Toward Biofabrication of Resorbable Implants Consisting of a Calcium Phosphate Cement and Fibrin—A Characterization In Vitro and In Vivo
Abstract
:1. Introduction
2. Results
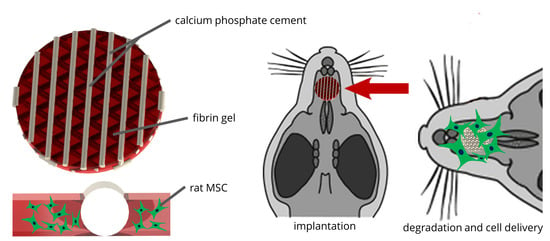
2.1. Development of the CPC-Fibrin Implants
2.2. Degradation Behavior of the Fibrin Hydrogel of the Cell-Laden Fibrin Hydrogel
2.2.1. Degradation of Fibrin Dependent on Cell Species and Cell Number
2.2.2. Degradation of rMSC-Laden vs. Cell-Free Fibrin in Presence of a CPC Construct
2.3. Cell Survival and Migration in Biphasic CPC-Fibrin Constructs
2.4. In Vivo Pilot Study
2.4.1. Study Design and Post-Operative Evaluation
2.4.2. Microcomputed Tomography and Histomorphology
2.4.3. Histomorphometry
3. Discussion
4. Materials and Methods
4.1. Fabrication of CPC-Fibrin Implants
4.2. Cell Culture
4.2.1. Cell Isolation and Expansion
4.2.2. Degradation Experiments
4.2.3. Cellular Behavior
4.3. In Vivo Application in a Rat Alveolar Cleft Model
4.4. Evaluation Methods
4.4.1. Microcomputed Tomography
4.4.2. Histology and Histological Analysis
4.5. Statistics
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CPC | Calcium phosphate cement |
| HAp | Hydroxyapatite |
| hMSC | Human mesenchymal stem cells |
| rMSC | Rat mesenchymal stem cells |
References
- Lilja, J. Alveolar Bone Grafting. Indian J. Plast. Surg. 2009, 42, 110–115. [Google Scholar] [CrossRef] [PubMed]
- Heinemann, S.; Rössler, S.; Lemm, M.; Ruhnow, M.; Nies, B. Properties of Injectable Ready-to-Use Calcium Phosphate Cement Based on Water-Immiscible Liquid. Acta Biomater. 2013, 9, 6199–6207. [Google Scholar] [CrossRef] [PubMed]
- Lode, A.; Meissner, K.; Luo, Y.; Sonntag, F.; Glorius, S.; Nies, B.; Vater, C.; Despang, F.; Hanke, T.; Gelinsky, M. Fabrication of Porous Scaffolds by Three-Dimensional Plotting of a Pasty Calcium Phosphate Bone Cement under Mild Conditions. J. Tissue Eng. Regen. Med. 2014, 8, 682–693. [Google Scholar] [CrossRef]
- Ahlfeld, T.; Akkineni, A.R.; Förster, Y.; Köhler, T.; Knaack, S.; Gelinsky, M.; Lode, A. Design and Fabrication of Complex Scaffolds for Bone Defect Healing: Combined 3D Plotting of a Calcium Phosphate Cement and a Growth Factor-Loaded Hydrogel. Ann. Biomed. Eng. 2017, 45, 224–236. [Google Scholar] [CrossRef] [PubMed]
- Ahlfeld, T.; Köhler, T.; Czichy, C.; Lode, A.; Gelinsky, M. A Methylcellulose Hydrogel as Support for 3D Plotting of Complex Shaped Calcium Phosphate Scaffolds. Gels 2018, 4, 68. [Google Scholar] [CrossRef] [Green Version]
- Korn, P.; Ahlfeld, T.; Lahmeyer, F.; Kilian, D.; Sembdner, P.; Stelzer, R.; Pradel, W.; Franke, A.; Rauner, M.; Range, U.; et al. 3D Printing of Bone Grafts for Cleft Alveolar Osteoplasty–In Vivo Evaluation in a Preclinical Model. Front. Bioeng. Biotechnol. 2020, 8, 217. [Google Scholar] [CrossRef]
- Kilian, D.; Ahlfeld, T.; Akkineni, A.R.; Lode, A.; Gelinsky, M. Three-Dimensional Bioprinting of Volumetric Tissues and Organs. MRS Bull. 2017, 42, 585–592. [Google Scholar] [CrossRef]
