The Human Tissue-Engineered Cornea (hTEC): Recent Progress
Abstract
:1. Introduction
1.1. Anatomy of the Human Cornea
1.1.1. Corneal Epithelium
1.1.2. Bowman’s Layer
1.1.3. Corneal Stroma
1.1.4. Descemet’s Membrane
1.1.5. Corneal Endothelium
1.2. Clinical Aspects of Corneal Blindness and Current Therapeutic Strategies
2. Tridimensional (3D) Scaffold Models of the Cornea
2.1. Silk-Based Corneal Implants
2.2. Chitin-Based Corneal Implants
2.3. Collagen-Chitosan Hydrogels
2.4. Fibrin-Agarose Hydrogels
2.5. Polyethylene Glycol and Polyacrylic Acid Hydrogels
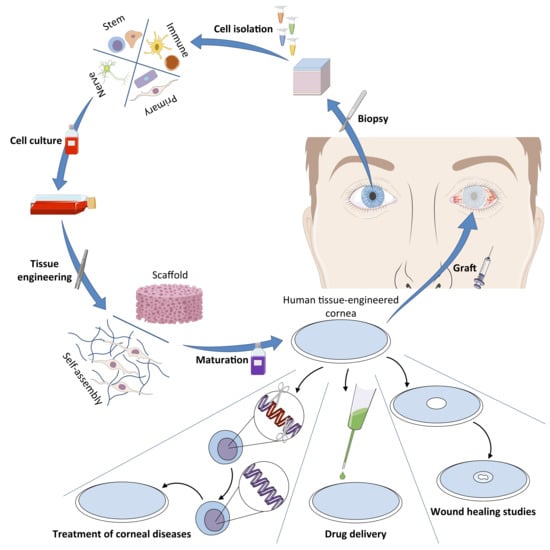
3. The Human Tissue-Engineered Cornea (hTEC)
3.1. The 3D Human Tissue-Engineered Cornea Generated by the Self-Assembly Method
3.1.1. Isolation and Culture of Human Corneal Cells (Epithelial, Stromal, and Endothelial Cells)
3.1.2. The Self-Assembly Procedure for the Reconstruction of hTECs
3.1.3. Characteristics and Advantages of the Human Tissue-Engineered Cornea
3.1.4. Recent Improvements Brought to the hTEC Model
3.1.5. What Are the ‘Missing Constituents?’
Corneal Stromal Stem Cells
Innervation
Immune Cells
4. The Future of the hTEC: Potential Applications and Uses
4.1. A Graftable Alternative in the Treatment of LSCD
4.2. A Model for the Study of Corneal Wound Healing
4.3. hTECs and Nanotechnologies: A Model for the Development of a New Drug Delivery System
5. Future Directions and Conclusions
6. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- DelMonte, D.W.; Kim, T. Anatomy and physiology of the cornea. J. Cataract. Refract. Surg. 2011, 37, 588–598. [Google Scholar] [CrossRef]
- Sridhar, M.S. Anatomy of cornea and ocular surface. Indian J. Ophthalmol. 2018, 66, 190–194. [Google Scholar] [CrossRef] [PubMed]
- Kurpakus-Wheater, M.; Kernacki, K.A.; Hazlett, L.D. Maintaining corneal integrity how the “window” stays clear. Prog. Histochem. Cytochem. 2001, 36, 185–259. [Google Scholar] [CrossRef]
- Jester, J.V.; Moller-Pedersen, T.; Huang, J.; Sax, C.M.; Kays, W.T.; Cavangh, H.D.; Petroll, W.M.; Piatigorsky, J. The cellular basis of corneal transparency: Evidence for ‘corneal crystallins’. J. Cell Sci. 1999, 112, 613–622. [Google Scholar] [PubMed]
- Meek, K.M.; Leonard, D.W.; Connon, C.J.; Dennis, S.; Khan, S. Transparency, swelling and scarring in the corneal stroma. Eye 2003, 17, 927–936. [Google Scholar] [CrossRef] [PubMed]
- Fischbarg, J. On the mechanism of fluid transport across corneal endothelium and epithelia in general. J. Exp. Zool. A Comp. Exp. Biol. 2003, 300, 30–40. [Google Scholar] [CrossRef]
- Jester, J.V. Corneal crystallins and the development of cellular transparency. Semin. Cell Dev. Biol. 2008, 19, 82–93. [Google Scholar] [CrossRef] [Green Version]
- Rufer, F.; Schroder, A.; Erb, C. White-to-white corneal diameter: Normal values in healthy humans obtained with the Orbscan II topography system. Cornea 2005, 24, 259–261. [Google Scholar] [CrossRef]
- Ehlers, N. The Precorneal Film. Biomicroscopical, Histological and Chemical Investigations. Acta Ophthalmol. Suppl. 1965, 81, 81–134. [Google Scholar]
- Rozsa, A.J.; Beuerman, R.W. Density and organization of free nerve endings in the corneal epithelium of the rabbit. Pain 1982, 14, 105–120. [Google Scholar] [CrossRef]
- Beuerman, R.W.; Pedroza, L. Ultrastructure of the human cornea. Microsc. Res. Tech. 1996, 33, 320–335. [Google Scholar] [CrossRef]
- Eghrari, A.O.; Riazuddin, S.A.; Gottsch, J.D. Overview of the Cornea: Structure, Function, and Development. Prog. Mol. Biol. Transl. Sci. 2015, 134, 7–23. [Google Scholar] [CrossRef] [PubMed]
- Dua, H.S.; Azuara-Blanco, A. Limbal stem cells of the corneal epithelium. Surv. Ophthalmol. 2000, 44, 415–425. [Google Scholar] [CrossRef]
- Wiley, L.; SundarRaj, N.; Sun, T.T.; Thoft, R.A. Regional heterogeneity in human corneal and limbal epithelia: An immunohistochemical evaluation. Investig. Ophthalmol. Vis. Sci. 1991, 32, 594–602. [Google Scholar]
- Hanna, C.; Bicknell, D.S.; O’Brien, J.E. Cell turnover in the adult human eye. Arch. Ophthalmol. 1961, 65, 695–698. [Google Scholar] [CrossRef]
- Osei-Bempong, C.; Figueiredo, F.C.; Lako, M. The limbal epithelium of the eye—A review of limbal stem cell biology, disease and treatment. Bioessays 2013, 35, 211–219. [Google Scholar] [CrossRef]
- Jacobsen, I.E.; Jensen, O.A.; Prause, J.U. Structure and composition of Bowman’s membrane. Study by frozen resin cracking. Acta Ophthalmol. 1984, 62, 39–53. [Google Scholar] [CrossRef]
- Nakayasu, K.; Tanaka, M.; Konomi, H.; Hayashi, T. Distribution of types I, II, III, IV and V collagen in normal and keratoconus corneas. Ophthalmic. Res. 1986, 18. [Google Scholar] [CrossRef]
- Marshall, G.E.; Konstas, A.G.; Lee, W.R. Immunogold fine structural localization of extracellular matrix components in aged human cornea. I. Types I-IV collagen and laminin. Graefes Arch. Clin. Exp. Ophthalmol. 1991, 229, 157–163. [Google Scholar] [CrossRef]
- Marshall, G.E.; Konstas, A.G.; Lee, W.R. Immunogold fine structural localization of extracellular matrix components in aged human cornea. II. Collagen types V and VI. Graefes Arch. Clin. Exp. Ophthalmol. 1991, 229, 164–171. [Google Scholar] [CrossRef]
- Lagali, N.; Germundsson, J.; Fagerholm, P. The role of Bowman’s layer in corneal regeneration after phototherapeutic keratectomy: A prospective study using in vivo confocal microscopy. Invesig. Ophthalmol. Vis. Sci. 2009, 50, 4192–4198. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Newsome, D.A.; Gross, J.; Hassell, J.R. Human corneal stroma contains three distinct collagens. Investig. Ophthalmol. Vis. Sci. 1982, 22, 376–381. [Google Scholar]
- Meek, K.M.; Fullwood, N.J. Corneal and scleral collagens—A microscopist’s perspective. Micron 2001, 32, 261–272. [Google Scholar] [CrossRef]
- Ruberti, J.W.; Sinha Roy, A.; Roberts, C.J. Corneal biomechanics and biomaterials. Annu. Rev. Biomed. Eng. 2011, 13, 269–295. [Google Scholar] [CrossRef] [PubMed]
- Morishige, N.; Takagi, Y.; Chikama, T.; Takahara, A.; Nishida, T. Three-dimensional analysis of collagen lamellae in the anterior stroma of the human cornea visualized by second harmonic generation imaging microscopy. Investig. Ophthalmol. Vis. Sci. 2011, 52, 911–915. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, S.; Mienaltowski, M.J.; Birk, D.E. Regulation of corneal stroma extracellular matrix assembly. Exp. Eye Res. 2015, 133, 69–80. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fini, M.E. Keratocyte and fibroblast phenotypes in the repairing cornea. Prog. Retin. Eye Res. 1999, 18, 529–551. [Google Scholar] [CrossRef]
- Fini, M.E.; Stramer, B.M. How the cornea heals: Cornea-specific repair mechanisms affecting surgical outcomes. Cornea 2005, 24, S2–S11. [Google Scholar] [CrossRef]
- Murphy, C.; Alvarado, J.; Juster, R. Prenatal and postnatal growth of the human Descemet’s membrane. Investig. Ophthalmol. Vis. Sci. 1984, 25, 1402–1415. [Google Scholar]
- Johnson, D.H.; Bourne, W.M.; Campbell, R.J. The ultrastructure of Descemet’s membrane. I. Changes with age in normal corneas. Arch. Ophthalmol. 1982, 100, 1942–1947. [Google Scholar] [CrossRef]
- Sawada, H.; Konomi, H.; Hirosawa, K. Characterization of the collagen in the hexagonal lattice of Descemet’s membrane: Its relation to type VIII collagen. J. Cell Biol. 1990, 110, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Tervo, T.; Sulonen, J.; Valtones, S.; Vannas, A.; Virtanen, I. Distribution of fibronectin in human and rabbit corneas. Exp. Eye Res. 1986, 42, 399–406. [Google Scholar] [CrossRef]
- Grewal, S.; Laibson, P.R.; Cohen, E.J.; Rapuano, C.J. Acute hydrops in the corneal ectasias: Associated factors and outcomes. Trans. Am. Ophthalmol. Soc. 1999, 97, 187–198, discussion 198–203. [Google Scholar] [CrossRef] [Green Version]
- Maharana, P.K.; Sharma, N.; Vajpayee, R.B. Acute corneal hydrops in keratoconus. Indian J. Ophthalmol. 2013, 61, 461–464. [Google Scholar] [CrossRef] [PubMed]
- Stiemke, M.M.; Edelhauser, H.F.; Geroski, D.H. The developing corneal endothelium: Correlation of morphology, hydration and Na/K ATPase pump site density. Curr. Eye Res. 1991, 10, 145–156. [Google Scholar] [CrossRef] [PubMed]
- Hirsch, M.; Renard, G.; Faure, J.P.; Pouliquen, Y. Study of the ultrastructure of the rabbit corneal endothelium by the freeze-fracture technique: Apical and lateral junctions. Exp. Eye Res. 1977, 25, 277–288. [Google Scholar] [CrossRef]
- Ottersen, O.P.; Vegge, T. Ultrastructure and distribution of intercellular junctions in corneal endothelium. Acta Ophthalmol. 1977, 55, 69–78. [Google Scholar] [CrossRef]
- Amann, J.; Holley, G.P.; Lee, S.B.; Edelhauser, H.F. Increased endothelial cell density in the paracentral and peripheral regions of the human cornea. Am. J. Ophthalmol. 2003, 135, 584–590. [Google Scholar] [CrossRef]
- Joyce, N.C. Proliferative capacity of the corneal endothelium. Prog. Retin. Eye Res. 2003, 22, 359–389. [Google Scholar] [CrossRef]
- Joyce, N.C. Cell cycle status in human corneal endothelium. Exp. Eye Res. 2005, 81, 629–638. [Google Scholar] [CrossRef]
- Yee, R.W.; Matsuda, M.; Schultz, R.O.; Edelhauser, H.F. Changes in the normal corneal endothelial cellular pattern as a function of age. Curr. Eye Res. 1985, 4, 671–678. [Google Scholar] [CrossRef] [PubMed]
- Bourne, W.M.; Nelson, L.R.; Hodge, D.O. Central corneal endothelial cell changes over a ten-year period. Investig. Ophthalmol. Vis. Sci. 1997, 38, 779–782. [Google Scholar]
- Abib, F.C.; Barreto Junior, J. Behavior of corneal endothelial density over a lifetime. J. Cataract. Refract. Surg. 2001, 27, 1574–1578. [Google Scholar] [CrossRef]
- Geroski, D.H.; Matsuda, M.; Yee, R.W.; Edelhauser, H.F. Pump function of the human corneal endothelium. Effects of age and cornea guttata. Ophthalmology 1985, 92, 759–763. [Google Scholar] [CrossRef]
- Watsky, M.A.; McDermott, M.L.; Edelhauser, H.F. In vitro corneal endothelial permeability in rabbit and human: The effects of age, cataract surgery and diabetes. Exp. Eye Res. 1989, 49, 751–767. [Google Scholar] [CrossRef]
- Flaxman, S.R.; Bourne, R.R.A.; Resnikoff, S.; Ackland, P.; Braithwaite, T.; Cicinelli, M.V.; Das, A.; Jonas, J.B.; Keeffe, J.; Kempen, J.H.; et al. Global causes of blindness and distance vision impairment 1990–2020: A systematic review and meta-analysis. Lancet Glob. Health 2017, 5, e1221–e1234. [Google Scholar] [CrossRef] [Green Version]
