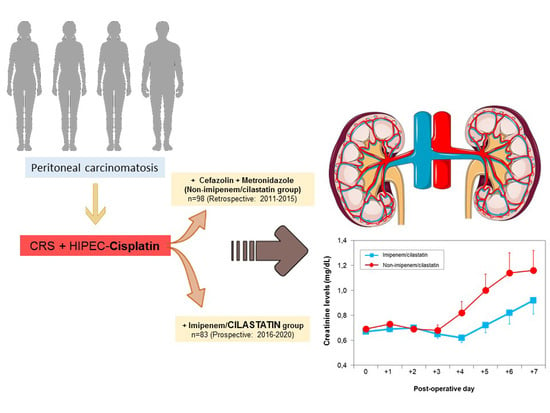
Effect of Cilastatin on Cisplatin-Induced Nephrotoxicity in Patients Undergoing Hyperthermic Intraperitoneal Chemotherapy
Abstract
:1. Introduction
2. Results and Discussion
2.1. Cisplatin-Induced Nephrotoxicity
2.2. Baseline Characteristics of the Study
2.3. Surgical and Anesthetic Data
2.4. HIPEC Procedure
2.5. Morbidity and Mortality
2.6. Renal Function and Protection with Cilastatin
2.7. Limitations of the Study
3. Materials and Methods
3.1. Anesthesia
3.2. CRS + HIPEC
3.3. Changes in the HIPEC Protocol
3.4. Data Collection
3.5. Statistical Analysis
3.6. Sample Size
4. Conclusions
5. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ACE-I | Angiotensin-converting enzyme inhibitors |
| AKI | Acute kidney injury |
| AKIN | Acute Kidney Injury Network |
| ARB | Angiotensin II receptor blockers |
| ASA | American Society of Anesthesiologists |
| BMI | Body mass index |
| BSA | Body surface area |
| CC score | Completeness of cytoreduction score |
| CRS | Cytoreductive surgery |
| CTCAE | Common Terminology Criteria for Adverse Events |
| DHP-I | Dehydropeptidase I |
| HIPEC | Hyperthermic intraperitoneal chemotherapy |
| HITOC | Hyperthermic intrathoracic chemotherapy |
| I/C | Imipenem/cilastatin |
| ICU | Intensive care unit |
| PCI | Peritoneal cancer index |
| RIFLE | Risk, Injury, Failure, Loss of kidney function, and End-stage kidney disease |
References
- Sadeghi, B.; Arvieux, C.; Glehen, O.; Beaujard, A.C.; Rivoire, M.; Baulieux, J.; Fontaumard, E.; Brachet, A.; Caillot, J.L.; Faure, J.L.; et al. Peritoneal carcinomatosis from non-gynecologic malignancies: Results of the EVOCAPE 1 multicentric prospective study. Cancer 2000, 88, 358–363. [Google Scholar] [CrossRef]
- Sugarbaker, P.H.; Cunliffe, W.J.; Belliveau, J.; de Bruijn, E.A.; Graves, T.; Mullins, R.E.; Schlag, P. Rationale for integrating early postoperative intraperitoneal chemotherapy into the surgical treatment of gastrointestinal cancer. Semin. Oncol. 1989, 16, 83–97. [Google Scholar] [PubMed]
- Jacquet, P.; Sugarbaker, P.H. Clinical research methodologies in diagnosis and staging of patients with peritoneal carcinomatosis. In Peritoneal Carcinomatosis: Principles of Management; Sugarbaker, P.H., Ed.; Kluwer Academic Publishers: Boston, MA, USA, 1996; pp. 359–374. ISBN 978-1-4613-1247-5. [Google Scholar]
- Sugarbaker, P.H. Peritonectomy procedures. Ann. Surg. 1995, 221, 29–42. [Google Scholar] [CrossRef] [PubMed]
- González-Moreno, S.; González-Bayón, L.A.; Ortega-Pérez, G. Hyperthermic intraperitoneal chemotherapy: Rationale and technique. World J. Gastrointest. Oncol. 2010, 2, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Yan, T.D.; Bijelic, L.; Sugarbaker, P.H. Critical analysis of treatment failure after complete cytoreductive surgery and perioperative intraperitoneal chemotherapy for peritoneal dissemination from appendiceal mucinous neoplasms. Ann. Surg. Oncol. 2007, 14, 2289–2299. [Google Scholar] [CrossRef]
