth factor-β |
| tPA | tissue plasminogen activator |
| TSG101 | tumor susceptibility gene 101 |
| Unc5A–D | uncoordinated receptor A–D |
| uPAR | urokinase receptor |
| VEGF-A | vascular endothelial growth factor A |
References
- Berdan, R.C.; Easaw, J.C.; Wang, R. Alterations in membrane potential after axotomy at different distances from the soma of an identified neuron and the effect of depolarization on neurite outgrowth and calcium channel expression. J. Neurophysiol. 1993, 69, 151–164. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Murashov, A. Molecular mechanisms of peripheral nerve regeneration: Emerging roles of microRNAs. Front. Physiol. 2013, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hanz, S.; Perlson, E.; Willis, D.; Zheng, J.Q.; Massarwa, R.; Huerta, J.J.; Koltzenburg, M.; Kohler, M.; van Minnen, J.; Twiss, J.L.; et al. Axoplasmic importins enable retrograde injury signaling in lesioned nerve. Neuron 2003, 40, 1095–1104. [Google Scholar] [CrossRef] [Green Version]
- Seijffers, R.; Allchorne, A.J.; Woolf, C.J. The transcription factor ATF-3 promotes neurite outgrowth. Mol. Cell. Neurosci. 2006, 32, 143–154. [Google Scholar] [CrossRef]
- Schmitt, A.B.; Breuer, S.; Liman, J.; Buss, A.; Schlangen, C.; Pech, K.; Hol, E.M.; Brook, G.A.; Noth, J.; Schwaiger, F.W. Identification of regeneration-associated genes after central and peripheral nerve injury in the adult rat. BMC Neurosci. 2003, 4, 8. [Google Scholar] [CrossRef] [Green Version]
- Faroni, A.; Mobasseri, S.A.; Kingham, P.J.; Reid, A.J. Peripheral nerve regeneration: Experimental strategies and future perspectives. Adv. Drug Deliv. Rev. 2015, 82-83, 160–167. [Google Scholar] [CrossRef]
- Funakoshi, H.; Frisen, J.; Barbany, G.; Timmusk, T.; Zachrisson, O.; Verge, V.M.; Persson, H. Differential expression of mRNAs for neurotrophins and their receptors after axotomy of the sciatic nerve. J. Cell Biol. 1993, 123, 455–465. [Google Scholar] [CrossRef] [PubMed]
- Jankowski, M.P.; McIlwrath, S.L.; Jing, X.; Cornuet, P.K.; Salerno, K.M.; Koerber, H.R.; Albers, K.M. Sox11 transcription factor modulates peripheral nerve regeneration in adult mice. Brain Res. 2009, 1256, 43–54. [Google Scholar] [CrossRef] [Green Version]
- Uchida, A.; Tashiro, T.; Komiya, Y.; Yorifuji, H.; Kishimoto, T.; Hisanaga, S. Morphological and biochemical changes of neurofilaments in aged rat sciatic nerve axons. J. Neurochem. 2004, 88, 735–745. [Google Scholar] [CrossRef]
- Navarro, X.; Vivo, M.; Valero-Cabre, A. Neural plasticity after peripheral nerve injury and regeneration. Prog. Neurobiol. 2007, 82, 163–201. [Google Scholar] [CrossRef]
- Mei, L.; Xiong, W.C. Neuregulin 1 in neural development, synaptic plasticity and schizophrenia. Nat. Rev. Neurosci. 2008, 9, 437–452. [Google Scholar] [CrossRef] [PubMed]
- Bolin, L.M.; Shooter, E.M. Neurons regulate Schwann cell genes by diffusible molecules. J. Cell Biol. 1993, 123, 237–243. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gomez-Sanchez, J.A.; Pilch, K.S.; van der Lans, M.; Fazal, S.V.; Benito, C.; Wagstaff, L.J.; Mirsky, R.; Jessen, K.R. After Nerve Injury, Lineage Tracing Shows That Myelin and Remak Schwann Cells Elongate Extensively and Branch to Form Repair Schwann Cells, Which Shorten Radically on Remyelination. J. Neurosci. 2017, 37, 9086–9099. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.L.; Yim, A.K.Y.; Kim, K.W.; Avey, D.; Czepielewski, R.S.; Colonna, M.; Milbrandt, J.; Randolph, G.J. Peripheral nerve resident macrophages share tissue-specific programming and features of activated microglia. Nat. Commun. 2020, 11, 2552. [Google Scholar] [CrossRef] [PubMed]