- Gain, P.; Jullienne, R.; He, Z.; Aldossary, M.; Acquart, S.; Cognasse, F.; Thuret, G. Global Survey of Corneal Transplantation and Eye Banking. JAMA Ophthalmol. 2016, 134, 167–173. [Google Scholar] [CrossRef] [Green Version]
- Oliva, M.S.; Schottman, T.; Gulati, M. Turning the tide of corneal blindness. Indian J. Ophthalmol. 2012, 60, 423–427. [Google Scholar] [CrossRef]
- Mathews, P.M.; Lindsley, K.; Aldave, A.J.; Akpek, E.K. Etiology of Global Corneal Blindness and Current Practices of Corneal Transplantation: A Focused Review. Cornea 2018, 37, 1198–1203. [Google Scholar] [CrossRef]
- Whitcher, J.P.; Srinivasan, M.; Upadhyay, M.P. Corneal blindness: A global perspective. Bull. World Health Organ. 2001, 79, 214–221. [Google Scholar]
- Wilson, S.L.; El Haj, A.J.; Yang, Y. Control of scar tissue formation in the cornea: Strategies in clinical and corneal tissue engineering. J. Funct. Biomater. 2012, 3, 642–687. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guilbert, E.; Bullet, J.; Sandali, O.; Basli, E.; Laroche, L.; Borderie, V.M. Long-term rejection incidence and reversibility after penetrating and lamellar keratoplasty. Am. J. Ophthalmol. 2013, 155, 560–569.e562. [Google Scholar] [CrossRef] [PubMed]
- Williams, K.A.; Lowe, M.; Bartlett, C.; Kelly, T.L.; Coster, D.J.; All, C. Risk factors for human corneal graft failure within the Australian corneal graft registry. Transplantation 2008, 86, 1720–1724. [Google Scholar] [CrossRef] [PubMed]
- Tan, D.T.; Janardhanan, P.; Zhou, H.; Chan, Y.H.; Htoon, H.M.; Ang, L.P.; Lim, L.S. Penetrating keratoplasty in Asian eyes: The Singapore Corneal Transplant Study. Ophthalmology 2008, 115, 975–982.e971. [Google Scholar] [CrossRef] [PubMed]
- Price, D.A.; Kelley, M.; Price, F.W., Jr.; Price, M.O. Five-Year Graft Survival of Descemet Membrane Endothelial Keratoplasty (EK) versus Descemet Stripping EK and the Effect of Donor Sex Matching. Ophthalmology 2018, 125, 1508–1514. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weisbrod, D.J.; Sit, M.; Naor, J.; Slomovic, A.R. Outcomes of repeat penetrating keratoplasty and risk factors for graft failure. Cornea 2003, 22, 429–434. [Google Scholar] [CrossRef] [PubMed]
- Yu, A.L.; Kaiser, M.; Schaumberger, M.; Messmer, E.; Kook, D.; Welge-Lussen, U. Donor-related risk factors and preoperative recipient-related risk factors for graft failure. Cornea 2014, 33, 1149–1156. [Google Scholar] [CrossRef] [Green Version]
- Gupta, N.; Vashist, P.; Ganger, A.; Tandon, R.; Gupta, S.K. Eye donation and eye banking in India. Natl. Med. J. India 2018, 31, 283–286. [Google Scholar] [CrossRef]
- Schrage, N.; Hille, K.; Cursiefen, C. Current treatment options with artificial corneas: Boston Kpro, Osteo-odontokeratoprosthesis, Miro Cornea(R) and KeraKlear(R). Ophthalmologe 2014, 111, 1010–1018. [Google Scholar] [CrossRef]
- Jiraskova, N.; Rozsival, P.; Burova, M.; Kalfertova, M. AlphaCor artificial cornea: Clinical outcome. Eye 2011, 25, 1138–1146. [Google Scholar] [CrossRef] [Green Version]
- Studeny, P.; Krizova, D.; Kuchynka, P. Use of PocketMaker Microkeratome for Creation of Corneal Pocket for Foldable Keratoprosthesis KeraKlear Implantation—Case Series. Open Ophthalmol. J. 2015, 9, 126–130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Casey, T.A. Osteo-odonto-keratoprosthesis. Proc. R. Soc. Med. 1966, 59, 530–531. [Google Scholar] [PubMed]
- Zerbe, B.L.; Belin, M.W.; Ciolino, J.B.; Boston Type 1 Keratoprosthesis Study Group. Results from the multicenter Boston Type 1 Keratoprosthesis Study. Ophthalmology 2006, 113, 1779–1784.e1. [Google Scholar] [CrossRef] [PubMed]
- Falcinelli, G.; Falsini, B.; Taloni, M.; Colliardo, P.; Falcinelli, G. Modified osteo-odonto-keratoprosthesis for treatment of corneal blindness: Long-term anatomical and functional outcomes in 181 cases. Arch Ophthalmol. 2005, 123, 1319–1329. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carlsson, D.J.; Li, F.; Shimmura, S.; Griffith, M. Bioengineered corneas: How close are we? Curr. Opin. Ophthalmol. 2003, 14, 192–197. [Google Scholar] [CrossRef]
- Li, F.; Carlsson, D.; Lohmann, C.; Suuronen, E.; Vascotto, S.; Kobuch, K.; Sheardown, H.; Munger, R.; Nakamura, M.; Griffith, M. Cellular and nerve regeneration within a biosynthetic extracellular matrix for corneal transplantation. Proc. Natl. Acad. Sci. USA 2003, 100, 15346–15351. [Google Scholar] [CrossRef] [Green Version]
- Merrett, K.; Fagerholm, P.; McLaughlin, C.R.; Dravida, S.; Lagali, N.; Shinozaki, N.; Watsky, M.A.; Munger, R.; Kato, Y.; Li, F.; et al. Tissue-engineered recombinant human collagen-based corneal substitutes for implantation: Performance of type I versus type III collagen. Investig. Ophthalmol. Vis. Sci. 2008, 49, 3887–3894. [Google Scholar] [CrossRef]
- Ihanamaki, T.; Pelliniemi, L.J.; Vuorio, E. Collagens and collagen-related matrix components in the human and mouse eye. Prog. Retin. Eye Res. 2004, 23, 403–434. [Google Scholar] [CrossRef]
- McLaughlin, C.R.; Acosta, M.C.; Luna, C.; Liu, W.; Belmonte, C.; Griffith, M.; Gallar, J. Regeneration of functional nerves within full thickness collagen-phosphorylcholine corneal substitute implants in guinea pigs. Biomaterials 2010, 31, 2770–2778. [Google Scholar] [CrossRef]
- Liu, W.; Deng, C.; McLaughlin, C.R.; Fagerholm, P.; Lagali, N.S.; Heyne, B.; Scaiano, J.C.; Watsky, M.A.; Kato, Y.; Munger, R.; et al. Collagen-phosphorylcholine interpenetrating network hydrogels as corneal substitutes. Biomaterials 2009, 30, 1551–1559. [Google Scholar] [CrossRef]
- Pellegrini, G.; Traverso, C.E.; Franzi, A.T.; Zingirian, M.; Cancedda, R.; De Luca, M. Long-term restoration of damaged corneal surfaces with autologous cultivated corneal epithelium. Lancet 1997, 349, 990–993. [Google Scholar] [CrossRef]
- Rama, P.; Bonini, S.; Lambiase, A.; Golisano, O.; Paterna, P.; De Luca, M.; Pellegrini, G. Autologous fibrin-cultured limbal stem cells permanently restore the corneal surface of patients with total limbal stem cell deficiency. Transplantation 2001, 72, 1478–1485. [Google Scholar] [CrossRef] [PubMed]
- Rama, P.; Matuska, S.; Paganoni, G.; Spinelli, A.; De Luca, M.; Pellegrini, G. Limbal stem-cell therapy and long-term corneal regeneration. N. Engl. J. Med. 2010, 363, 147–155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jacob, S.; Dhawan, P.; Tsatsos, M.; Agarwal, A.; Narasimhan, S.; Kumar, A. Fibrin Glue-Assisted Closure of Macroperforation in Predescemetic Deep Anterior Lamellar Keratoplasty with a Donor Obtained From Small Incision Lenticule Extraction. Cornea 2019. [Google Scholar] [CrossRef]
- Elhamaky, T.R.; Elbarky, A.M. Outcomes of Vertical Split Conjunctival Autograft Using Fibrin Glue in Treatment of Primary Double-Headed Pterygia. J. Ophthalmol. 2018, 2018, 9341846. [Google Scholar] [CrossRef] [Green Version]
- Yeung, A.M.; Faraj, L.A.; McIntosh, O.D.; Dhillon, V.K.; Dua, H.S. Fibrin glue inhibits migration of ocular surface epithelial cells. Eye 2016, 30, 1389–1394. [Google Scholar] [CrossRef] [Green Version]
- Dereli Can, G.; Akdere, O.E.; Can, M.E.; Aydin, B.; Cagil, N.; Gumusderelioglu, M. A completely human-derived biomaterial mimicking limbal niche: Platelet-rich fibrin gel. Exp. Eye Res. 2018, 173. [Google Scholar] [CrossRef]
- Meyer-Blazejewska, E.A.; Kruse, F.E.; Bitterer, K.; Meyer, C.; Hofmann-Rummelt, C.; Wunsch, P.H.; Schlotzer-Schrehardt, U. Preservation of the limbal stem cell phenotype by appropriate culture techniques. Investig. Ophthalmol. Vis. Sci. 2010, 51, 765–774. [Google Scholar] [CrossRef]
- Kesting, M.R.; Wolff, K.D.; Nobis, C.P.; Rohleder, N.H. Amniotic membrane in oral and maxillofacial surgery. Oral. Maxillofac. Surg. 2014, 18, 153–164. [Google Scholar] [CrossRef]
- Keene, D.R.; Sakai, L.Y.; Lunstrum, G.P.; Morris, N.P.; Burgeson, R.E. Type VII collagen forms an extended network of anchoring fibrils. J. Cell Biol. 1987, 104, 611–621. [Google Scholar] [CrossRef] [Green Version]
- Boudreau, N.; Sympson, C.J.; Werb, Z.; Bissell, M.J. Suppression of ICE and apoptosis in mammary epithelial cells by extracellular matrix. Science 1995, 267, 891–893. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Solomon, A.; Rosenblatt, M.; Monroy, D.; Ji, Z.; Pflugfelder, S.C.; Tseng, S.C. Suppression of interleukin 1alpha and interleukin 1beta in human limbal epithelial cells cultured on the amniotic membrane stromal matrix. Br. J. Ophthalmol. 2001, 85, 444–449. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hao, Y.; Ma, D.H.; Hwang, D.G.; Kim, W.S.; Zhang, F. Identification of antiangiogenic and antiinflammatory proteins in human amniotic membrane. Cornea 2000, 19, 348–352. [Google Scholar] [CrossRef] [PubMed]
- Galask, R.P.; Snyder, I.S. Antimicrobial factors in amniotic fluid. Am. J. Obstet. Gynecol. 1970, 106, 59–65. [Google Scholar] [CrossRef]
- Houlihan, J.M.; Biro, P.A.; Harper, H.M.; Jenkinson, H.J.; Holmes, C.H. The human amnion is a site of MHC class Ib expression: Evidence for the expression of HLA-E and HLA-G. J. Immunol. 1995, 154, 5665–5674. [Google Scholar]
- Akle, C.A.; Adinolfi, M.; Welsh, K.I.; Leibowitz, S.; McColl, I. Immunogenicity of human amniotic epithelial cells after transplantation into volunteers. Lancet 1981, 2, 1003–1005. [Google Scholar] [CrossRef]
- Berguiga, M.; Mameletzi, E.; Nicolas, M.; Rivier, D.; Majo, F. Long-term follow-up of multilayer amniotic membrane transplantation (MLAMT) for non-traumatic corneal perforations or deep ulcers with descemetocele. Klin. Monbl. Augenheilkd. 2013, 230, 413–418. [Google Scholar] [CrossRef]
- Siu, G.D.; Young, A.L.; Cheng, L.L. Long-term symptomatic relief of bullous keratopathy with amniotic membrane transplant. Int. Ophthalmol. 2015, 35, 777–783. [Google Scholar] [CrossRef]
- Anderson, D.F.; Prabhasawat, P.; Alfonso, E.; Tseng, S.C. Amniotic membrane transplantation after the primary surgical management of band keratopathy. Cornea 2001, 20, 354–361. [Google Scholar] [CrossRef]
- Sharma, N.; Mohanty, S.; Jhanji, V.; Vajpayee, R.B. Amniotic membrane transplantation with or without autologous cultivated limbal stem cell transplantation for the management of partial limbal stem cell deficiency. Clin. Ophthalmol. 2018, 12, 2103–2106. [Google Scholar] [CrossRef] [Green Version]
- Shortt, A.J.; Bunce, C.; Levis, H.J.; Blows, P.; Dore, C.J.; Vernon, A.; Secker, G.A.; Tuft, S.J.; Daniels, J.T. Three-year outcomes of cultured limbal epithelial allografts in aniridia and Stevens-Johnson syndrome evaluated using the Clinical Outcome Assessment in Surgical Trials assessment tool. Stem Cells Transl. Med. 2014, 3, 265–275. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, T.; Inatomi, T.; Sotozono, C.; Amemiya, T.; Kanamura, N.; Kinoshita, S. Transplantation of cultivated autologous oral mucosal epithelial cells in patients with severe ocular surface disorders. Br. J. Ophthalmol. 2004, 88, 1280–1284. [Google Scholar] [CrossRef] [PubMed]