- Groll, J.; Burdick, J.A.; Cho, D.-W.; Derby, B.; Gelinsky, M.; Heilshorn, S.C.; Juengst, T.; Malda, J.; Mironov, V.A.; Nakayama, K.; et al. A Definition of Bioinks and Their Distinction from Biomaterial Inks. Biofabrication 2018, 11, 013001. [Google Scholar] [CrossRef]
- Ahlfeld, T.; Doberenz, F.; Kilian, D.; Vater, C.; Korn, P.; Lauer, G.; Lode, A. Michael Gelinsky Bioprinting of Mineralized Constructs Utilizing Multichannel Plotting of a Self-Setting Calcium Phosphate Cement and a Cell-Laden Bioink. Biofabrication 2018, 10, 045002. [Google Scholar] [CrossRef]
- Khodakaram-Tafti, A.; Mehrabani, D.; Shaterzadeh-Yazdi, H. An Overview on Autologous Fibrin Glue in Bone Tissue Engineering of Maxillofacial Surgery. Dent. Res. J. 2017, 14, 79–86. [Google Scholar]
- Noori, A.; Ashrafi, S.J.; Vaez-Ghaemi, R.; Hatamian-Zaremi, A.; Webster, T.J. A Review of Fibrin and Fibrin Composites for Bone Tissue Engineering. Int. J. Nanomed. 2017, 12, 4937–4961. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laurens, N.; Koolwijk, P.; Maat, M.P.M.D. Fibrin Structure and Wound Healing. J. Thromb. Haemost. 2006, 4, 932–939. [Google Scholar] [CrossRef] [PubMed]
- Palta, S.; Saroa, R.; Palta, A. Overview of the Coagulation System. Indian J. Anaesth. 2014, 58, 515–523. [Google Scholar] [CrossRef] [PubMed]
- Böcker, W.; Yin, Z.; Drosse, I.; Haasters, F.; Rossmann, O.; Wierer, M.; Popov, C.; Locher, M.; Mutschler, W.; Docheva, D.; et al. Introducing a Single-Cell-Derived Human Mesenchymal Stem Cell Line Expressing HTERT after Lentiviral Gene Transfer. J. Cell. Mol. Med. 2008, 12, 1347–1359. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Korn, P.; Schulz, M.C.; Range, U.; Lauer, G.; Pradel, W. Efficacy of Tissue Engineered Bone Grafts Containing Mesenchymal Stromal Cells for Cleft Alveolar Osteoplasty in a Rat Model. J. Craniomaxillofac. Surg. 2014, 42, 1277–1285. [Google Scholar] [CrossRef] [PubMed]
- Korn, P.; Hauptstock, M.; Range, U.; Kunert-Keil, C.; Pradel, W.; Lauer, G.; Schulz, M.C. Application of Tissue-Engineered Bone Grafts for Alveolar Cleft Osteoplasty in a Rodent Model. Clin. Oral Investig. 2017, 21, 2521–2534. [Google Scholar] [CrossRef]
- Lee, A.; Hudson, A.R.; Shiwarski, D.J.; Tashman, J.W.; Hinton, T.J.; Yerneni, S.; Bliley, J.M.; Campbell, P.G.; Feinberg, A.W. 3D Bioprinting of Collagen to Rebuild Components of the Human Heart. Science 2019, 365, 482–487. [Google Scholar] [CrossRef]
- de Melo, B.A.G.; Jodat, Y.A.; Cruz, E.M.; Benincasa, J.C.; Shin, S.R.; Porcionatto, M.A. Strategies to Use Fibrinogen as Bioink for 3D Bioprinting Fibrin-Based Soft and Hard Tissues. Acta Biomater. 2020, 117, 60–76. [Google Scholar] [CrossRef]
- Schuurman, W.; Khristov, V.; Pot, M.W.; van Weeren, P.R.; Dhert, W.J.A.; Malda, J. Bioprinting of Hybrid Tissue Constructs with Tailorable Mechanical Properties. Biofabrication 2011, 3, 021001. [Google Scholar] [CrossRef]