- Cattin, A.L.; Burden, J.J.; Van Emmenis, L.; Mackenzie, F.E.; Hoving, J.J.; Garcia Calavia, N.; Guo, Y.; McLaughlin, M.; Rosenberg, L.H.; Quereda, V.; et al. Macrophage-Induced Blood Vessels Guide Schwann Cell-Mediated Regeneration of Peripheral Nerves. Cell 2015, 162, 1127–1139. [Google Scholar] [CrossRef] [Green Version]
- Liu, P.; Peng, J.; Han, G.H.; Ding, X.; Wei, S.; Gao, G.; Huang, K.; Chang, F.; Wang, Y. Role of macrophages in peripheral nerve injury and repair. Neural Regen. Res. 2019, 14, 1335–1342. [Google Scholar] [CrossRef] [PubMed]
- Barouch, R.; Appel, E.; Kazimirsky, G.; Brodie, C. Macrophages express neurotrophins and neurotrophin receptors. Regulation of nitric oxide production by NT-3. J. Neuroimmunol. 2001, 112, 72–77. [Google Scholar] [CrossRef]
- Kirsch, M.; Terheggen, U.; Hofmann, H.D. Ciliary neurotrophic factor is an early lesion-induced retrograde signal for axotomized facial motoneurons. Mol. Cell. Neurosci. 2003, 24, 130–138. [Google Scholar] [CrossRef]
- Leibinger, M.; Andreadaki, A.; Diekmann, H.; Fischer, D. Neuronal STAT3 activation is essential for CNTF- and inflammatory stimulation-induced CNS axon regeneration. Cell Death Dis. 2013, 4, e805. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Verrilli, M.A.; Court, F. Transfer of Vesicles From Schwann Cells to Axons: A Novel Mechanism of Communication in the Peripheral Nervous System. Front. Physiol. 2012, 3. [Google Scholar] [CrossRef] [Green Version]
- Chernousov, M.A.; Yu, W.M.; Chen, Z.L.; Carey, D.J.; Strickland, S. Regulation of Schwann cell function by the extracellular matrix. Glia 2008, 56, 1498–1507. [Google Scholar] [CrossRef] [PubMed]
- Kaur, B.; Khwaja, F.W.; Severson, E.A.; Matheny, S.L.; Brat, D.J.; Van Meir, E.G. Hypoxia and the hypoxia-inducible-factor pathway in glioma growth and angiogenesis. Neuro-Oncology 2005, 7, 134–153. [Google Scholar] [CrossRef] [Green Version]
- Tang, B.L. Inhibitors of neuronal regeneration: Mediators and signaling mechanisms. Neurochem. Int. 2003, 42, 189–203. [Google Scholar] [CrossRef]
- Zheng, J.Q.; Kelly, T.K.; Chang, B.; Ryazantsev, S.; Rajasekaran, A.K.; Martin, K.C.; Twiss, J.L. A functional role for intra-axonal protein synthesis during axonal regeneration from adult sensory neurons. J. Neurosci. 2001, 21, 9291–9303. [Google Scholar] [CrossRef]
- Letourneau, P.C.; Shattuck, T.A. Distribution and possible interactions of actin-associated proteins and cell adhesion molecules of nerve growth cones. Development 1989, 105, 505–519. [Google Scholar] [CrossRef] [PubMed]
- Klimovich, P.S.; Semina, E.V.; Karagyaur, M.N.; Rysenkova, K.D.; Sysoeva, V.Y.; Mironov, N.A.; Sagaradze, G.D.; Az’muko, A.A.; Popov, V.S.; Rubina, K.A.; et al. Urokinase receptor regulates nerve regeneration through its interaction with alpha5beta1-integrin. Biomed. Pharmacother. 2020, 125, 110008. [Google Scholar] [CrossRef] [PubMed]
- Allodi, I.; Udina, E.; Navarro, X. Specificity of peripheral nerve regeneration: Interactions at the axon level. Prog. Neurobiol. 2012, 98, 16–37. [Google Scholar] [CrossRef] [PubMed]