- McDermott, A.M. Defensins and other antimicrobial peptides at the ocular surface. Ocul. Surf. 2004, 2, 229–247. [Google Scholar] [CrossRef] [Green Version]
- Chen, H.C.; Chen, H.L.; Lai, J.Y.; Chen, C.C.; Tsai, Y.J.; Kuo, M.T.; Chu, P.H.; Sun, C.C.; Chen, J.K.; Ma, D.H. Persistence of transplanted oral mucosal epithelial cells in human cornea. Investig. Ophthalmol. Vis. Sci. 2009, 50, 4660–4668. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gaddipati, S.; Muralidhar, R.; Sangwan, V.S.; Mariappan, I.; Vemuganti, G.K.; Balasubramanian, D. Oral epithelial cells transplanted on to corneal surface tend to adapt to the ocular phenotype. Indian J. Ophthalmol. 2014, 62, 644–648. [Google Scholar] [CrossRef] [PubMed]
- Ricardo, J.R.; Cristovam, P.C.; Filho, P.A.; Farias, C.C.; de Araujo, A.L.; Loureiro, R.R.; Covre, J.L.; de Barros, J.N.; Barreiro, T.P.; dos Santos, M.S.; et al. Transplantation of conjunctival epithelial cells cultivated ex vivo in patients with total limbal stem cell deficiency. Cornea 2013, 32, 221–228. [Google Scholar] [CrossRef] [PubMed]
- Meinel, L.; Hofmann, S.; Karageorgiou, V.; Kirker-Head, C.; McCool, J.; Gronowicz, G.; Zichner, L.; Langer, R.; Vunjak-Novakovic, G.; Kaplan, D.L. The inflammatory responses to silk films in vitro and in vivo. Biomaterials 2005, 26, 147–155. [Google Scholar] [CrossRef]
- Wang, Y.; Rudym, D.D.; Walsh, A.; Abrahamsen, L.; Kim, H.J.; Kim, H.S.; Kirker-Head, C.; Kaplan, D.L. In vivo degradation of three-dimensional silk fibroin scaffolds. Biomaterials 2008, 29, 3415–3428. [Google Scholar] [CrossRef] [Green Version]
- Hannah, P.; Gopinath, A.; Kaplan, D.L.; Negro, L.D.; Omenetto, F.G. Nano- and Micropatterning of Optically Transparent, Mechanically Robust, Biocompatible Silk Fibroin Films. Adv. Mater. 2008, 20, 3070–3072. [Google Scholar]
- Omenetto, F.G.; Kaplan, D.L. New opportunities for an ancient material. Science 2010, 329, 528–531. [Google Scholar] [CrossRef] [Green Version]
- Muffly, T.M.; Tizzano, A.P.; Walters, M.D. The history and evolution of sutures in pelvic surgery. J. R. Soc. Med. 2011, 104, 107–112. [Google Scholar] [CrossRef] [PubMed]
- Servoli, E.; Maniglio, D.; Motta, A.; Predazzer, R.; Migliaresi, C. Surface properties of silk fibroin films and their interaction with fibroblasts. Macromol. Biosci. 2005, 5, 1175–1183. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, B.D.; Marchant, J.K.; Pindrus, M.A.; Omenetto, F.G.; Kaplan, D.L. Silk film biomaterials for cornea tissue engineering. Biomaterials 2009, 30, 1299–1308. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gil, E.S.; Park, S.H.; Marchant, J.; Omenetto, F.; Kaplan, D.L. Response of human corneal fibroblasts on silk film surface patterns. Macromol. Biosci. 2010, 10, 664–673. [Google Scholar] [CrossRef] [Green Version]
- Gupta, M.K.; Khokhar, S.K.; Phillips, D.M.; Sowards, L.A.; Drummy, L.F.; Kadakia, M.P.; Naik, R.R. Patterned silk films cast from ionic liquid solubilized fibroin as scaffolds for cell growth. Langmuir 2007, 23, 1315–1319. [Google Scholar] [CrossRef]
- Harkin, D.G.; George, K.A.; Madden, P.W.; Schwab, I.R.; Hutmacher, D.W.; Chirila, T.V. Silk fibroin in ocular tissue reconstruction. Biomaterials 2011, 32, 2445–2458. [Google Scholar] [CrossRef]
- Wang, L.; Ma, R.; Du, G.; Guo, H.; Huang, Y. Biocompatibility of helicoidal multilamellar arginine-glycine-aspartic acid-functionalized silk biomaterials in a rabbit corneal model. J. Biomed. Mater. Res. B Appl. Biomater. 2015, 103, 204–211. [Google Scholar] [CrossRef]
- Guan, L.; Ge, H.; Tang, X.; Su, S.; Tian, P.; Xiao, N.; Zhang, H.; Zhang, L.; Liu, P. Use of a silk fibroin-chitosan scaffold to construct a tissue-engineered corneal stroma. Cells Tissues Organs 2013, 198, 190–197. [Google Scholar] [CrossRef]
- Gil, E.S.; Mandal, B.B.; Park, S.H.; Marchant, J.K.; Omenetto, F.G.; Kaplan, D.L. Helicoidal multi-lamellar features of RGD-functionalized silk biomaterials for corneal tissue engineering. Biomaterials 2010, 31, 8953–8963. [Google Scholar] [CrossRef] [Green Version]
- Higa, K.; Takeshima, N.; Moro, F.; Kawakita, T.; Kawashima, M.; Demura, M.; Shimazaki, J.; Asakura, T.; Tsubota, K.; Shimmura, S. Porous silk fibroin film as a transparent carrier for cultivated corneal epithelial sheets. J. Biomater. Sci. Polym. Ed. 2011, 22, 2261–2276. [Google Scholar] [CrossRef]
- Wang, S.; Ghezzi, C.E.; Gomes, R.; Pollard, R.E.; Funderburgh, J.L.; Kaplan, D.L. In vitro 3D corneal tissue model with epithelium, stroma, and innervation. Biomaterials 2017, 112, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vazquez, N.; Rodriguez-Barrientos, C.A.; Aznar-Cervantes, S.D.; Chacon, M.; Cenis, J.L.; Riestra, A.C.; Sanchez-Avila, R.M.; Persinal, M.; Brea-Pastor, A.; Fernandez-Vega Cueto, L.; et al. Silk Fibroin Films for Corneal Endothelial Regeneration: Transplant in a Rabbit Descemet Membrane Endothelial Keratoplasty. Investig. Ophthalmol. Vis. Sci. 2017, 58, 3357–3365. [Google Scholar] [CrossRef] [PubMed]
- Shadforth, A.M.A.; Suzuki, S.; Theodoropoulos, C.; Richardson, N.A.; Chirila, T.V.; Harkin, D.G. A Bruch’s membrane substitute fabricated from silk fibroin supports the function of retinal pigment epithelial cells in vitro. J. Tissue Eng. Regen. Med. 2017, 11, 1915–1924. [Google Scholar] [CrossRef] [PubMed]
- Tran, S.H.; Wilson, C.G.; Seib, F.P. A Review of the Emerging Role of Silk for the Treatment of the Eye. Pharm. Res. 2018, 35, 248. [Google Scholar] [CrossRef] [Green Version]
- Chirila, T.V.; Suzuki, S.; Bray, L.J.; Barnett, N.L.; Harkin, D.G. Evaluation of silk sericin as a biomaterial: In vitro growth of human corneal limbal epithelial cells on Bombyx mori sericin membranes. Prog. Biomater. 2013, 2, 14. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, T.A.; Aljaeid, B.M. Preparation, characterization, and potential application of chitosan, chitosan derivatives, and chitosan metal nanoparticles in pharmaceutical drug delivery. Drug Des. Devel. Ther. 2016, 10, 483–507. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fonseca-Santos, B.; Chorilli, M. An overview of carboxymethyl derivatives of chitosan: Their use as biomaterials and drug delivery systems. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 77, 1349–1362. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Irimia, T.; Dinu-Pirvu, C.E.; Ghica, M.V.; Lupuleasa, D.; Muntean, D.L.; Udeanu, D.I.; Popa, L. Chitosan-Based In Situ Gels for Ocular Delivery of Therapeutics: A State-of-the-Art Review. Mar. Drugs 2018, 16, 373. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yeh, L.K.; Chen, Y.H.; Chiu, C.S.; Hu, F.R.; Young, T.H.; Wang, I.J. The phenotype of bovine corneal epithelial cells on chitosan membrane. J. Biomed. Mater. Res. A 2009, 90, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Liu, W.; Han, B.; Yang, C.; Ma, Q.; Song, F.; Bi, Q. An in situ formed biodegradable hydrogel for reconstruction of the corneal endothelium. Colloids Surf. B Biointerfaces 2011, 82. [Google Scholar] [CrossRef]
- Liang, Y.; Xu, W.; Han, B.; Li, N.; Zhao, W.; Liu, W. Tissue-engineered membrane based on chitosan for repair of mechanically damaged corneal epithelium. J. Mater. Sci. Mater. Med. 2014, 25, 2163–2171. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Liu, K.; Li, T.; Zhang, W.; Dong, Y.; Lv, J.; Wang, W.; Sun, J.; Li, M.; Wang, M.; et al. An in situ hydrogel based on carboxymethyl chitosan and sodium alginate dialdehyde for corneal wound healing after alkali burn. J. Biomed. Mater. Res. A 2019, 107, 742–754. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, Y.; Hu, Y.; Cai, J.; Ma, S.; Wang, X. Coagulation property of hyaluronic acid-collagen/chitosan complex film. J. Mater. Sci. Mater. Med. 2008, 19, 3621–3629. [Google Scholar] [CrossRef] [PubMed]
- de Mesquita, J.P.; Donnici, C.L.; Pereira, F.V. Biobased nanocomposites from layer-by-layer assembly of cellulose nanowhiskers with chitosan. Biomacromolecules 2010, 11, 473–480. [Google Scholar] [CrossRef]
- Zhou, Y.; Yang, D.; Chen, X.; Xu, Q.; Lu, F.; Nie, J. Electrospun water-soluble carboxyethyl chitosan/poly(vinyl alcohol) nanofibrous membrane as potential wound dressing for skin regeneration. Biomacromolecules 2008, 9, 349–354. [Google Scholar] [CrossRef]
- Li, W.; Long, Y.; Liu, Y.; Long, K.; Liu, S.; Wang, Z.; Wang, Y.; Ren, L. Fabrication and characterization of chitosan-collagen crosslinked membranes for corneal tissue engineering. J. Biomater. Sci. Polym. Ed. 2014, 25, 1962–1972. [Google Scholar] [CrossRef]
- Rafat, M.; Li, F.; Fagerholm, P.; Lagali, N.S.; Watsky, M.A.; Munger, R.; Matsuura, T.; Griffith, M. PEG-stabilized carbodiimide crosslinked collagen-chitosan hydrogels for corneal tissue engineering. Biomaterials 2008, 29, 3960–3972. [Google Scholar] [CrossRef]
- Ye, J.; Shi, X.; Chen, X.; Xie, J.; Wang, C.; Yao, K.; Gao, C.; Gou, Z. Chitosan-modified, collagen-based biomimetic nanofibrous membranes as selective cell adhering wound dressings in the treatment of chemically burned corneas. J. Mater. Chem. B 2014, 2, 4226–4236. [Google Scholar] [CrossRef]
- Meana, A.; Iglesias, J.; Del Rio, M.; Larcher, F.; Madrigal, B.; Fresno, M.F.; Martin, C.; San Roman, F.; Tevar, F. Large surface of cultured human epithelium obtained on a dermal matrix based on live fibroblast-containing fibrin gels. Burns 1998, 24, 621–630. [Google Scholar] [CrossRef]
- Llames, S.G.; Del Rio, M.; Larcher, F.; Garcia, E.; Garcia, M.; Escamez, M.J.; Jorcano, J.L.; Holguin, P.; Meana, A. Human plasma as a dermal scaffold for the generation of a completely autologous bioengineered skin. Transplantation 2004, 77, 350–355. [Google Scholar] [CrossRef]
- Chen, J.; Li, Q.; Xu, J.; Huang, Y.; Ding, Y.; Deng, H.; Zhao, S.; Chen, R. Study on biocompatibility of complexes of collagen-chitosan-sodium hyaluronate and cornea. Artif. Organs 2005, 29, 104–113. [Google Scholar] [CrossRef] [PubMed]
- Aufderheide, A.C.; Athanasiou, K.A. Comparison of scaffolds and culture conditions for tissue engineering of the knee meniscus. Tissue Eng. 2005, 11, 1095–1104. [Google Scholar] [CrossRef] [PubMed]