- Van Driel, W.J.; Koole, S.N.; Sikorska, K.; Schagen van Leeuwen, J.H.; Schreuder, H.W.R.; Hermans, H.M.R.; de Hingh, I.H.J.T.; van der Velden, J.; Arts, H.J.; Massuger, L.F.A.G.; et al. Hyperthermic Intraperitoneal Chemotherapy in Ovarian Cancer. N. Engl. J. Med. 2018, 378, 230–240. [Google Scholar] [CrossRef]
- Yan, T.D.; Deraco, M.; Baratti, D.; Kusamura, S.; Elias, D.; Glehen, O.; Gilly, F.N.; Levine, E.A.; Shen, P.; Mohamed, F.; et al. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for malignant peritoneal mesothelioma: Multi-institutional experience. J. Clin. Oncol. 2009, 27, 6237–6242. [Google Scholar] [CrossRef] [PubMed]
- Elias, D.; Gilly, F.; Boutitie, F.; Quenet, F.; Bereder, J.M.; Mansvelt, B.; Lorimier, G.; Dubè, P.; Glehen, O. Peritoneal colorectal carcinomatosis treated with surgery and perioperative intraperitoneal chemotherapy: Retrospective analysis of 523 patients from a multicentric French study. J. Clin. Oncol. 2010, 28, 63–68. [Google Scholar] [CrossRef] [Green Version]
- Nadler, A.; McCart, J.A.; Govindarajan, A. Peritoneal Carcinomatosis from Colon Cancer: A Systematic Review of the Data for Cytoreduction and Intraperitoneal Chemotherapy. Clin. Colon Rectal Surg. 2015, 28, 234–246. [Google Scholar] [CrossRef] [Green Version]
- Takemoto, M.; Kuroda, M.; Urano, M.; Nishimura, Y.; Kawasaki, S.; Kato, H.; Okumura, Y.; Akaki, S.; Kanazawa, S.; Asaumi, J.; et al. The effect of various chemotherapeutic agents given with mild hyperthermia on different types of tumours. Int. J. Hyperth. 2003, 19, 193–203. [Google Scholar] [CrossRef]
- Oh, G.S.; Kim, H.J.; Shen, A.; Lee, S.B.; Khadka, D.; Pandit, A.; So, H.S. Cisplatin-induced Kidney Dysfunction and Perspectives on Improving Treatment Strategies. Electrolyte Blood Press. 2014, 12, 55–65. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Canda, A.E.; Sokmen, S.; Terzi, C.; Arslan, C.; Oztop, I.; Karabulut, B.; Ozzeybek, D.; Sarioglu, S.; Fuzun, M. Complications and toxicities after cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. Ann. Surg. Oncol. 2013, 20, 1082–1087. [Google Scholar] [CrossRef] [PubMed]
- Arjona-Sánchez, A.; Cadenas-Febres, A.; Cabrera-Bermon, J.; Muñoz-Casares, F.C.; Casado-Adam, A.; Sánchez-Hidalgo, J.M.; López-Andreu, M.; Briceño-Delgado, J.; Rufián-Peña, S. Assessment of RIFLE and AKIN criteria to define acute renal dysfunction for HIPEC procedures for ovarian and non ovarian peritoneal malignances. Eur. J. Surg. Oncol. 2016, 42, 869–876. [Google Scholar] [CrossRef] [PubMed]