- Lemons, M.L.; Abanto, M.L.; Dambrouskas, N.; Clements, C.C.; Deloughery, Z.; Garozzo, J.; Condic, M.L. Integrins and cAMP mediate netrin-induced growth cone collapse. Brain Res. 2013, 1537, 46–58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dun, X.-P.; Parkinson, D. Classic axon guidance molecules control correct nerve bridge tissue formation and precise axon regeneration. Neural Regen. Res. 2020, 15, 6–9. [Google Scholar] [CrossRef] [PubMed]
- Dun, X.P.; Parkinson, D.B. Role of Netrin-1 Signaling in Nerve Regeneration. Int. J. Mol. Sci. 2017, 18, 491. [Google Scholar] [CrossRef] [Green Version]
- Parrinello, S.; Napoli, I.; Ribeiro, S.; Wingfield Digby, P.; Fedorova, M.; Parkinson, D.B.; Doddrell, R.D.; Nakayama, M.; Adams, R.H.; Lloyd, A.C. EphB signaling directs peripheral nerve regeneration through Sox2-dependent Schwann cell sorting. Cell 2010, 143, 145–155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dun, X.-p.; Carr, L.; Woodley, P.K.; Barry, R.W.; Drake, L.K.; Mindos, T.; Roberts, S.L.; Lloyd, A.C.; Parkinson, D.B. Macrophage-Derived Slit3 Controls Cell Migration and Axon Pathfinding in the Peripheral Nerve Bridge. Cell Rep. 2019, 26, 1458–1472. [Google Scholar] [CrossRef] [Green Version]
- Tannemaat, M.R.; Korecka, J.; Ehlert, E.M.E.; Mason, M.R.J.; van Duinen, S.G.; Boer, G.J.; Malessy, M.J.A.; Verhaagen, J. Human Neuroma Contains Increased Levels of Semaphorin 3A, Which Surrounds Nerve Fibers and Reduces Neurite Extension In Vitro. J. Neurosci. 2007, 27, 14260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Semina, E.; Rubina, K.; Sysoeva, V.; Rysenkova, K.; Klimovich, P.; Plekhanova, O.; Tkachuk, V. Urokinase and urokinase receptor participate in regulation of neuronal migration, axon growth and branching. Eur. J. Cell Biol. 2016, 95, 295–310. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klimovich, P.S.; Semina, E.V. Mechanisms of Participation of the Urokinase Receptor in Directed Axonal Growth. Mol. Biol. 2020, 54, 89–98. [Google Scholar] [CrossRef]
- Ogier, M.; Kron, M.; Katz, D.M. Neurotrophic factors in development and regulation of respiratory control. Compr. Physiol. 2013, 3, 1125–1134. [Google Scholar] [CrossRef] [PubMed]
- Gordon, T. The physiology of neural injury and regeneration: The role of neurotrophic factors. J. Commun. Disord. 2010, 43, 265–273. [Google Scholar] [CrossRef] [PubMed]
- Terada, Y.; Morita-Takemura, S.; Isonishi, A.; Tanaka, T.; Okuda, H.; Tatsumi, K.; Shinjo, T.; Kawaguchi, M.; Wanaka, A. NGF and BDNF expression in mouse DRG after spared nerve injury. Neurosci. Lett. 2018, 686, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Sterne, G.D.; Coulton, G.R.; Brown, R.A.; Green, C.J.; Terenghi, G. Neurotrophin-3-enhanced nerve regeneration selectively improves recovery of muscle fibers expressing myosin heavy chains 2b. J. Cell Biol. 1997, 139, 709–715. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Erickson, J.T.; Brosenitsch, T.A.; Katz, D.M. Brain-derived neurotrophic factor and glial cell line-derived neurotrophic factor are required simultaneously for survival of dopaminergic primary sensory neurons in vivo. J. Neurosci. 2001, 21, 581–589. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raivich, G.; Kreutzberg, G.W. Pathophysiology of glial growth factor receptors. Glia 1994, 11, 129–146. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.Y.; Luo, X.G.; Xian, C.J.; Liu, Z.H.; Zhou, X.F. Endogenous BDNF is required for myelination and regeneration of injured sciatic nerve in rodents. Eur. J. Neurosci. 2000, 12, 4171–4180. [Google Scholar] [PubMed]