- Alaminos, M.; Del Carmen Sanchez-Quevedo, M.; Munoz-Avila, J.I.; Serrano, D.; Medialdea, S.; Carreras, I.; Campos, A. Construction of a complete rabbit cornea substitute using a fibrin-agarose scaffold. Investig. Ophthalmol. Vis. Sci. 2006, 47, 3311–3317. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reichl, S.; Bednarz, J.; Muller-Goymann, C.C. Human corneal equivalent as cell culture model for in vitro drug permeation studies. Br. J. Ophthalmol. 2004, 88, 560–565. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cardona Jde, L.; Ionescu, A.M.; Gomez-Sotomayor, R.; Gonzalez-Andrades, M.; Campos, A.; Alaminos, M.; Perez Mdel, M. Transparency in a fibrin and fibrin-agarose corneal stroma substitute generated by tissue engineering. Cornea 2011, 30, 1428–1435. [Google Scholar] [CrossRef]
- Rico-Sanchez, L.; Garzon, I.; Gonzalez-Andrades, M.; Ruiz-Garcia, A.; Punzano, M.; Lizana-Moreno, A.; Munoz-Avila, J.I.; Sanchez-Quevedo, M.C.; Martinez-Atienza, J.; Lopez-Navas, L.; et al. Successful development and clinical translation of a novel anterior lamellar artificial cornea. J. Tissue Eng. Regen. Med. 2019. [Google Scholar] [CrossRef] [Green Version]
- Myung, D.; Koh, W.; Bakri, A.; Zhang, F.; Marshall, A.; Ko, J.; Noolandi, J.; Carrasco, M.; Cochran, J.R.; Frank, C.W.; et al. Design and fabrication of an artificial cornea based on a photolithographically patterned hydrogel construct. Biomed. Microdevices 2007, 9, 911–922. [Google Scholar] [CrossRef]
- Myung, D.; Farooqui, N.; Waters, D.; Schaber, S.; Koh, W.; Carrasco, M.; Noolandi, J.; Frank, C.W.; Ta, C.N. Glucose-permeable interpenetrating polymer network hydrogels for corneal implant applications: A pilot study. Curr. Eye Res. 2008, 33, 29–43. [Google Scholar] [CrossRef]
- Myung, D.; Farooqui, N.; Zheng, L.L.; Koh, W.; Gupta, S.; Bakri, A.; Noolandi, J.; Cochran, J.R.; Frank, C.W.; Ta, C.N. Bioactive interpenetrating polymer network hydrogels that support corneal epithelial wound healing. J. Biomed. Mater. Res. A 2009, 90, 70–81. [Google Scholar] [CrossRef] [Green Version]
- Parke-Houben, R.; Fox, C.H.; Zheng, L.L.; Waters, D.J.; Cochran, J.R.; Ta, C.N.; Frank, C.W. Interpenetrating polymer network hydrogel scaffolds for artificial cornea periphery. J. Mater. Sci. Mater. Med. 2015, 26, 107. [Google Scholar] [CrossRef]
- Tan, X.W.; Hartman, L.; Tan, K.P.; Poh, R.; Myung, D.; Zheng, L.L.; Waters, D.; Noolandi, J.; Beuerman, R.W.; Frank, C.W.; et al. In vivo biocompatibility of two PEG/PAA interpenetrating polymer networks as corneal inlays following deep stromal pocket implantation. J. Mater. Sci. Mater. Med. 2013, 24, 967–977. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hartmann, L.; Watanabe, K.; Zheng, L.L.; Kim, C.Y.; Beck, S.E.; Huie, P.; Noolandi, J.; Cochran, J.R.; Ta, C.N.; Frank, C.W. Toward the development of an artificial cornea: Improved stability of interpenetrating polymer networks. J. Biomed. Mater. Res. B Appl. Biomater. 2011, 98, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.L.; Vanchinathan, V.; Dalal, R.; Noolandi, J.; Waters, D.J.; Hartmann, L.; Cochran, J.R.; Frank, C.W.; Yu, C.Q.; Ta, C.N. Biocompatibility of poly(ethylene glycol) and poly(acrylic acid) interpenetrating network hydrogel by intrastromal implantation in rabbit cornea. J. Biomed. Mater. Res. A 2015, 103, 3157–3165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zieske, J.D.; Mason, V.S.; Wasson, M.E.; Meunier, S.F.; Nolte, C.J.; Fukai, N.; Olsen, B.R.; Parenteau, N.L. Basement membrane assembly and differentiation of cultured corneal cells: Importance of culture environment and endothelial cell interaction. Exp. Cell Res. 1994, 214, 621–633. [Google Scholar] [CrossRef]
- Ren, R.; Hutcheon, A.E.; Guo, X.Q.; Saeidi, N.; Melotti, S.A.; Ruberti, J.W.; Zieske, J.D.; Trinkaus-Randall, V. Human primary corneal fibroblasts synthesize and deposit proteoglycans in long-term 3-D cultures. Dev. Dyn. 2008, 237, 2705–2715. [Google Scholar] [CrossRef] [Green Version]
- Guo, X.; Hutcheon, A.E.; Melotti, S.A.; Zieske, J.D.; Trinkaus-Randall, V.; Ruberti, J.W. Morphologic characterization of organized extracellular matrix deposition by ascorbic acid-stimulated human corneal fibroblasts. Investig. Ophthalmol. Vis. Sci. 2007, 48, 4050–4060. [Google Scholar] [CrossRef]
- Priyadarsini, S.; Sarker-Nag, A.; Rowsey, T.G.; Ma, J.X.; Karamichos, D. Establishment of a 3D In Vitro Model to Accelerate the Development of Human Therapies against Corneal Diabetes. PLoS ONE 2016, 11, e0168845. [Google Scholar] [CrossRef]
- Karamichos, D.; Zareian, R.; Guo, X.; Hutcheon, A.E.; Ruberti, J.W.; Zieske, J.D. Novel in Vitro Model for Keratoconus Disease. J. Funct. Biomater. 2012, 3, 760–775. [Google Scholar] [CrossRef] [Green Version]
- Priyadarsini, S.; Nicholas, S.E.; Karamichos, D. 3D Stacked Construct: A Novel Substitute for Corneal Tissue Engineering. Methods Mol. Biol. 2018, 1697, 173–180. [Google Scholar] [CrossRef]
- McKay, T.B.; Karamichos, D.; Hutcheon, A.E.K.; Guo, X.; Zieske, J.D. Corneal Epithelial-Stromal Fibroblast Constructs to Study Cell-Cell Communication in Vitro. Bioengineering 2019, 6, 110. [Google Scholar] [CrossRef] [Green Version]
- Hutcheon, A.E.K.; Zieske, J.D.; Guo, X. 3D in vitro model for human corneal endothelial cell maturation. Exp. Eye Res. 2019, 184, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Theriault, M.; Gendron, S.P.; Brunette, I.; Rochette, P.J.; Proulx, S. Function-Related Protein Expression in Fuchs Endothelial Corneal Dystrophy Cells and Tissue Models. Am. J. Pathol. 2018, 188, 1703–1712. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peh, G.S.; Chng, Z.; Ang, H.P.; Cheng, T.Y.; Adnan, K.; Seah, X.Y.; George, B.L.; Toh, K.P.; Tan, D.T.; Yam, G.H.; et al. Propagation of human corneal endothelial cells: A novel dual media approach. Cell Transplant. 2015, 24, 287–304. [Google Scholar] [CrossRef] [PubMed]
- Stoesser, T.R.; Church, R.L.; Brown, S.I. Partial characterization of human collagen and procollagen secreted by human corneal stromal fibroblasts in cell culture. Investig. Ophthalmol. Vis. Sci. 1978, 17, 264–271. [Google Scholar]
- L’Heureux, N.; Paquet, S.; Labbe, R.; Germain, L.; Auger, F.A. A completely biological tissue-engineered human blood vessel. FASEB J. 1998, 12, 47–56. [Google Scholar] [CrossRef]
- Michel, M.; L’Heureux, N.; Pouliot, R.; Xu, W.; Auger, F.A.; Germain, L. Characterization of a new tissue-engineered human skin equivalent with hair. In Vitro Cell Dev. Biol. Anim. 1999, 35, 318–326. [Google Scholar] [CrossRef]
- Russell, S.B.; Russell, J.D.; Trupin, K.M. Collagen synthesis in human fibroblasts: Effects of ascorbic acid and regulation by hydrocortisone. J. Cell. Physiol. 1981, 109, 121–131. [Google Scholar] [CrossRef]
- Hata, R.; Senoo, H. L-ascorbic acid 2-phosphate stimulates collagen accumulation, cell proliferation, and formation of a three-dimensional tissuelike substance by skin fibroblasts. J. Cell. Physiol. 1989, 138, 8–16. [Google Scholar] [CrossRef]
- Geesin, J.C.; Darr, D.; Kaufman, R.; Murad, S.; Pinnell, S.R. Ascorbic acid specifically increases type I and type III procollagen messenger RNA levels in human skin fibroblast. J. Investig. Dermatol. 1988, 90, 420–424. [Google Scholar] [CrossRef] [Green Version]
- Chan, D.; Lamande, S.R.; Cole, W.G.; Bateman, J.F. Regulation of procollagen synthesis and processing during ascorbate-induced extracellular matrix accumulation in vitro. Biochem. J. 1990, 269, 175–181. [Google Scholar] [CrossRef]
- Dube, J.; Bourget, J.M.; Gauvin, R.; Lafrance, H.; Roberge, C.J.; Auger, F.A.; Germain, L. Progress in developing a living human tissue-engineered tri-leaflet heart valve assembled from tissue produced by the self-assembly approach. Acta Biomater. 2014, 10, 3563–3570. [Google Scholar] [CrossRef] [PubMed]
- Laflamme, K.; Roberge, C.J.; Labonte, J.; Pouliot, S.; D’Orleans-Juste, P.; Auger, F.A.; Germain, L. Tissue-engineered human vascular media with a functional endothelin system. Circulation 2005, 111, 459–464. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vermette, M.; Trottier, V.; Menard, V.; Saint-Pierre, L.; Roy, A.; Fradette, J. Production of a new tissue-engineered adipose substitute from human adipose-derived stromal cells. Biomaterials 2007, 28, 2850–2860. [Google Scholar] [CrossRef] [PubMed]
- Carrier, P.; Deschambeault, A.; Talbot, M.; Giasson, C.J.; Auger, F.A.; Guerin, S.L.; Germain, L. Characterization of wound reepithelialization using a new human tissue-engineered corneal wound healing model. Investig. Ophthalmol. Vis. Sci. 2008, 49, 1376–1385. [Google Scholar] [CrossRef]
- Magnan, M.; Levesque, P.; Gauvin, R.; Dube, J.; Barrieras, D.; El-Hakim, A.; Bolduc, S. Tissue engineering of a genitourinary tubular tissue graft resistant to suturing and high internal pressures. Tissue Eng. Part A 2009, 15, 197–202. [Google Scholar] [CrossRef] [Green Version]
- Prunieras, M.; Regnier, M.; Fougere, S.; Woodley, D. Keratinocytes synthesize basal-lamina proteins in culture. J. Investig. Dermatol. 1983, 81, 74s–81s. [Google Scholar] [CrossRef] [Green Version]
- Proulx, S.; d’Arc Uwamaliya, J.; Carrier, P.; Deschambeault, A.; Audet, C.; Giasson, C.J.; Guerin, S.L.; Auger, F.A.; Germain, L. Reconstruction of a human cornea by the self-assembly approach of tissue engineering using the three native cell types. Mol. Vis. 2010, 16, 2192–2201. [Google Scholar]
- Pedersen, I.B.; Ivarsen, A.; Hjortdal, J. Graft rejection and failure following endothelial keratoplasty (DSAEK) and penetrating keratoplasty for secondary endothelial failure. Acta Ophthalmol. 2015, 93, 172–177. [Google Scholar] [CrossRef] [Green Version]
- Heinzelmann, S.; Bohringer, D.; Eberwein, P.; Lapp, T.; Reinhard, T.; Maier, P. Descemet membrane endothelial keratoplasty for graft failure following penetrating keratoplasty. Graefes Arch. Clin. Exp. Ophthalmol. 2017, 255, 979–985. [Google Scholar] [CrossRef]
- Chakrabarty, K.; Shetty, R.; Ghosh, A. Corneal cell therapy: With iPSCs, it is no more a far-sight. Stem Cell Res. Ther. 2018, 9, 287. [Google Scholar] [CrossRef] [Green Version]
- Couture, C.; Desjardins, P.; Zaniolo, K.; Germain, L.; Guerin, S.L. Enhanced wound healing of tissue-engineered human corneas through altered phosphorylation of the CREB and AKT signal transduction pathways. Acta Biomater. 2018, 73, 312–325. [Google Scholar] [CrossRef] [PubMed]
- Lake, J.; Zaniolo, K.; Gaudreault, M.; Carrier, P.; Deschambault, A.; Bazin, R.; Germain, L.; Salesse, C.; Guerin, S.L. Expression of the alpha5 integrin gene in corneal epithelial cells cultured on tissue-engineered human extracellular matrices. Biomaterials 2013, 34, 6367–6376. [Google Scholar] [CrossRef] [PubMed]