- Han, J.; Kim, D.S.; Jang, H.; Kim, H.-R.; Kang, H.-W. Bioprinting of Three-Dimensional Dentin–Pulp Complex with Local Differentiation of Human Dental Pulp Stem Cells. J. Tissue Eng. 2019, 10, 2041731419845849. [Google Scholar] [CrossRef]
- Ahlfeld, T.; Cubo-Mateo, N.; Cometta, S.; Guduric, V.; Vater, C.; Bernhardt, A.; Akkineni, A.R.; Lode, A.; Gelinsky, M. A Novel Plasma-Based Bioink Stimulates Cell Proliferation and Differentiation in Bioprinted, Mineralized Constructs. ACS Appl. Mater. Interfaces 2020, 12, 12557–12572. [Google Scholar] [CrossRef] [PubMed]
- Cui, G.; Li, J.; Lei, W.; Bi, L.; Tang, P.; Liang, Y.; Tao, S.; Wang, Y. The Mechanical and Biological Properties of an Injectable Calcium Phosphate Cement-Fibrin Glue Composite for Bone Regeneration. J. Biomed. Mater. Res. B Appl. Biomater. 2010, 92B, 377–385. [Google Scholar] [CrossRef] [PubMed]
- Xiu, J.; Fan, J.; Li, J.; Cui, G.; Lei, W. Different Angiogenic Abilities of Self-Setting Calcium Phosphate Cement Scaffolds Consisting of Different Proportions of Fibrin Glue. Biomed. Res. Int. 2014, 2014, 785146. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Zhang, C.; Wang, P.; Wang, L.; Bao, C.; Weir, M.D.; Reynolds, M.A.; Ren, K.; Zhao, L.; Xu, H.H.K. Engineering Bone Regeneration with Novel Cell-Laden Hydrogel Microfiber-Injectable Calcium Phosphate Scaffold. Mater. Sci. Eng. C 2017, 75, 895–905. [Google Scholar] [CrossRef] [Green Version]
- Bernhardt, A.; Schumacher, M.; Gelinsky, M. Formation of Osteoclasts on Calcium Phosphate Bone Cements and Polystyrene Depends on Monocyte Isolation Conditions. Tissue Eng. Part C Methods 2014, 21, 160–170. [Google Scholar] [CrossRef]
- Reitmaier, S.; Kovtun, A.; Schuelke, J.; Kanter, B.; Lemm, M.; Hoess, A.; Heinemann, S.; Nies, B.; Ignatius, A. Strontium(II) and Mechanical Loading Additively Augment Bone Formation in Calcium Phosphate Scaffolds. J. Orthop. Res. 2018, 36, 106–117. [Google Scholar] [CrossRef] [Green Version]
- Heissig, B.; Salama, Y.; Takahashi, S.; Osada, T.; Hattori, K. The Multifaceted Role of Plasminogen in Inflammation. Cell. Signal. 2020, 75, 109761. [Google Scholar] [CrossRef]
- Carrion, B.; Janson, I.A.; Kong, Y.P.; Putnam, A.J. A Safe and Efficient Method to Retrieve Mesenchymal Stem Cells from Three-Dimensional Fibrin Gels. Tissue Eng. Part C Methods 2013, 20, 252–263. [Google Scholar] [CrossRef] [Green Version]
- Mehanna, R.A.; Nabil, I.; Attia, N.; Bary, A.A.; Razek, K.A.; Ahmed, T.A.E.; Elsayed, F. The Effect of Bone Marrow-Derived Mesenchymal Stem Cells and Their Conditioned Media Topically Delivered in Fibrin Glue on Chronic Wound Healing in Rats. BioMed Res. Int. 2015, 2015. [Google Scholar] [CrossRef]
- Shamsul, B.S.; Chowdhury, S.R.; Hamdan, M.Y.; Ruszymah, B.H.I. Effect of Cell Density on Formation of Three-Dimensional Cartilaginous Constructs Using Fibrin & Human Osteoarthritic Chondrocytes. Ind. J. Med. Res. 2019, 149, 641. [Google Scholar] [CrossRef]