- McGregor, C.E.; English, A.W. The Role of BDNF in Peripheral Nerve Regeneration: Activity-Dependent Treatments and Val66Met. Front. Cell Neurosci. 2018, 12, 522. [Google Scholar] [CrossRef] [Green Version]
- Vogelin, E.; Baker, J.M.; Gates, J.; Dixit, V.; Constantinescu, M.A.; Jones, N.F. Effects of local continuous release of brain derived neurotrophic factor (BDNF) on peripheral nerve regeneration in a rat model. Exp. Neurol. 2006, 199, 348–353. [Google Scholar] [CrossRef] [PubMed]
- Karagyaur, M.; Rostovtseva, A.; Semina, E.; Klimovich, P.; Balabanyan, V.; Makarevich, P.; Popov, V.; Stambolsky, D.; Tkachuk, V. A Bicistronic Plasmid Encoding Brain-Derived Neurotrophic Factor and Urokinase Plasminogen Activator Stimulates Peripheral Nerve Regeneration After Injury. J. Pharmacol. Exp. Ther. 2020, 372, 248–255. [Google Scholar] [CrossRef] [PubMed]
- Karagyaur, M.; Dyikanov, D.; Makarevich, P.; Semina, E.; Stambolsky, D.; Plekhanova, O.; Kalinina, N.; Tkachuk, V. Non-viral transfer of BDNF and uPA stimulates peripheral nerve regeneration. Biomed. Pharmacother. 2015, 74, 63–70. [Google Scholar] [CrossRef]
- Mills, C.D.; Allchorne, A.J.; Griffin, R.S.; Woolf, C.J.; Costigan, M. GDNF selectively promotes regeneration of injury-primed sensory neurons in the lesioned spinal cord. Mol. Cell. Neurosci. 2007, 36, 185–194. [Google Scholar] [CrossRef] [Green Version]
- Klimovich, P.; Rubina, K.; Sysoeva, V.; Semina, E. Three-Dimensional Model of Dorsal Root Ganglion Explant as a Method of Studying Neurotrophic Factors in Regenerative Medicine. Biomedicines 2020, 8, 49. [Google Scholar] [CrossRef] [Green Version]
- Gupta, R.; Gray, M.; Chao, T.; Bear, D.; Modafferi, E.; Mozaffar, T. Schwann cells upregulate vascular endothelial growth factor secondary to chronic nerve compression injury. Muscle Nerve 2005, 31, 452–460. [Google Scholar] [CrossRef]
- Homs, J.; Ariza, L.; Pages, G.; Udina, E.; Navarro, X.; Chillon, M.; Bosch, A. Schwann cell targeting via intrasciatic injection of AAV8 as gene therapy strategy for peripheral nerve regeneration. Gene Ther. 2011, 18, 622–630. [Google Scholar] [CrossRef] [Green Version]
- Tham, S.; Dowsing, B.; Finkelstein, D.; Donato, R.; Cheema, S.S.; Bartlett, P.F.; Morrison, W.A. Leukemia inhibitory factor enhances the regeneration of transected rat sciatic nerve and the function of reinnervated muscle. J. Neurosci. Res. 1997, 47, 208–215. [Google Scholar] [CrossRef]
- Mohiuddin, L.; Delcroix, J.D.; Fernyhough, P.; Tomlinson, D.R. Focally administered nerve growth factor suppresses molecular regenerative responses of axotomized peripheral afferents in rats. Neuroscience 1999, 91, 265–271. [Google Scholar] [CrossRef]
- Johnstone, R.M. Exosomes biological significance: A concise review. Blood Cells Mol. Dis. 2006, 36, 315–321. [Google Scholar] [CrossRef] [PubMed]
- Nawaz, M.; Fatima, F.; Vallabhaneni, K.; Penfornis, P.; Valadi, H.; Ekström, K.; Kholia, S.; Whitt, J.; Fernandes, J.; Pochampally, R.; et al. Extracellular Vesicles: Evolving Factors in Stem Cell Biology. Stem Cell Int. 2016, 2016. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qing, L.; Chen, H.; Tang, J.; Jia, X. Exosomes and Their MicroRNA Cargo: New Players in Peripheral Nerve Regeneration. Neurorehabil. Neural Repair 2018, 32, 765–776. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wahlgren, J.; Statello, L.; Skogberg, G.; Telemo, E.; Valadi, H. Delivery of Small Interfering RNAs to Cells via Exosomes. In SiRNA Delivery Methods: Methods and Protocols; Shum, K., Rossi, J., Eds.; Springer: New York, NY, USA, 2016; pp. 105–125. [Google Scholar]