- Camaño, S.; Lazaro, A.; Moreno-Gordaliza, E.; Torres, A.M.; de Lucas, C.; Humanes, B.; Lazaro, J.A.; Gomez-Gomez, M.M.; Bosca, L.; Tejedor, A. Cilastatin attenuates cisplatin-induced proximal tubular cell damage. J. Pharmacol. Exp. Ther. 2010, 334, 419–429. [Google Scholar] [CrossRef] [PubMed]
- Humanes, B.; Camaño, S.; Lara, J.M.; Sabbisetti, V.; González-Nicolás, M.A.; Bonventre, J.V.; Tejedor, A.; Lázaro, A. Cisplatin-induced renal inflammation is ameliorated by cilastatin nephroprotection. Nephrol. Dial. Transplant. 2017, 32, 1645–1655. [Google Scholar] [CrossRef] [Green Version]
- Moreno-Gordaliza, E.; Esteban-Fernández, D.; Lázaro, A.; Aboulmagd, S.; Humanes, B.; Tejedor, A.; Linscheid, M.W.; Gómez-Gómez, M.M. Lipid imaging for visualizating cilastatin amelioration of cisplatin-induced nephrotoxicity. Lipid Res. 2018, 59, 1561–1574. [Google Scholar] [CrossRef] [Green Version]
- Moreno-Gordaliza, E.; Giesen, C.; Lázaro, A.; Esteban-Fernández, D.; Humanes, B.; Cañas, B.; Panne, U.; Tejedor, A.; Jakubowski, N.; Gómez-Gómez, M.M. Elemental bioimaging in kidney by LA-ICP-MS as a tool to study nephrotoxicity and renal protective strategies in cisplatin therapies. Anal. Chem. 2011, 83, 7933–7940. [Google Scholar] [CrossRef]
- Humanes, B.; Lazaro, A.; Camaño, S.; Moreno-Gordaliz, E.; Lazaro, J.A.; Blanco-Codesido, M.; Lara, J.M.; Ortiz, A.; Gomez-Gomez, M.M.; Martín-Vasallo, P.; et al. Cilastatin protects against cisplatin-induced nephrotoxicity without compromising its anticancer efficiency in rats. Kidney Int. 2012, 82, 652–663. [Google Scholar] [CrossRef] [Green Version]
- González-Nicolás, M.A.; González-Guerrero, C.; Pérez-Fernández, V.A.; Lázaro, A. Cilastatin: A potential treatment strategy against COVI-19 that may decrease viral replication and protect from the cytokine storm. Clin. Kidney J. 2020, 13, 903–905. [Google Scholar] [CrossRef]
- Tejedor, A.; Torres, A.M.; Castilla, M.; Lazaro, J.A.; de Lucas, C.; Caramelo, C. Cilastatin protection against cyclosporin A-induced nephrotoxicity: Clinical evidence. Curr. Med. Res. Opin. 2007, 23, 505–513. [Google Scholar] [CrossRef]
- Carmellini, M.; Matteucci, E.; Boggi, U.; Cecconi, S.; Giampietro, O.; Mosca, F. Imipenem/cilastatin reduces cyclosporin-induced tubular damage in kidney transplant recipients. Transplant. Proc. 1998, 30, 2034–2035. [Google Scholar] [CrossRef]
- Markewitz, A.; Hammer, C.; Pfeiffer, M.; Zahn, S.; Drechsel, J.; Reichenspurner, H.; Reichart, B. Reduction of cyclosporine induced nephrotoxicity by cilastatin following clinical heart transplantation. Transplantation 1994, 57, 865–870. [Google Scholar] [CrossRef] [PubMed]
- Zacharias, M.; Mugawar, M.; Herbison, G.P.; Walker, R.J.; Hovhannisyan, K.; Sivalingam, P.; Conlon, N.P. Interventions for protecting renal function in the perioperative period. Cochrane Database Syst. Rev. 2013, 9, CD003590. [Google Scholar] [CrossRef]