- Couture, C.; Zaniolo, K.; Carrier, P.; Lake, J.; Patenaude, J.; Germain, L.; Guerin, S.L. The tissue-engineered human cornea as a model to study expression of matrix metalloproteinases during corneal wound healing. Biomaterials 2016, 78, 86–101. [Google Scholar] [CrossRef]
- Guillemette, M.D.; Cui, B.; Roy, E.; Gauvin, R.; Giasson, C.J.; Esch, M.B.; Carrier, P.; Deschambeault, A.; Dumoulin, M.; Toner, M.; et al. Surface topography induces 3D self-orientation of cells and extracellular matrix resulting in improved tissue function. Integr. Biol. 2009, 1, 196–204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carrier, P.; Deschambeault, A.; Audet, C.; Talbot, M.; Gauvin, R.; Giasson, C.J.; Auger, F.A.; Guerin, S.L.; Germain, L. Impact of cell source on human cornea reconstructed by tissue engineering. Investig. Ophthalmol. Vis. Sci. 2009, 50, 2645–2652. [Google Scholar] [CrossRef] [Green Version]
- Zaniolo, K.; Carrier, P.; Guerin, S.L.; Auger, F.A.; Germain, L. A tissue-engineered corneal wound healing model for the characterization of reepithelialization. Methods Mol. Biol. 2013, 1037, 59–78. [Google Scholar] [CrossRef]
- Rheinwald, J.G.; Green, H. Serial cultivation of strains of human epidermal keratinocytes: The formation of keratinizing colonies from single cells. Cell 1975, 6, 331–343. [Google Scholar] [CrossRef]
- Kruse, F.E.; Tseng, S.C. Growth factors modulate clonal growth and differentiation of cultured rabbit limbal and corneal epithelium. Investig. Ophthalmol. Vis. Sci. 1993, 34, 1963–1976. [Google Scholar]
- Tseng, S.C.; Kruse, F.E.; Merritt, J.; Li, D.Q. Comparison between serum-free and fibroblast-cocultured single-cell clonal culture systems: Evidence showing that epithelial anti-apoptotic activity is present in 3T3 fibroblast-conditioned media. Curr. Eye Res. 1996, 15, 973–984. [Google Scholar] [CrossRef]
- Balasubramanian, S.; Jasty, S.; Sitalakshmi, G.; Madhavan, H.N.; Krishnakumar, S. Influence of feeder layer on the expression of stem cell markers in cultured limbal corneal epithelial cells. Indian J. Med. Res. 2008, 128, 616–622. [Google Scholar]
- Martin, M.J.; Muotri, A.; Gage, F.; Varki, A. Human embryonic stem cells express an immunogenic nonhuman sialic acid. Nat. Med. 2005, 11, 228–232. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.X.; Gao, X.W.; Ren, B.; Cai, Y.; Li, W.J.; Yang, Y.L.; Li, Y.J. Comparative analysis of different feeder layers with 3T3 fibroblasts for culturing rabbits limbal stem cells. Int. J. Ophthalmol. 2017, 10, 1021–1027. [Google Scholar] [CrossRef] [PubMed]
- Mendoza, R.; Vaughan, A.E.; Miller, A.D. The left half of the XMRV retrovirus is present in an endogenous retrovirus of NIH/3T3 Swiss mouse cells. J. Virol. 2011, 85, 9247–9248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Omoto, M.; Miyashita, H.; Shimmura, S.; Higa, K.; Kawakita, T.; Yoshida, S.; McGrogan, M.; Shimazaki, J.; Tsubota, K. The use of human mesenchymal stem cell-derived feeder cells for the cultivation of transplantable epithelial sheets. Investig. Ophthalmol. Vis. Sci. 2009, 50, 2109–2115. [Google Scholar] [CrossRef] [PubMed]
- Sugiyama, H.; Maeda, K.; Yamato, M.; Hayashi, R.; Soma, T.; Hayashida, Y.; Yang, J.; Shirakabe, M.; Matsuyama, A.; Kikuchi, A.; et al. Human adipose tissue-derived mesenchymal stem cells as a novel feeder layer for epithelial cells. J. Tissue Eng. Regen. Med. 2008, 2, 445–449. [Google Scholar] [CrossRef] [PubMed]
- O’Callaghan, A.R.; Morgan, L.; Daniels, J.T.; Lewis, M.P. Human-derived feeder fibroblasts for the culture of epithelial cells for clinical use. Regen. Med. 2016, 11, 529–543. [Google Scholar] [CrossRef] [Green Version]
- Sharma, S.M.; Fuchsluger, T.; Ahmad, S.; Katikireddy, K.R.; Armant, M.; Dana, R.; Jurkunas, U.V. Comparative analysis of human-derived feeder layers with 3T3 fibroblasts for the ex vivo expansion of human limbal and oral epithelium. Stem Cell Rev. Rep. 2012, 8, 696–705. [Google Scholar] [CrossRef]
- Shirzadeh, E.; Heidari Keshel, S.; Ezzatizadeh, V.; Jabbehdari, S.; Baradaran-Rafii, A. Unrestricted somatic stem cells, as a novel feeder layer: Ex vivo culture of human limbal stem cells. J. Cell. Biochem. 2018, 119, 2666–2678. [Google Scholar] [CrossRef]
- Nakajima, R.; Takeda, S. Fabrication of corneal epithelial cell sheets maintaining colony-forming cells without feeder cells by oxygen-controlled method. Exp. Eye Res. 2014, 118, 53–60. [Google Scholar] [CrossRef]
- Le-Bel, G.; Cortez Ghio, S.; Guerin, L.P.; Bisson, F.; Germain, L.; Guerin, S.L. Irradiated Human Fibroblasts as a Substitute Feeder Layer to Irradiated Mouse 3T3 for the Culture of Human Corneal Epithelial Cells: Impact on the Stability of the Transcription Factors Sp1 and NFI. Int. J. Mol. Sci. 2019, 20, 6296. [Google Scholar] [CrossRef] [Green Version]
- Bisson, F.; Rochefort, E.; Lavoie, A.; Larouche, D.; Zaniolo, K.; Simard-Bisson, C.; Damour, O.; Auger, F.A.; Guerin, S.L.; Germain, L. Irradiated human dermal fibroblasts are as efficient as mouse fibroblasts as a feeder layer to improve human epidermal cell culture lifespan. Int. J. Mol. Sci. 2013, 14, 4684–4704. [Google Scholar] [CrossRef] [PubMed]
- Auxenfans, C.; Thepot, A.; Justin, V.; Hautefeuille, A.; Shahabeddin, L.; Damour, O.; Hainaut, P. Characterisation of human fibroblasts as keratinocyte feeder layer using p63 isoforms status. Biomed. Mater. Eng. 2009, 19, 365–372. [Google Scholar] [CrossRef] [PubMed]
- Cortez Ghio, S.; Cantin-Warren, L.; Guignard, R.; Larouche, D.; Germain, L. Are the Effects of the Cholera Toxin and Isoproterenol on Human Keratinocytes’ Proliferative Potential Dependent on Whether They Are Co-Cultured with Human or Murine Fibroblast Feeder Layers? Int. J. Mol. Sci. 2018, 19, 2174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Black, A.F.; Bouez, C.; Perrier, E.; Schlotmann, K.; Chapuis, F.; Damour, O. Optimization and characterization of an engineered human skin equivalent. Tissue Eng. 2005, 11, 723–733. [Google Scholar] [CrossRef] [PubMed]
- Green, H. Cyclic AMP in relation to proliferation of the epidermal cell: A new view. Cell 1978, 15, 801–811. [Google Scholar] [CrossRef]
- Takagi, R.; Yamato, M.; Murakami, D.; Kondo, M.; Yang, J.; Ohki, T.; Nishida, K.; Kohno, C.; Okano, T. Preparation of keratinocyte culture medium for the clinical applications of regenerative medicine. J. Tissue Eng. Regen. Med. 2011, 5, e63–e73. [Google Scholar] [CrossRef] [PubMed]
- Ghoubay-Benallaoua, D.; Pecha, F.; Goldschmidt, P.; Fialaire-Legendre, A.; Chaumeil, C.; Laroche, L.; Borderie, V.M. Effects of isoproterenol and cholera toxin on human limbal epithelial cell cultures. Curr. Eye Res. 2012, 37, 644–653. [Google Scholar] [CrossRef]
- Joyce, N.C.; Zhu, C.C. Human corneal endothelial cell proliferation: Potential for use in regenerative medicine. Cornea 2004, 23, S8–S19. [Google Scholar] [CrossRef]
- Zhu, C.; Joyce, N.C. Proliferative response of corneal endothelial cells from young and older donors. Investig. Ophthalmol. Vis. Sci. 2004, 45, 1743–1751. [Google Scholar] [CrossRef]
- Engelmann, K.; Bohnke, M.; Friedl, P. Isolation and long-term cultivation of human corneal endothelial cells. Investig. Ophthalmol. Vis. Sci. 1988, 29, 1656–1662. [Google Scholar]
- Roy, O.; Leclerc, V.B.; Bourget, J.M.; Theriault, M.; Proulx, S. Understanding the process of corneal endothelial morphological change in vitro. Investig. Ophthalmol. Vis. Sci. 2015, 56, 1228–1237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Okumura, N.; Kay, E.P.; Nakahara, M.; Hamuro, J.; Kinoshita, S.; Koizumi, N. Inhibition of TGF-beta signaling enables human corneal endothelial cell expansion in vitro for use in regenerative medicine. PLoS ONE 2013, 8, e58000. [Google Scholar] [CrossRef] [PubMed]
- Leung, E.W.; Rife, L.; Smith, R.E.; Kay, E.P. Extracellular matrix components in retrocorneal fibrous membrane in comparison to corneal endothelium and Descemet’s membrane. Mol. Vis. 2000, 6, 15–23. [Google Scholar] [PubMed]
- Beaulieu Leclerc, V.; Roy, O.; Santerre, K.; Proulx, S. TGF-beta1 promotes cell barrier function upon maturation of corneal endothelial cells. Sci. Rep. 2018, 8, 4438. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Funderburgh, M.L.; Mann, M.M.; SundarRaj, N.; Funderburgh, J.L. Multipotent stem cells in human corneal stroma. Stem Cells 2005, 23, 1266–1275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mathews, S.; Chidambaram, J.D.; Lanjewar, S.; Mascarenhas, J.; Prajna, N.V.; Muthukkaruppan, V.; Chidambaranathan, G.P. In vivo confocal microscopic analysis of normal human anterior limbal stroma. Cornea 2015, 34, 464–470. [Google Scholar] [CrossRef] [Green Version]
- Xie, H.T.; Chen, S.Y.; Li, G.G.; Tseng, S.C. Limbal epithelial stem/progenitor cells attract stromal niche cells by SDF-1/CXCR4 signaling to prevent differentiation. Stem Cells 2011, 29, 1874–1885. [Google Scholar] [CrossRef]
- Li, G.G.; Zhu, Y.T.; Xie, H.T.; Chen, S.Y.; Tseng, S.C. Mesenchymal stem cells derived from human limbal niche cells. Investig. Ophthalmol. Vis. Sci. 2012, 53, 5686–5697. [Google Scholar] [CrossRef] [Green Version]
- Basu, S.; Hertsenberg, A.J.; Funderburgh, M.L.; Burrow, M.K.; Mann, M.M.; Du, Y.; Lathrop, K.L.; Syed-Picard, F.N.; Adams, S.M.; Birk, D.E.; et al. Human limbal biopsy-derived stromal stem cells prevent corneal scarring. Sci. Transl. Med. 2014, 6, 266ra172. [Google Scholar] [CrossRef] [Green Version]
- Du, Y.; Sundarraj, N.; Funderburgh, M.L.; Harvey, S.A.; Birk, D.E.; Funderburgh, J.L. Secretion and organization of a cornea-like tissue in vitro by stem cells from human corneal stroma. Investig. Ophthalmol. Vis. Sci. 2007, 48, 5038–5045. [Google Scholar] [CrossRef]
- Golebiewska, A.; Brons, N.H.; Bjerkvig, R.; Niclou, S.P. Critical appraisal of the side population assay in stem cell and cancer stem cell research. Cell Stem Cell 2011, 8, 136–147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amano, S.; Yamagami, S.; Mimura, T.; Uchida, S.; Yokoo, S. Corneal stromal and endothelial cell precursors. Cornea 2006, 25, S73–S77. [Google Scholar] [CrossRef] [PubMed]
- Long, C.J.; Roth, M.R.; Tasheva, E.S.; Funderburgh, M.; Smit, R.; Conrad, G.W.; Funderburgh, J.L. Fibroblast growth factor-2 promotes keratan sulfate proteoglycan expression by keratocytes in vitro. J. Biol. Chem. 2000, 275, 13918–13923. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Funderburgh, J.L.; Caterson, B.; Conrad, G.W. Keratan sulfate proteoglycan during embryonic development of the chicken cornea. Dev. Biol. 1986, 116, 267–277. [Google Scholar] [CrossRef]