- Kopper, P.H. Role of Calcium in Fibrin Formation. Nature 1963, 198, 493–494. [Google Scholar] [CrossRef] [PubMed]
- Bruce, S. The Effect of Calcium on Fibrinolysis in Vitro. J. Clin. Pathol. 1964, 17, 282–286. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kilian, D.; Ahlfeld, T.; Akkineni, A.R.; Bernhardt, A.; Gelinsky, M.; Lode, A. 3D Bioprinting of Osteochondral Tissue Substitutes–in Vitro -Chondrogenesis in Multi-Layered Mineralized Constructs. Sci. Rep. 2020, 10, 8277. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.H.; Wang, P.; Wang, L.; Bao, C.; Chen, Q.; Weir, M.D.; Chow, L.C.; Zhao, L.; Zhou, X.; Reynolds, M.A. Calcium Phosphate Cements for Bone Engineering and Their Biological Properties. Bone Res. 2017, 5, 17056. [Google Scholar] [CrossRef] [Green Version]
- Moussa, M.; Carrel, J.-P.; Scherrer, S.; Cattani-Lorente, M.; Wiskott, A.; Durual, S. Medium-Term Function of a 3D Printed TCP/HA Structure as a New Osteoconductive Scaffold for Vertical Bone Augmentation: A Simulation by BMP-2 Activation. Materials 2015, 8, 2174–2190. [Google Scholar] [CrossRef] [Green Version]
- Donath, K.; Breuner, G. A Method for the Study of Undecalcified Bones and Teeth with Attached Soft Tissues. The Sage-Schliff (Sawing and Grinding) Technique. J. Oral Pathol. 1982, 11, 318–326. [Google Scholar] [CrossRef]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahlfeld, T.; Lode, A.; Richter, R.F.; Pradel, W.; Franke, A.; Rauner, M.; Stadlinger, B.; Lauer, G.; Gelinsky, M.; Korn, P. Toward Biofabrication of Resorbable Implants Consisting of a Calcium Phosphate Cement and Fibrin—A Characterization In Vitro and In Vivo. Int. J. Mol. Sci. 2021, 22, 1218. https://doi.org/10.3390/ijms22031218
Ahlfeld T, Lode A, Richter RF, Pradel W, Franke A, Rauner M, Stadlinger B, Lauer G, Gelinsky M, Korn P. Toward Biofabrication of Resorbable Implants Consisting of a Calcium Phosphate Cement and Fibrin—A Characterization In Vitro and In Vivo. International Journal of Molecular Sciences. 2021; 22(3):1218. https://doi.org/10.3390/ijms22031218
Chicago/Turabian StyleAhlfeld, Tilman, Anja Lode, Richard Frank Richter, Winnie Pradel, Adrian Franke, Martina Rauner, Bernd Stadlinger, Günter Lauer, Michael Gelinsky, and Paula Korn. 2021. "Toward Biofabrication of Resorbable Implants Consisting of a Calcium Phosphate Cement and Fibrin—A Characterization In Vitro and In Vivo" International Journal of Molecular Sciences 22, no. 3: 1218. https://doi.org/10.3390/ijms22031218
APA StyleAhlfeld, T., Lode, A., Richter, R. F., Pradel, W., Franke, A., Rauner, M., Stadlinger, B., Lauer, G., Gelinsky, M., & Korn, P. (2021). Toward Biofabrication of Resorbable Implants Consisting of a Calcium Phosphate Cement and Fibrin—A Characterization In Vitro and In Vivo. International Journal of Molecular Sciences, 22(3), 1218. https://doi.org/10.3390/ijms22031218