- Dagel, T.; Misirlioglu, S.; Tanju, S.; Afsar, B.; Selcukbiricik, F.; Erus, S.; Vatansever, D.; Balik, E.; Taskira, C.; Dilege, S.; et al. Hyperthermic intraperitoneal chemotherapy is an independent risk factor for development of acute kidney injury. J BUON 2018, 23, 1528–1533. [Google Scholar] [PubMed]
- Hakeam, H.A.; Breakiet, M.; Azzam, A.; Nadeem, A.; Amin, T. The incidence of cisplatin nephrotoxicity post hyperthermic intraperitoneal chemotherapy (HIPEC) and cytoreductive surgery. Renal Fail 2014, 36, 1486–1491. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barton, C.D.; Pizer, B.; Jones, C.; Oni, L.; Pirmohamed, M.; Hawcutt, D.B. Identifying cisplatin-induced kidney damage in paediatric oncology patients. Pediatr. Nephrol. 2018, 33, 1467–1474. [Google Scholar] [CrossRef] [Green Version]
- Zivanovic, O.; Abramian, A.; Kullmann, M.; Fuhrmann, C.; Coch, C.; Hoeller, T.; Ruehs, H.; Keyver-Paik, M.D.; Rudlowski, C.; Weber, S.; et al. HIPEC ROC I: A phase I study of cisplatin administered as hyperthermic intraoperative intraperitoneal chemoperfusion followed by postoperative intravenous platinum-based chemotherapy in patients with platinum-sensitive recurrent epithelial ovarian cancer. Int. J. Cancer 2015, 136, 699–708. [Google Scholar] [CrossRef] [Green Version]
- Colantonio, L.; Claroni, C.; Fabrizi, L.; Marcelli, M.E.; Sofra, M.; Giannarelli, D.; Garofalo, A.; Forastiere, E. A randomized trial of goal directed vs. standard fluid therapy in cytoreductive surgery with hyperthermic intraperitoneal chemotherapy. J. Gastrointest. Surg. 2015, 19, 722–729. [Google Scholar] [CrossRef]
- Dickey, D.T.; Wu, Y.J.; Muldoon, L.L.; Neuwelt, E.A. Protection against cisplatin-induced toxicities by N-acetylcysteine and sodium thiosulfate as assessed at the molecular, cellular, and in vivo levels. J. Pharmacol. Exp. Ther. 2005, 314, 1052–1058. [Google Scholar] [CrossRef] [Green Version]
- Bouhadjari, N.; Gabato, W.; Calabrese, D.; Msika, S.; Keita, H. Hyperthermic intraperitoneal chemotherapy with cisplatin: Amifostine prevents acute severe renal impairment. Eur. J. Surg. Oncol. 2016, 42, 219–223. [Google Scholar] [CrossRef]
- Vaira, M.; Barone, R.; Aghemo, B.; Mioli, P.R.; De Simone, M. Renal protection with amifostine during intraoperative peritoneal chemohyperthermia (IPCH) with cisplatin (CDDP) for peritoneal carcinosis. Phase 1 study. Minerva Med. 2001, 92, 207–211. [Google Scholar] [PubMed]
- Gruss, E.; Tomás, J.F.; Bernis, C.; Rodriguez, F.; Traver, J.A.; Fernández-Rañada, J.M. Nephroprotective effect of cilastatin in allogeneic bone marrow transplantation. Results from a retrospective analysis. Bone Marrow Transplant. 1996, 18, 761–765. [Google Scholar] [PubMed]