- Chakravarti, S.; Magnuson, T.; Lass, J.H.; Jepsen, K.J.; LaMantia, C.; Carroll, H. Lumican regulates collagen fibril assembly: Skin fragility and corneal opacity in the absence of lumican. J. Cell Biol. 1998, 141, 1277–1286. [Google Scholar] [CrossRef]
- Funderburgh, J.L. Keratan sulfate: Structure, biosynthesis, and function. Glycobiology 2000, 10, 951–958. [Google Scholar] [CrossRef]
- Kao, W.W.; Liu, C.Y. Roles of lumican and keratocan on corneal transparency. Glycoconj. J. 2002, 19, 275–285. [Google Scholar] [CrossRef]
- Carlson, E.C.; Liu, C.Y.; Chikama, T.; Hayashi, Y.; Kao, C.W.; Birk, D.E.; Funderburgh, J.L.; Jester, J.V.; Kao, W.W. Keratocan, a cornea-specific keratan sulfate proteoglycan, is regulated by lumican. J. Biol. Chem. 2005, 280, 25541–25547. [Google Scholar] [CrossRef] [Green Version]
- Chakravarti, S.; Zhang, G.; Chervoneva, I.; Roberts, L.; Birk, D.E. Collagen fibril assembly during postnatal development and dysfunctional regulation in the lumican-deficient murine cornea. Dev. Dyn. 2006, 235, 2493–2506. [Google Scholar] [CrossRef]
- Wu, J.; Du, Y.; Mann, M.M.; Funderburgh, J.L.; Wagner, W.R. Corneal stromal stem cells versus corneal fibroblasts in generating structurally appropriate corneal stromal tissue. Exp. Eye Res. 2014, 120, 71–81. [Google Scholar] [CrossRef] [Green Version]
- Wu, J.; Du, Y.; Watkins, S.C.; Funderburgh, J.L.; Wagner, W.R. The engineering of organized human corneal tissue through the spatial guidance of corneal stromal stem cells. Biomaterials 2012, 33, 1343–1352. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karamichos, D.; Funderburgh, M.L.; Hutcheon, A.E.; Zieske, J.D.; Du, Y.; Wu, J.; Funderburgh, J.L. A role for topographic cues in the organization of collagenous matrix by corneal fibroblasts and stem cells. PLoS ONE 2014, 9, e86260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, J.; Rnjak-Kovacina, J.; Du, Y.; Funderburgh, M.L.; Kaplan, D.L.; Funderburgh, J.L. Corneal stromal bioequivalents secreted on patterned silk substrates. Biomaterials 2014, 35, 3744–3755. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karamichos, D.; Guo, X.Q.; Hutcheon, A.E.; Zieske, J.D. Human corneal fibrosis: An in vitro model. Investig. Ophthalmol. Vis. Sci. 2010, 51, 1382–1388. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karamichos, D.; Hutcheon, A.E.; Zieske, J.D. Transforming growth factor-beta3 regulates assembly of a non-fibrotic matrix in a 3D corneal model. J. Tissue Eng. Regen. Med. 2011, 5, e228–e238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karamichos, D.; Rich, C.B.; Zareian, R.; Hutcheon, A.E.; Ruberti, J.W.; Trinkaus-Randall, V.; Zieske, J.D. TGF-beta3 stimulates stromal matrix assembly by human corneal keratocyte-like cells. Investig. Ophthalmol. Vis. Sci. 2013, 54, 6612–6619. [Google Scholar] [CrossRef] [Green Version]
- Muller, L.J.; Marfurt, C.F.; Kruse, F.; Tervo, T.M. Corneal nerves: Structure, contents and function. Exp. Eye Res. 2003, 76, 521–542. [Google Scholar] [CrossRef]
- Marfurt, C.F.; Cox, J.; Deek, S.; Dvorscak, L. Anatomy of the human corneal innervation. Exp. Eye Res. 2010, 90, 478–492. [Google Scholar] [CrossRef]
- Han, K.Y.; Tran, J.A.; Chang, J.H.; Azar, D.T.; Zieske, J.D. Potential role of corneal epithelial cell-derived exosomes in corneal wound healing and neovascularization. Sci. Rep. 2017, 7, 40548. [Google Scholar] [CrossRef] [Green Version]
- Cruzat, A.; Pavan-Langston, D.; Hamrah, P. In vivo confocal microscopy of corneal nerves: Analysis and clinical correlation. Semin. Ophthalmol. 2010, 25, 171–177. [Google Scholar] [CrossRef] [Green Version]
- Cruzat, A.; Qazi, Y.; Hamrah, P. In Vivo Confocal Microscopy of Corneal Nerves in Health and Disease. Ocul. Surf. 2017, 15, 15–47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stapleton, F.; Marfurt, C.; Golebiowski, B.; Rosenblatt, M.; Bereiter, D.; Begley, C.; Dartt, D.; Gallar, J.; Belmonte, C.; Hamrah, P.; et al. The TFOS International Workshop on Contact Lens Discomfort: Report of the subcommittee on neurobiology. Investig. Ophthalmol. Vis. Sci. 2013, 54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dieckmann, G.; Goyal, S.; Hamrah, P. Neuropathic Corneal Pain: Approaches for Management. Ophthalmology 2017, 124, S34–S47. [Google Scholar] [CrossRef] [PubMed]
- Stepp, M.A.; Zieske, J.D.; Trinkaus-Randall, V.; Kyne, B.M.; Pal-Ghosh, S.; Tadvalkar, G.; Pajoohesh-Ganji, A. Wounding the cornea to learn how it heals. Exp. Eye Res. 2014, 121, 178–193. [Google Scholar] [CrossRef] [Green Version]
- Ghezzi, C.E.; Marelli, B.; Omenetto, F.G.; Funderburgh, J.L.; Kaplan, D.L. 3D Functional Corneal Stromal Tissue Equivalent Based on Corneal Stromal Stem Cells and Multi-Layered Silk Film Architecture. PLoS ONE 2017, 12, e0169504. [Google Scholar] [CrossRef] [Green Version]
- Gosselin, E.A.; Torregrosa, T.; Ghezzi, C.E.; Mendelsohn, A.C.; Gomes, R.; Funderburgh, J.L.; Kaplan, D.L. Multi-layered silk film coculture system for human corneal epithelial and stromal stem cells. J. Tissue Eng. Regen. Med. 2018, 12, 285–295. [Google Scholar] [CrossRef] [Green Version]
- Siran, W.; Ghezzi, C.E.; Cairns, D.M.; Pollard, R.E.; Chen, Y.; Gomes, R.; McKay, T.B.; Pouli, D.; Jamali, A.; Georgakoudi, I.; et al. Human Corneal Tissue Model for Nociceptive Assessments. Adv. Healthc. Mater. 2018, 7, e1800488. [Google Scholar] [CrossRef]
- Sharif, R.; Priyadarsini, S.; Rowsey, T.G.; Ma, J.X.; Karamichos, D. Corneal Tissue Engineering: An In Vitro Model of the Stromal-nerve Interactions of the Human Cornea. J. Vis. Exp. 2018. [Google Scholar] [CrossRef]
- Martini, R. Expression and functional roles of neural cell surface molecules and extracellular matrix components during development and regeneration of peripheral nerves. J. Neurocytol. 1994, 23, 1–28. [Google Scholar] [CrossRef]
- Chen, P.; Cescon, M.; Bonaldo, P. The Role of Collagens in Peripheral Nerve Myelination and Function. Mol. Neurobiol. 2015, 52, 216–225. [Google Scholar] [CrossRef]
- Hori, J.; Vega, J.L.; Masli, S. Review of ocular immune privilege in the year 2010: Modifying the immune privilege of the eye. Ocul. Immunol. Inflamm. 2010, 18, 325–333. [Google Scholar] [CrossRef] [PubMed]
- Niederkorn, J.Y. Corneal transplantation and immune privilege. Int. Rev. Immunol. 2013, 32, 57–67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gell, P.G.H.; Coombs, R.R.A. Clinical Aspects of Immunology; F. A. Davis: Philadelphia, PA, USA, 1963. [Google Scholar]
- Ellenberg, D.; Azar, D.T.; Hallak, J.A.; Tobaigy, F.; Han, K.Y.; Jain, S.; Zhou, Z.; Chang, J.H. Novel aspects of corneal angiogenic and lymphangiogenic privilege. Prog. Retin. Eye Res. 2010, 29, 208–248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hamrah, P.; Huq, S.O.; Liu, Y.; Zhang, Q.; Dana, M.R. Corneal immunity is mediated by heterogeneous population of antigen-presenting cells. J. Leukoc. Biol. 2003, 74, 172–178. [Google Scholar] [CrossRef] [PubMed]
- Brissette-Storkus, C.S.; Reynolds, S.M.; Lepisto, A.J.; Hendricks, R.L. Identification of a novel macrophage population in the normal mouse corneal stroma. Investig. Ophthalmol. Vis. Sci. 2002, 43, 2264–2271. [Google Scholar]
- Hamrah, P.; Zhang, Q.; Liu, Y.; Dana, M.R. Novel characterization of MHC class II-negative population of resident corneal Langerhans cell-type dendritic cells. Investig. Ophthalmol. Vis. Sci. 2002, 43, 639–646. [Google Scholar]
- Chen, W.; Hara, K.; Tian, Q.; Zhao, K.; Yoshitomi, T. Existence of small slow-cycling Langerhans cells in the limbal basal epithelium that express ABCG2. Exp. Eye Res. 2007, 84, 626–634. [Google Scholar] [CrossRef]
- Hamrah, P.; Liu, Y.; Zhang, Q.; Dana, M.R. The corneal stroma is endowed with a significant number of resident dendritic cells. Investig. Ophthalmol. Vis. Sci. 2003, 44, 581–589. [Google Scholar] [CrossRef]
- Ueta, M.; Koga, A.; Kikuta, J.; Yamada, K.; Kojima, S.; Shinomiya, K.; Ishii, M.; Kinoshita, S. Intravital imaging of the cellular dynamics of LysM-positive cells in a murine corneal suture model. Br. J. Ophthalmol. 2016, 100, 432–435. [Google Scholar] [CrossRef]
- Mott, K.R.; Osorio, Y.; Brown, D.J.; Morishige, N.; Wahlert, A.; Jester, J.V.; Ghiasi, H. The corneas of naive mice contain both CD4+ and CD8+ T cells. Mol. Vis. 2007, 13, 1802–1812. [Google Scholar]
- Tran, M.T.; Tellaetxe-Isusi, M.; Elner, V.; Strieter, R.M.; Lausch, R.N.; Oakes, J.E. Proinflammatory cytokines induce RANTES and MCP-1 synthesis in human corneal keratocytes but not in corneal epithelial cells. Beta-chemokine synthesis in corneal cells. Investig. Ophthalmol. Vis. Sci. 1996, 37, 987–996. [Google Scholar]
- Hong, J.W.; Liu, J.J.; Lee, J.S.; Mohan, R.R.; Mohan, R.R.; Woods, D.J.; He, Y.G.; Wilson, S.E. Proinflammatory chemokine induction in keratocytes and inflammatory cell infiltration into the cornea. Investig. Ophthalmol. Vis. Sci. 2001, 42, 2795–2803. [Google Scholar]
- Stapleton, W.M.; Chaurasia, S.S.; Medeiros, F.W.; Mohan, R.R.; Sinha, S.; Wilson, S.E. Topical interleukin-1 receptor antagonist inhibits inflammatory cell infiltration into the cornea. Exp. Eye Res. 2008, 86, 753–757. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ebihara, N.; Matsuda, A.; Nakamura, S.; Matsuda, H.; Murakami, A. Role of the IL-6 classic- and trans-signaling pathways in corneal sterile inflammation and wound healing. Investig. Ophthalmol. Vis. Sci. 2011, 52, 8549–8557. [Google Scholar] [CrossRef]
- Sotozono, C.; He, J.; Matsumoto, Y.; Kita, M.; Imanishi, J.; Kinoshita, S. Cytokine expression in the alkali-burned cornea. Curr. Eye Res. 1997, 16, 670–676. [Google Scholar] [CrossRef]
- O’Brien, T.P.; Li, Q.; Ashraf, M.F.; Matteson, D.M.; Stark, W.J.; Chan, C.C. Inflammatory response in the early stages of wound healing after excimer laser keratectomy. Arch. Ophthalmol. 1998, 116, 1470–1474. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Burns, A.R.; Smith, C.W. Two waves of neutrophil emigration in response to corneal epithelial abrasion: Distinct adhesion molecule requirements. Investig. Ophthalmol. Vis. Sci. 2006, 47, 1947–1955. [Google Scholar] [CrossRef]