- Santonocito, M.; Vento, M.; Guglielmino, M.R.; Battaglia, R.; Wahlgren, J.; Ragusa, M.; Barbagallo, D.; Borzi, P.; Rizzari, S.; Maugeri, M.; et al. Molecular characterization of exosomes and their microRNA cargo in human follicular fluid: Bioinformatic analysis reveals that exosomal microRNAs control pathways involved in follicular maturation. Fertil. Steril. 2014, 102, 1751–1761. [Google Scholar] [CrossRef]
- Kingham, P.; Ching, R. The role of exosomes in peripheral nerve regeneration. Neural Regen. Res. 2015, 10, 743. [Google Scholar] [CrossRef]
- Lopez-Verrilli, M.A.; Picou, F.; Court, F.A. Schwann cell-derived exosomes enhance axonal regeneration in the peripheral nervous system. Glia 2013, 61, 1795–1806. [Google Scholar] [CrossRef]
- Wei, Z.; Fan, B.; Ding, H.; Liu, Y.; Tang, H.; Pan, D.; Shi, J.; Zheng, P.; Shi, H.; Wu, H.; et al. Proteomics analysis of Schwann cell-derived exosomes: A novel therapeutic strategy for central nervous system injury. Mol. Cell Biochem. 2019, 457, 51–59. [Google Scholar] [CrossRef] [Green Version]
- Semina, E.V.; Rysenkova, K.D.; Troyanovskiy, K.E.; Shmakova, A.A.; Rubina, K.A. MicroRNAs in Cancer: From Gene Expression Regulation to the Metastatic Niche Reprogramming. Biochemistry 2021, 86, 785–799. [Google Scholar] [CrossRef] [PubMed]
- Yu, B.; Zhou, S.; Wang, Y.; Qian, T.; Ding, G.; Ding, F.; Gu, X. miR-221 and miR-222 promote Schwann cell proliferation and migration by targeting LASS2 after sciatic nerve injury. J. Cell Sci. 2012, 125, 2675–2683. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, S.; Zhang, R.; Yuan, Y.; Yi, S.; Chen, Q.; Gong, L.; Liu, J.; Ding, F.; Cao, Z.; Gu, X. MiR-340 Regulates Fibrinolysis and Axon Regrowth Following Sciatic Nerve Injury. Mol. Neurobiol. 2017, 54, 4379–4389. [Google Scholar] [CrossRef] [PubMed]
- Yi, S.; Yuan, Y.; Chen, Q.; Wang, X.; Gong, L.; Liu, J.; Gu, X.; Li, S. Regulation of Schwann cell proliferation and migration by miR-1 targeting brain-derived neurotrophic factor after peripheral nerve injury. Sci. Rep. 2016, 6, 29121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, S.; Wang, X.; Gu, Y.; Chen, C.; Wang, Y.; Liu, J.; Hu, W.; Yu, B.; Wang, Y.; Ding, F.; et al. Let-7 microRNAs regenerate peripheral nerve regeneration by targeting nerve growth factor. Mol. Ther. 2015, 23, 423–433. [Google Scholar] [CrossRef] [Green Version]
- Zhan, C.; Ma, C.B.; Yuan, H.M.; Cao, B.Y.; Zhu, J.J. Macrophage-derived microvesicles promote proliferation and migration of Schwann cell on peripheral nerve repair. Biochem. Biophys. Res. Commun. 2015, 468, 343–348. [Google Scholar] [CrossRef]
- Simeoli, R.; Montague, K.; Jones, H.R.; Castaldi, L.; Chambers, D.; Kelleher, J.H.; Vacca, V.; Pitcher, T.; Grist, J.; Al-Ahdal, H.; et al. Exosomal cargo including microRNA regulates sensory neuron to macrophage communication after nerve trauma. Nat. Commun. 2017, 8, 1778. [Google Scholar] [CrossRef] [Green Version]
- Lopez-Verrilli, M.A.; Caviedes, A.; Cabrera, A.; Sandoval, S.; Wyneken, U.; Khoury, M. Mesenchymal stem cell-derived exosomes from different sources selectively promote neuritic outgrowth. Neuroscience 2016, 320, 129–139. [Google Scholar] [CrossRef]
- Kalinina, N.I.; Sysoeva, V.Y.; Rubina, K.A.; Parfenova, Y.V.; Tkachuk, V.A. Mesenchymal stem cells in tissue growth and repair. Acta Nat. 2011, 3, 30–37. [Google Scholar] [CrossRef]