- Cata, J.P.; Zavala, A.M.; Van Meter, A.; Williams, U.U.; Soliz, J.; Hernandez, M.; Owusu-Agyemang, P. Identification of risk factors associated with postoperative acute kidney injury after cytoreductive surgery with hyperthermic intraperitoneal chemotherapy: A retrospective study. Int. J. Hyperth. 2018, 34, 538–544. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- I-Lin Sin, E.; Shulyn Chia, C.; Hwei Ching Tan, G.; Chee Soo, K.; Ching-Ching Teo, M. Acute kidney injury in ovarian cancer patients undergoing cytoreductive surgery and hyperthermic intra-peritoneal chemotherapy. Int. J. Hyperth. 2017, 33, 690–695. [Google Scholar] [CrossRef] [PubMed]
- Galfetti, E.; Cerutti, A.; Ghielmini, M.; Zucca, E.; Wannesson, L. Risk factors for renal toxicity after inpatient cisplatin administration. BMC Pharmacol. Toxicol. 2020, 21, 19. [Google Scholar] [CrossRef] [PubMed]
- Cascales-Campos, P.A.; López-López, V.; Muñoz-Casares, F.C.; Feliciangeli, E.; Torres Melero, J.; Barrios, P.; Morales, R.; Ramos, I.; Ortega, G.; Camps, B.; et al. Morbidity and mortality outcomes after cytoreductive surgery and hyperthermic intraperitoneal chemotherapy in patients aged 75 years and over: Spanish group of peritoneal cancer surgery (GECOP) multicenter study. Surg. Oncol. 2016, 25, 111–116. [Google Scholar] [CrossRef]
- Eng, O.S.; Dumitra, S.; O’Leary, M.; Raoof, M.; Wakabayashi, M.; Dellinger, T.H.; Han, E.S.; Lee, S.J.; Paz, I.B.; Lee, B. Association of fluid administration with morbidity in cytoreductive surgery with hyperthermic intraperitoneal chemotherapy. JAMA Surg. 2017, 152, 1156–1160. [Google Scholar] [CrossRef]
- Cornelison, T.L.; Reed, E. Nephrotoxicity and hydration management for cisplatin, carboplatin, and ormaplatin. Gynecol. Oncol. 1993, 50, 147–158. [Google Scholar] [CrossRef]
- Giglio, M.; Dalfino, L.; Puntillo, F.; Brienza, N. Hemodynamic goal-directed therapy and postoperative kidney injury: An updated meta-analysis with trial sequential analysis. Crit. Care 2019, 23, 232. [Google Scholar] [CrossRef] [Green Version]
- Crona, D.J.; Faso, A.; Nishijima, T.F.; Mcgraw, K.A.; Galsky, M.; Milowsky, M.I. A Systematic Review of Strategies to Prevent Cisplatin-Induced Nephrotoxicity. Oncologist 2017, 22, 609–619. [Google Scholar] [CrossRef] [Green Version]
- Bush, K.; Fisher, J.F. Epidemiological expansion, structural studies, and clinical challenges of new β-lactamases from gram-negative bacteria. Annu. Rev. Microbiol. 2011, 65, 455–478. [Google Scholar] [CrossRef] [PubMed]
- Hoste, E.A.; Clermont, G.; Kersten, A.; Venkataraman, R.; Angus, D.C.; De Bacquer, D.; Kellum, J.A. RIFLE criteria for acute kidney injury are associated with hospital mortality in critically ill patients: A cohort analysis. Crit. Care 2006, 10, R73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Riordan, A.; Wong, V.; McQuillan, R.; McCormick, P.A.; Hegarty, J.E.; Watson, A.J. Acute renal disease, as defined by the RIFLE criteria, post-liver transplantation. Am. J. Transplant. 2007, 7, 168–176. [Google Scholar] [CrossRef] [PubMed]