- Cursiefen, C.; Chen, L.; Borges, L.P.; Jackson, D.; Cao, J.; Radziejewski, C.; D’Amore, P.A.; Dana, M.R.; Wiegand, S.J.; Streilein, J.W. VEGF-A stimulates lymphangiogenesis and hemangiogenesis in inflammatory neovascularization via macrophage recruitment. J. Clin. Investig. 2004, 113, 1040–1050. [Google Scholar] [CrossRef] [Green Version]
- Watari, K.; Nakao, S.; Fotovati, A.; Basaki, Y.; Hosoi, F.; Bereczky, B.; Higuchi, R.; Miyamoto, T.; Kuwano, M.; Ono, M. Role of macrophages in inflammatory lymphangiogenesis: Enhanced production of vascular endothelial growth factor C and D through NF-kappaB activation. Biochem. Biophys. Res. Commun. 2008, 377, 826–831. [Google Scholar] [CrossRef]
- Maruyama, K.; Ii, M.; Cursiefen, C.; Jackson, D.G.; Keino, H.; Tomita, M.; Van Rooijen, N.; Takenaka, H.; D’Amore, P.A.; Stein-Streilein, J.; et al. Inflammation-induced lymphangiogenesis in the cornea arises from CD11b-positive macrophages. J. Clin. Investig. 2005, 115, 2363–2372. [Google Scholar] [CrossRef]
- Barnett, F.H.; Rosenfeld, M.; Wood, M.; Kiosses, W.B.; Usui, Y.; Marchetti, V.; Aguilar, E.; Friedlander, M. Macrophages form functional vascular mimicry channels in vivo. Sci. Rep. 2016, 6, 36659. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Li, B.; Jiang, H.; Wang, Y.; Qu, M.; Duan, H.; Zhou, Q.; Shi, W. Macrophage depletion impairs corneal wound healing after autologous transplantation in mice. PLoS ONE 2013, 8, e61799. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bellner, L.; Marrazzo, G.; van Rooijen, N.; Dunn, M.W.; Abraham, N.G.; Schwartzman, M.L. Heme oxygenase-2 deletion impairs macrophage function: Implication in wound healing. FASEB J. 2015, 29, 105–115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamagami, S.; Hamrah, P.; Miyamoto, K.; Miyazaki, D.; Dekaris, I.; Dawson, T.; Lu, B.; Gerard, C.; Dana, M.R. CCR5 chemokine receptor mediates recruitment of MHC class II-positive Langerhans cells in the mouse corneal epithelium. Investig. Ophthalmol. Vis. Sci. 2005, 46, 1201–1207. [Google Scholar] [CrossRef]
- Chen, W.; Lin, H.; Dong, N.; Sanae, T.; Liu, Z.; Yoshitomi, T. Cauterization of central cornea induces recruitment of major histocompatibility complex class II+ Langerhans cells from limbal basal epithelium. Cornea 2010, 29, 73–79. [Google Scholar] [CrossRef]
- Hamrah, P.; Liu, Y.; Zhang, Q.; Dana, M.R. Alterations in corneal stromal dendritic cell phenotype and distribution in inflammation. Arch. Ophthalmol. 2003, 121, 1132–1140. [Google Scholar] [CrossRef] [Green Version]
- Liu, Q.; Smith, C.W.; Zhang, W.; Burns, A.R.; Li, Z. NK cells modulate the inflammatory response to corneal epithelial abrasion and thereby support wound healing. Am. J. Pathol. 2012, 181, 452–462. [Google Scholar] [CrossRef] [Green Version]
- Gao, Y.; Li, Z.; Hassan, N.; Mehta, P.; Burns, A.R.; Tang, X.; Smith, C.W. NK cells are necessary for recovery of corneal CD11c+ dendritic cells after epithelial abrasion injury. J. Leukoc. Biol. 2013, 94, 343–351. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Burns, A.R.; Rumbaut, R.E.; Smith, C.W. Gamma delta T cells are necessary for platelet and neutrophil accumulation in limbal vessels and efficient epithelial repair after corneal abrasion. Am. J. Pathol. 2007, 171, 838–845. [Google Scholar] [CrossRef] [Green Version]
- Byeseda, S.E.; Burns, A.R.; Dieffenbaugher, S.; Rumbaut, R.E.; Smith, C.W.; Li, Z. ICAM-1 is necessary for epithelial recruitment of gammadelta T cells and efficient corneal wound healing. Am. J. Pathol. 2009, 175, 571–579. [Google Scholar] [CrossRef] [Green Version]
- O’Brien, R.L.; Taylor, M.A.; Hartley, J.; Nuhsbaum, T.; Dugan, S.; Lahmers, K.; Aydintug, M.K.; Wands, J.M.; Roark, C.L.; Born, W.K. Protective role of gammadelta T cells in spontaneous ocular inflammation. Investig. Ophthalmol. Vis. Sci. 2009, 50, 3266–3274. [Google Scholar] [CrossRef] [PubMed]
- Bechetoille, N.; Dezutter-Dambuyant, C.; Damour, O.; Andre, V.; Orly, I.; Perrier, E. Effects of solar ultraviolet radiation on engineered human skin equivalent containing both Langerhans cells and dermal dendritic cells. Tissue Eng. 2007, 13, 2667–2679. [Google Scholar] [CrossRef] [PubMed]
- Chau, D.Y.; Johnson, C.; MacNeil, S.; Haycock, J.W.; Ghaemmaghami, A.M. The development of a 3D immunocompetent model of human skin. Biofabrication 2013, 5, 035011. [Google Scholar] [CrossRef] [PubMed]
- Goyal, S.; Chauhan, S.K.; Zhang, Q.; Dana, R. Amelioration of murine dry eye disease by topical antagonist to chemokine receptor 2. Arch. Ophthalmol. 2009, 127, 882–887. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ouwehand, K.; Spiekstra, S.W.; Waaijman, T.; Breetveld, M.; Scheper, R.J.; de Gruijl, T.D.; Gibbs, S. CCL5 and CCL20 mediate immigration of Langerhans cells into the epidermis of full thickness human skin equivalents. Eur. J. Cell Biol. 2012, 91, 765–773. [Google Scholar] [CrossRef] [PubMed]
- Ouwehand, K.; Spiekstra, S.W.; Waaijman, T.; Scheper, R.J.; de Gruijl, T.D.; Gibbs, S. Technical advance: Langerhans cells derived from a human cell line in a full-thickness skin equivalent undergo allergen-induced maturation and migration. J. Leukoc. Biol. 2011, 90, 1027–1033. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laubach, V.; Zoller, N.; Rossberg, M.; Gorg, K.; Kippenberger, S.; Bereiter-Hahn, J.; Kaufmann, R.; Bernd, A. Integration of Langerhans-like cells into a human skin equivalent. Arch. Dermatol. Res. 2011, 303, 135–139. [Google Scholar] [CrossRef]
- Kosten, I.J.; Spiekstra, S.W.; de Gruijl, T.D.; Gibbs, S. MUTZ-3 derived Langerhans cells in human skin equivalents show differential migration and phenotypic plasticity after allergen or irritant exposure. Toxicol. Appl. Pharmacol. 2015, 287, 35–42. [Google Scholar] [CrossRef] [Green Version]
- Linde, N.; Gutschalk, C.M.; Hoffmann, C.; Yilmaz, D.; Mueller, M.M. Integrating macrophages into organotypic co-cultures: A 3D in vitro model to study tumor-associated macrophages. PLoS ONE 2012, 7, e40058. [Google Scholar] [CrossRef]
- Kuhbacher, A.; Henkel, H.; Stevens, P.; Grumaz, C.; Finkelmeier, D.; Burger-Kentischer, A.; Sohn, K.; Rupp, S. Central Role for Dermal Fibroblasts in Skin Model Protection against Candida albicans. J. Infect. Dis. 2017, 215, 1742–1752. [Google Scholar] [CrossRef]
- Lorthois, I.; Simard, M.; Morin, S.; Pouliot, R. Infiltration of T Cells into a Three-Dimensional Psoriatic Skin Model Mimics Pathological Key Features. Int. J. Mol. Sci. 2019, 20, 1670. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Funderburgh, J.L.; Funderburgh, M.L.; Mann, M.M.; Corpuz, L.; Roth, M.R. Proteoglycan expression during transforming growth factor beta -induced keratocyte-myofibroblast transdifferentiation. J. Biol. Chem. 2001, 276, 44173–44178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Funderburgh, J.L.; Mann, M.M.; Funderburgh, M.L. Keratocyte phenotype mediates proteoglycan structure: A role for fibroblasts in corneal fibrosis. J. Biol. Chem. 2003, 278, 45629–45637. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haagdorens, M.; Van Acker, S.I.; Van Gerwen, V.; Ni Dhubhghaill, S.; Koppen, C.; Tassignon, M.J.; Zakaria, N. Limbal Stem Cell Deficiency: Current Treatment Options and Emerging Therapies. Stem Cells Int. 2016, 2016, 9798374. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pellegrini, G.; Rama, P.; Matuska, S.; Lambiase, A.; Bonini, S.; Pocobelli, A.; Colabelli, R.G.; Spadea, L.; Fasciani, R.; Balestrazzi, E.; et al. Biological parameters determining the clinical outcome of autologous cultures of limbal stem cells. Regen. Med. 2013, 8, 553–567. [Google Scholar] [CrossRef]
- Anderson, D.F.; Ellies, P.; Pires, R.T.; Tseng, S.C. Amniotic membrane transplantation for partial limbal stem cell deficiency. Br. J. Ophthalmol. 2001, 85, 567–575. [Google Scholar] [CrossRef] [Green Version]
- Tseng, S.C.; Prabhasawat, P.; Barton, K.; Gray, T.; Meller, D. Amniotic membrane transplantation with or without limbal allografts for corneal surface reconstruction in patients with limbal stem cell deficiency. Arch Ophthalmol 1998, 116, 431–441. [Google Scholar] [CrossRef]
- Sangwan, V.S.; Basu, S.; Vemuganti, G.K.; Sejpal, K.; Subramaniam, S.V.; Bandyopadhyay, S.; Krishnaiah, S.; Gaddipati, S.; Tiwari, S.; Balasubramanian, D. Clinical outcomes of xeno-free autologous cultivated limbal epithelial transplantation: A 10-year study. Br. J. Ophthalmol. 2011, 95, 1525–1529. [Google Scholar] [CrossRef]
- Barut Selver, O.; Yagci, A.; Egrilmez, S.; Gurdal, M.; Palamar, M.; Cavusoglu, T.; Ates, U.; Veral, A.; Guven, C.; Wolosin, J.M. Limbal Stem Cell Deficiency and Treatment with Stem Cell Transplantation. Turk. J. Ophthalmol. 2017, 47, 285–291. [Google Scholar] [CrossRef]
- Rama, P.; Giannini, R.; Bruni, A.; Gatto, C.; Tiso, R.; Ponzin, D. Further evaluation of amniotic membrane banking for transplantation in ocular surface diseases. Cell Tissue Bank. 2001, 2, 155–163. [Google Scholar] [CrossRef]
- Tseng, S.C.; Chen, S.Y.; Shen, Y.C.; Chen, W.L.; Hu, F.R. Critical appraisal of ex vivo expansion of human limbal epithelial stem cells. Curr. Mol. Med. 2010, 10, 841–850. [Google Scholar] [CrossRef] [PubMed]
- Shortt, A.J.; Tuft, S.J.; Daniels, J.T. Ex vivo cultured limbal epithelial transplantation. A clinical perspective. Ocul. Surf. 2010, 8, 80–90. [Google Scholar] [CrossRef]
- Shanbhag, S.S.; Nikpoor, N.; Rao Donthineni, P.; Singh, V.; Chodosh, J.; Basu, S. Autologous limbal stem cell transplantation: A systematic review of clinical outcomes with different surgical techniques. Br. J. Ophthalmol. 2019. [Google Scholar] [CrossRef] [PubMed]
- Basu, S.; Mohan, S.; Bhalekar, S.; Singh, V.; Sangwan, V. Simple limbal epithelial transplantation (SLET) in failed cultivated limbal epithelial transplantation (CLET) for unilateral chronic ocular burns. Br. J. Ophthalmol. 2018, 102, 1640–1645. [Google Scholar] [CrossRef] [PubMed]
- Le-Bel, G.; Guerin, L.P.; Carrier, P.; Mouriaux, F.; Germain, L.; Guerin, S.L.; Bazin, R. Grafting of an autologous tissue-engineered human corneal epithelium to a patient with limbal stem cell deficiency (LSCD). Am. J. Ophthalmol. Case Rep. 2019, 15, 100532. [Google Scholar] [CrossRef] [PubMed]
- Sangwan, V.S.; Vemuganti, G.K.; Singh, S.; Balasubramanian, D. Successful reconstruction of damaged ocular outer surface in humans using limbal and conjuctival stem cell culture methods. Biosci. Rep. 2003, 23, 169–174. [Google Scholar] [CrossRef]
- Nakamura, T.; Inatomi, T.; Sotozono, C.; Koizumi, N.; Kinoshita, S. Successful primary culture and autologous transplantation of corneal limbal epithelial cells from minimal biopsy for unilateral severe ocular surface disease. Acta Ophthalmol. Scand. 2004, 82, 468–471. [Google Scholar] [CrossRef]