- Lopatina, T.; Kalinina, N.; Karagyaur, M.; Stambolsky, D.; Rubina, K.; Revischin, A.; Pavlova, G.; Parfyonova, Y.; Tkachuk, V. Adipose-derived stem cells stimulate regeneration of peripheral nerves: BDNF secreted by these cells promotes nerve healing and axon growth de novo. PLoS ONE 2011, 6, e17899. [Google Scholar] [CrossRef]
- Wei, J.J.; Chen, Y.F.; Xue, C.L.; Ma, B.T.; Shen, Y.M.; Guan, J.; Bao, X.J.; Wu, H.; Han, Q.; Wang, R.Z.; et al. Protection of Nerve Injury with Exosome Extracted from Mesenchymal Stem Cell. Zhongguo Yi Xue Ke Xue Yuan Xue Bao 2016, 38, 33–36. [Google Scholar] [CrossRef] [PubMed]
- Mao, Q.; Nguyen, P.D.; Shanti, R.M.; Shi, S.; Shakoori, P.; Zhang, Q.; Le, A.D. Gingiva-Derived Mesenchymal Stem Cell-Extracellular Vesicles Activate Schwann Cell Repair Phenotype and Promote Nerve Regeneration. Tissue Eng. Part. A 2019, 25, 887–900. [Google Scholar] [CrossRef] [Green Version]
- Ferguson, S.W.; Wang, J.; Lee, C.J.; Liu, M.; Neelamegham, S.; Canty, J.M.; Nguyen, J. The microRNA regulatory landscape of MSC-derived exosomes: A systems view. Sci. Rep. 2018, 8, 1419. [Google Scholar] [CrossRef] [PubMed]
- Xin, H.; Li, Y.; Liu, Z.; Wang, X.; Shang, X.; Cui, Y.; Zhang, Z.G.; Chopp, M. MiR-133b promotes neural plasticity and functional recovery after treatment of stroke with multipotent mesenchymal stromal cells in rats via transfer of exosome-enriched extracellular particles. Stem Cells 2013, 31, 2737–2746. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mead, B.; Tomarev, S. Bone Marrow-Derived Mesenchymal Stem Cells-Derived Exosomes Promote Survival of Retinal Ganglion Cells Through miRNA-Dependent Mechanisms. Stem Cells Transl. Med. 2017, 6, 1273–1285. [Google Scholar] [CrossRef]
- Toh, W.S.; Lai, R.C.; Hui, J.H.P.; Lim, S.K. MSC exosome as a cell-free MSC therapy for cartilage regeneration: Implications for osteoarthritis treatment. Semin. Cell Dev. Biol. 2017, 67, 56–64. [Google Scholar] [CrossRef]
- Zhang, B.; Yin, Y.; Lai, R.C.; Tan, S.S.; Choo, A.B.; Lim, S.K. Mesenchymal stem cells secrete immunologically active exosomes. Stem. Cells Dev. 2014, 23, 1233–1244. [Google Scholar] [CrossRef]
- Ti, D.; Hao, H.; Tong, C.; Liu, J.; Dong, L.; Zheng, J.; Zhao, Y.; Liu, H.; Fu, X.; Han, W. LPS-preconditioned mesenchymal stromal cells modify macrophage polarization for resolution of chronic inflammation via exosome-shuttled let-7b. J. Transl. Med. 2015, 13, 308. [Google Scholar] [CrossRef] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Klimovich, P.; Rubina, K.; Sysoeva, V.; Semina, E. New Frontiers in Peripheral Nerve Regeneration: Concerns and Remedies. Int. J. Mol. Sci. 2021, 22, 13380. https://doi.org/10.3390/ijms222413380
Klimovich P, Rubina K, Sysoeva V, Semina E. New Frontiers in Peripheral Nerve Regeneration: Concerns and Remedies. International Journal of Molecular Sciences. 2021; 22(24):13380. https://doi.org/10.3390/ijms222413380
Chicago/Turabian StyleKlimovich, Polina, Kseniya Rubina, Veronika Sysoeva, and Ekaterina Semina. 2021. "New Frontiers in Peripheral Nerve Regeneration: Concerns and Remedies" International Journal of Molecular Sciences 22, no. 24: 13380. https://doi.org/10.3390/ijms222413380
APA StyleKlimovich, P., Rubina, K., Sysoeva, V., & Semina, E. (2021). New Frontiers in Peripheral Nerve Regeneration: Concerns and Remedies. International Journal of Molecular Sciences, 22(24), 13380. https://doi.org/10.3390/ijms222413380