- Silver, S.A.; Harel, Z.; McArthur, E.; Nash, D.M.; Acedillo, R.; Kitchlu, A.; Garg, A.X.; Chertow, G.M.; Bell, C.M.; Wald, R. Causes of Death after a Hospitalization with AKI. J. Am. Soc. Nephrol. 2018, 29, 1001–1010. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bucaloiu, I.D.; Kirchner, H.L.; Norfolk, E.R.; Hartle, J.E.; Perkins, R.M. Increased risk of death and de novo chronic kidney disease following reversible acute kidney injury. Kidney Int. 2012, 81, 477–485. [Google Scholar] [CrossRef] [Green Version]
- Humanes, B.; Jado, J.C.; Camaño, S.; López-Parra, V.; Torres, A.M.; Álvarez-Sala, L.A.; Cercenado, E.; Tejedor, A.; Lázaro, A. Protective Effects of Cilastatin against Vancomycin-Induced Nephrotoxicity. Biomed. Res. Int. 2015, 2015, 704382. [Google Scholar] [CrossRef] [Green Version]
- Jado, J.C.; Humanes, B.; González-Nicolás, M.A.; Camaño, S.; Lara, J.M.; López, B.; Cercenado, E.; García-Bordas, J.; Tejedor, A.; Lázaro, A. Nephroprotective Effect of Cilastatin against Gentamicin-Induced Renal Injury In Vitro and In Vivo without Altering Its Bactericidal Efficiency. Antioxidants 2020, 9, 821. [Google Scholar] [CrossRef]
- Pérez, M.; Castilla, M.; Torres, A.M.; Lázaro, J.A.; Sarmiento, E.; Tejedor, A. Inhibition of brush border dipeptidase with cilastatin reduces toxic accumulation of cyclosporin A in kidney proximal tubule epithelial cells. Nephrol. Dial. Transplant. 2004, 19, 2445–2455. [Google Scholar] [CrossRef] [Green Version]
- Lazaro, A.; Camaño, S.; Humanes, B.; Tejedor, A. Novel strategies in drug-induced acute kidney injury. In Pharmacology; Gallelli, L., Ed.; Intech: Rijeka, Croatia, 2012; pp. 381–396. [Google Scholar] [CrossRef] [Green Version]
- Dimanche-Boitrel, M.T.; Meurette, O.; Rebillard, A.; Lacour, S. Role of early plasma membrane events in chemotherapy-induced cell death. Drug Resist. Update 2005, 8, 5–14. [Google Scholar] [CrossRef]
- Holditch, S.J.; Brown, C.N.; Lombardi, A.M.; Nguyen, K.N.; Edelstein, C.L. Recent Advances in Models, Mechanisms, Biomarkers, and Interventions in Cisplatin-Induced Acute Kidney Injury. Int. J. Mol. Sci. 2019, 20, 3011. [Google Scholar] [CrossRef] [Green Version]
- Hori, Y.; Aoki, N.; Kuwahara, S.; Hosojima, M.; Kaseda, R.; Goto, S.; Iida, T.; De, S.; Kabasawa, H.; Kaneko, R.; et al. Megalin Blockade with Cilastatin Suppresses Drug-Induced Nephrotoxicity. J. Am. Soc. Nephrol. 2017, 28, 1783–1791. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lopes, J.A.; Jorge, S. The RIFLE and AKIN classifications for acute kidney injury: A critical and comprehensive review. Clin. Kidney J. 2013, 6, 8–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Portilla, A.G.; Shigeki, K.; Dario, B.; Marcello, D. The intraoperative staging systems in the management of peritoneal surface malignancy. J. Surg. Oncol. 2008, 98, 228–231. [Google Scholar] [CrossRef] [PubMed]
- González-Moreno, S.; Kusamura, S.; Baratti, D.; Deraco, M. Postoperative residual disease evaluation in the locoregional treatment of peritoneal surface malignancy. J. Surg. Oncol. 2008, 98, 237–241. [Google Scholar] [CrossRef] [PubMed]
- Sugarbaker, P.H. Technical Handbook for the Integration of Cytoreductive Surgery and Perioperative Intraperitoneal Chemotherapy into the Surgical Management of Gastrointestinal and Gynecologic Malignancy, 4th ed.; The Ludann Company: Grand Rapids, MI, USA, 2005; pp. 52–56. [Google Scholar]
- Cashin, P.H.; Ehrsson, H.; Wallin, I.; Nygren, P.; Mahteme, H. Pharmacokinetics of cisplatin during hyperthermic intraperitoneal treatment of peritoneal carcinomatosis. Eur. J. Clin. Pharmacol. 2013, 69, 533–540. [Google Scholar] [CrossRef] [PubMed]





| Patient Characteristics | Non-I/C Group (n = 98) | I/C Group (n = 83) | p-Value |
|---|---|---|---|
| Age (years) | 53.22 ± 10.94 | 56.79 ± 11.42 | 0.034 |
| Patients > 65 years | 12 (12.4) | 21 (25.3) | 0.025 |
| ASA, n (%) | |||
| I | 1 (1) | 2 (2.4) | 0.06 |
| II | 69 (70.4) | 69 (83.1) | |
| III | 28 (28.6) | 12 (14.5) | |
| Sex, n (%) | |||
| Female | 91 (92.9) | 80 (94) | 0.76 |
| Male | 7 (7.1) | 5 (6) | |
| Comorbidity associated with nephrotoxicity, n (%) | 23 (24) | 23 (27.7) | 0.56 |
| BMI (kg/m2) | 24.5 ± 6.29 | 25.4 ± 5.3 | 0.33 |
| BSA (m2) | 1.65 ± 0.17 | 1.63 ± 0.21 | 0.47 |
| Previous chemotherapy with cisplatin | 71 (72.4) | 67 (80.7) | 0.12 |
| Number of cycles of previous cisplatin chemotherapy | 4.35 ± 3.78 | 4.21 ± 2.5 | 0.76 |
| Tumor: | - | - | 0.87 |
| Ovarian | 82 | 69 | - |
| Colon adenocarcinoma | 1 | 1 | - |
| Appendix | 2 | 1 | - |
| Gastric tumor | 1 | 3 | - |
| Mesothelioma | 10 | 8 | - |
| Other | 2 | 1 | - |
| PCI | 15.36 ± 10.23 | 15.25 ± 9.80 | 0.94 |
| Duration of anesthesia (min) | 682.7 ± 127.77 | 614.28 ± 105.90 | 0.0001 |
| Duration of surgery (min) | 566.29 ± 126.13 | 497.39 ± 101.87 | 0.0001 |
| Duration of HIPEC (min) | 68.50 ± 13.84 | 60.72 ± 6.5 | 0.0001 |
| Perioperative urine output (mL) | 1223.33 ± 387.08 | 1464.28 ± 592.36 | 0.18 |
| Urine output during HIPEC (mL) | 891.39 ± 375.51 | 845.48 ± 354.55 | 0.40 |
| Urine output during HIPEC > 150 mL/15 min, n (%) | 70 (76.1) | 66 (80.5) | 0.60 |
| Intraoperative administration of diuretic (%) | 53 (71.6) | 66 (82.5) | 0.14 |
| Perioperative fluid balance (mL) | 6897.22 ± 2849.94 | 5127.81 ± 1493.38 | 0.0001 |
| Crystalloids (mL) | 5781.25 ± 2516.55 | 3905.06 ± 1220.63 | 0.0001 |
| Colloids, n (%) | 66 (66) | 46 (54) | 0.31 |
| Vasopressor, n (%) | 17 (17.1) | 25 (29) | 0.11 |
| Dose of cisplatin (mg) | 142.23 ± 34.35 | 142.09 ± 32.28 | 0.97 |
| Dose of cisplatin (mg/BSA) | 85.27 ± 18.86 | 87.21 ± 18.5 | 0.50 |
| Cisplatin + doxorubicin (%) | 53 (53.5) | 8 (9.4) | 0.0001 |
| Stay in intensive care > 3 days, n (%) | 27 (27.6) | 12 (14.1) | 0.02 |
| Length of hospital stay | 24.11 ± 30 | 13.52 ± 10.9 | 0.005 |
| 90-day mortality, n (%) | 3 (3) | 0 | 0.15 |
| Major complications, n (%) | 18 (18.4) | 8 (9.6) | 0.07 |
| Time | Non-I/C Group (n = 98) | I/C Group (n = 83) | p-Value |
|---|---|---|---|
| Baseline | 0.69 ± 0.13 | 0.67 ± 0.13 | 0.57 |
| Day 1 | 0.73 ± 0.23 | 0.769 ± 0.22 | 0.31 |
| Day 2 | 0.69 ± 0.25 | 0.70 ± 0.32 | 0.89 |
| Day 3 | 0.68 ± 0.40 | 0.65 ± 0.30 | 0.58 |
| Day 4 | 0.82 ± 0.78 | 0.62 ± 0.33 | 0.04 * |
| Day 5 | 1.00 ± 1.02 | 0.72 ± 0.51 | 0.06 ** |
| Day 6 | 1.14 ± 1.25 | 0.82 ± 0.67 | 0.09 ** |
| Day 7 | 1.16 ± 1.28 | 0.92 ± 0.89 | 0.2 |
| Category | Non-I/C Group (n = 98) | I/C Group (n = 83) | p-Value |
|---|---|---|---|
| No renal failure | 73 | 64 | 0.83 |
| Risk | 8 | 7 | |
| Injury | 10 | 6 | |
| Failure | 3 | 4 | |
| Loss | 4 | 2 | |
| ESRD | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zaballos, M.; Power, M.; Canal-Alonso, M.I.; González-Nicolás, M.Á.; Vasquez-Jimenez, W.; Lozano-Lominchar, P.; Cabrerizo-Torrente, P.; Palencia-García, N.; Gago-Quiroga, S.; Ginel-Feito, M.D.; et al. Effect of Cilastatin on Cisplatin-Induced Nephrotoxicity in Patients Undergoing Hyperthermic Intraperitoneal Chemotherapy. Int. J. Mol. Sci. 2021, 22, 1239. https://doi.org/10.3390/ijms22031239
Zaballos M, Power M, Canal-Alonso MI, González-Nicolás MÁ, Vasquez-Jimenez W, Lozano-Lominchar P, Cabrerizo-Torrente P, Palencia-García N, Gago-Quiroga S, Ginel-Feito MD, et al. Effect of Cilastatin on Cisplatin-Induced Nephrotoxicity in Patients Undergoing Hyperthermic Intraperitoneal Chemotherapy. International Journal of Molecular Sciences. 2021; 22(3):1239. https://doi.org/10.3390/ijms22031239
Chicago/Turabian StyleZaballos, Matilde, Mercedes Power, María Iluminada Canal-Alonso, María Ángeles González-Nicolás, Wenceslao Vasquez-Jimenez, Pablo Lozano-Lominchar, Pilar Cabrerizo-Torrente, Natividad Palencia-García, Susana Gago-Quiroga, María Dolores Ginel-Feito, and et al. 2021. "Effect of Cilastatin on Cisplatin-Induced Nephrotoxicity in Patients Undergoing Hyperthermic Intraperitoneal Chemotherapy" International Journal of Molecular Sciences 22, no. 3: 1239. https://doi.org/10.3390/ijms22031239
APA StyleZaballos, M., Power, M., Canal-Alonso, M. I., González-Nicolás, M. Á., Vasquez-Jimenez, W., Lozano-Lominchar, P., Cabrerizo-Torrente, P., Palencia-García, N., Gago-Quiroga, S., Ginel-Feito, M. D., Jiménez, C., Lázaro, A., & González-Bayón, L. (2021). Effect of Cilastatin on Cisplatin-Induced Nephrotoxicity in Patients Undergoing Hyperthermic Intraperitoneal Chemotherapy. International Journal of Molecular Sciences, 22(3), 1239. https://doi.org/10.3390/ijms22031239