- Zakaria, N.; Ni Dhubhghaill, S.; Taal, M.; Berneman, Z.; Koppen, C.; Tassignon, M.J. Optical Coherence Tomography in Cultivated Limbal Epithelial Stem Cell Transplantation Surgery. Asia Pac. J. Ophthalmol. 2015, 4, 339–345. [Google Scholar] [CrossRef]
- Zakaria, N.; Possemiers, T.; Dhubhghaill, S.N.; Leysen, I.; Rozema, J.; Koppen, C.; Timmermans, J.P.; Berneman, Z.; Tassignon, M.J. Results of a phase I/II clinical trial: Standardized, non-xenogenic, cultivated limbal stem cell transplantation. J. Transl. Med. 2014, 12, 58. [Google Scholar] [CrossRef] [Green Version]
- Espana, E.M.; Di Pascuale, M.; Grueterich, M.; Solomon, A.; Tseng, S.C. Keratolimbal allograft in corneal reconstruction. Eye 2004, 18, 406–417. [Google Scholar] [CrossRef]
- Boulze Pankert, M.; Goyer, B.; Zaguia, F.; Bareille, M.; Perron, M.C.; Liu, X.; Cameron, J.D.; Proulx, S.; Brunette, I. Biocompatibility and functionality of a tissue-engineered living corneal stroma transplanted in the feline eye. Investig. Ophthalmol. Vis. Sci. 2014, 55, 6908–6920. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Du, Y.; Carlson, E.C.; Funderburgh, M.L.; Birk, D.E.; Pearlman, E.; Guo, N.; Kao, W.W.; Funderburgh, J.L. Stem cell therapy restores transparency to defective murine corneas. Stem Cells 2009, 27, 1635–1642. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, H.; Chen, Y.; Wang, P.; Li, B.; Wang, W.; Su, Y.; Sheng, M. Efficacy and safety of deep anterior lamellar keratoplasty vs. penetrating keratoplasty for keratoconus: A meta-analysis. PLoS ONE 2015, 10, e0113332. [Google Scholar] [CrossRef]
- Feizi, S.; Javadi, M.A.; Javadi, F.; Jafarinasab, M.R. Deep Anterior Lamellar Keratoplasty in Keratoconic Patients with versus without Vernal Keratoconjunctivitis. J. Ophthalmic. Vis. Res. 2015, 10, 112–117. [Google Scholar] [CrossRef]
- Germain, L.; Carrier, P.; Auger, F.A.; Salesse, C.; Guerin, S.L. Can we produce a human corneal equivalent by tissue engineering? Prog. Retin. Eye Res. 2000, 19, 497–527. [Google Scholar] [CrossRef]
- Griffith, M.; Osborne, R.; Munger, R.; Xiong, X.; Doillon, C.J.; Laycock, N.L.; Hakim, M.; Song, Y.; Watsky, M.A. Functional human corneal equivalents constructed from cell lines. Science 1999, 286, 2169–2172. [Google Scholar] [CrossRef]
- Liu, C.Y.; Kao, W.W. Corneal Epithelial Wound Healing. Prog. Mol. Biol. Transl. Sci. 2015, 134, 61–71. [Google Scholar] [CrossRef]
- Streuli, C. Extracellular matrix remodelling and cellular differentiation. Curr. Opin. Cell Biol. 1999, 11, 634–640. [Google Scholar] [CrossRef]
- Fini, M.E.; Girard, M.T.; Matsubara, M. Collagenolytic/gelatinolytic enzymes in corneal wound healing. Acta Ophthalmol. Suppl. 1992, 70, 26–33. [Google Scholar] [CrossRef]
- Theocharis, A.D.; Skandalis, S.S.; Gialeli, C.; Karamanos, N.K. Extracellular matrix structure. Adv. Drug Deliv. Rev. 2016, 97, 4–27. [Google Scholar] [CrossRef]
- Murakami, J.; Nishida, T.; Otori, T. Coordinated appearance of beta 1 integrins and fibronectin during corneal wound healing. J. Lab. Clin. Med. 1992, 120, 86–93. [Google Scholar] [PubMed]
- Kang, S.J.; Kim, E.K.; Kim, H.B. Expression and distribution of extracellular matrices during corneal wound healing after keratomileusis in rabbits. Ophthalmologica 1999, 213, 20–24. [Google Scholar] [CrossRef] [PubMed]
- Zieske, J.D.; Higashijima, S.C.; Spurr-Michaud, S.J.; Gipson, I.K. Biosynthetic responses of the rabbit cornea to a keratectomy wound. Investig. Ophthalmol. Vis. Sci. 1987, 28, 1668–1677. [Google Scholar]
- Koivisto, L.; Heino, J.; Hakkinen, L.; Larjava, H. Integrins in Wound Healing. Adv. Wound Care 2014, 3, 762–783. [Google Scholar] [CrossRef] [Green Version]
- Carracedo, S.; Lu, N.; Popova, S.N.; Jonsson, R.; Eckes, B.; Gullberg, D. The fibroblast integrin alpha11beta1 is induced in a mechanosensitive manner involving activin A and regulates myofibroblast differentiation. J. Biol. Chem. 2010, 285, 10434–10445. [Google Scholar] [CrossRef] [Green Version]
- Brazzell, R.K.; Stern, M.E.; Aquavella, J.V.; Beuerman, R.W.; Baird, L. Human recombinant epidermal growth factor in experimental corneal wound healing. Investig. Ophthalmol. Vis. Sci. 1991, 32, 336–340. [Google Scholar]
- Burling, K.; Seguin, M.A.; Marsh, P.; Brinkman, K.; Madigan, J.; Thurmond, M.; Moon-Massat, P.; Mannis, M.; Murphy, C.J. Effect of topical administration of epidermal growth factor on healing of corneal epithelial defects in horses. Am. J. Vet. Res. 2000, 61, 1150–1155. [Google Scholar] [CrossRef]
- Grant, M.B.; Khaw, P.T.; Schultz, G.S.; Adams, J.L.; Shimizu, R.W. Effects of epidermal growth factor, fibroblast growth factor, and transforming growth factor-beta on corneal cell chemotaxis. Investig. Ophthalmol. Vis. Sci. 1992, 33, 3292–3301. [Google Scholar]
- Kim, M.J.; Jun, R.M.; Kim, W.K.; Hann, H.J.; Chong, Y.H.; Park, H.Y.; Chung, J.H. Optimal concentration of human epidermal growth factor (hEGF) for epithelial healing in experimental corneal alkali wounds. Curr. Eye Res. 2001, 22, 272–279. [Google Scholar] [CrossRef]
- Maldonado, B.A.; Furcht, L.T. Epidermal growth factor stimulates integrin-mediated cell migration of cultured human corneal epithelial cells on fibronectin and arginine-glycine-aspartic acid peptide. Investig. Ophthalmol. Vis. Sci. 1995, 36, 2120–2126. [Google Scholar]
- Nelson, J.D.; Silverman, V.; Lima, P.H.; Beckman, G. Corneal epithelial wound healing: A tissue culture assay on the effect of antibiotics. Curr. Eye Res. 1990, 9, 277–285. [Google Scholar] [CrossRef] [PubMed]
- Simmons, S.J.; Jumblatt, M.M.; Neufeld, A.H. Corneal epithelial wound closure in tissue culture: An in vitro model of ocular irritancy. Toxicol. Appl. Pharmacol. 1987, 88, 13–23. [Google Scholar] [CrossRef]
- Taliana, L.; Evans, M.D.; Dimitrijevich, S.D.; Steele, J.G. Vitronectin or fibronectin is required for corneal fibroblast-seeded collagen gel contraction. Investig. Ophthalmol. Vis. Sci. 2000, 41, 103–109. [Google Scholar]
- Zieske, J.D.; Hutcheon, A.E.; Guo, X.; Chung, E.H.; Joyce, N.C. TGF-beta receptor types I and II are differentially expressed during corneal epithelial wound repair. Investig. Ophthalmol. Vis. Sci. 2001, 42, 1465–1471. [Google Scholar]
- Ghezzi, C.E.; Rnjak-Kovacina, J.; Kaplan, D.L. Corneal Tissue Engineering: Recent Advances and Future Perspectives. Tissue Eng. Part B Rev. 2015. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Germain, L.; Auger, F.A.; Grandbois, E.; Guignard, R.; Giasson, M.; Boisjoly, H.; Guerin, S.L. Reconstructed human cornea produced in vitro by tissue engineering. Pathobiology 1999, 67, 140–147. [Google Scholar] [CrossRef] [PubMed]
- Germain, L.; Giasson, C.; Carrier, P.; Guérin, S.L.; Salesse, C.; Auger, F.A. Tissue Engineering of Human Cornea; Marcel Dekker: New York, NY, USA, 2003. [Google Scholar]
- Paquet, C.; Larouche, D.; Bisson, F.; Proulx, S.; Simard-Bisson, C.; Gaudreault, M.; Robitaille, H.; Carrier, P.; Martel, I.; Duranceau, L.; et al. Tissue engineering of skin and cornea: Development of new models for in vitro studies. Ann. N. Y. Acad. Sci. 2010, 1197, 166–177. [Google Scholar] [CrossRef] [PubMed]
- Gaudreault, M.; Carrier, P.; Larouche, K.; Leclerc, S.; Giasson, M.; Germain, L.; Guerin, S.L. Influence of sp1/sp3 expression on corneal epithelial cells proliferation and differentiation properties in reconstructed tissues. Investig. Ophthalmol. Vis. Sci. 2003, 44, 1447–1457. [Google Scholar] [CrossRef] [Green Version]
- Brizzi, M.F.; Defilippi, P.; Rosso, A.; Venturino, M.; Garbarino, G.; Miyajima, A.; Silengo, L.; Tarone, G.; Pegoraro, L. Integrin-mediated adhesion of endothelial cells induces JAK2 and STAT5A activation: Role in the control of c-fos gene expression. Mol. Biol. Cell 1999, 10, 3463–3471. [Google Scholar] [CrossRef] [Green Version]
- Yee, K.L.; Weaver, V.M.; Hammer, D.A. Integrin-mediated signalling through the MAP-kinase pathway. IET Syst. Biol. 2008, 2, 8–15. [Google Scholar] [CrossRef]
- Zeller, K.S.; Idevall-Hagren, O.; Stefansson, A.; Velling, T.; Jackson, S.P.; Downward, J.; Tengholm, A.; Johansson, S. PI3-kinase p110alpha mediates beta1 integrin-induced Akt activation and membrane protrusion during cell attachment and initial spreading. Cell Signal. 2010, 22, 1838–1848. [Google Scholar] [CrossRef] [PubMed]
- Fong, Y.C.; Liu, S.C.; Huang, C.Y.; Li, T.M.; Hsu, S.F.; Kao, S.T.; Tsai, F.J.; Chen, W.C.; Chen, C.Y.; Tang, C.H. Osteopontin increases lung cancer cells migration via activation of the alphavbeta3 integrin/FAK/Akt and NF-kappaB-dependent pathway. Lung Cancer 2009, 64, 263–270. [Google Scholar] [CrossRef] [PubMed]
- Guenther, C.; Faisal, I.; Uotila, L.M.; Asens, M.L.; Harjunpaa, H.; Savinko, T.; Ohman, T.; Yao, S.; Moser, M.; Morris, S.W.; et al. A beta2-Integrin/MRTF-A/SRF Pathway Regulates Dendritic Cell Gene Expression, Adhesion, and Traction Force Generation. Front. Immunol. 2019, 10, 1138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Desjardins, P.; Couture, C.; Germain, L.; Guerin, S.L. Contribution of the WNK1 kinase to corneal wound healing using the tissue-engineered human cornea as an in vitro model. J. Tissue Eng. Regen. Med. 2019. [Google Scholar] [CrossRef]
- Manning, G.; Whyte, D.B.; Martinez, R.; Hunter, T.; Sudarsanam, S. The protein kinase complement of the human genome. Science 2002, 298, 1912–1934. [Google Scholar] [CrossRef] [Green Version]
- Richardson, C.; Alessi, D.R. The regulation of salt transport and blood pressure by the WNK-SPAK/OSR1 signalling pathway. J. Cell Sci. 2008, 121, 3293–3304. [Google Scholar] [CrossRef] [Green Version]
- Zagorska, A.; Pozo-Guisado, E.; Boudeau, J.; Vitari, A.C.; Rafiqi, F.H.; Thastrup, J.; Deak, M.; Campbell, D.G.; Morrice, N.A.; Prescott, A.R.; et al. Regulation of activity and localization of the WNK1 protein kinase by hyperosmotic stress. J. Cell Biol. 2007, 176, 89–100. [Google Scholar] [CrossRef] [Green Version]
- Rodan, A.R.; Jenny, A. WNK Kinases in Development and Disease. Curr. Top. Dev. Biol. 2017, 123, 1–47. [Google Scholar] [CrossRef] [Green Version]
- Kochl, R.; Thelen, F.; Vanes, L.; Brazao, T.F.; Fountain, K.; Xie, J.; Huang, C.L.; Lyck, R.; Stein, J.V.; Tybulewicz, V.L. WNK1 kinase balances T cell adhesion versus migration in vivo. Nat. Immunol. 2016, 17, 1075–1083. [Google Scholar] [CrossRef] [Green Version]
- Cuddapah, V.A.; Sontheimer, H. Ion channels and transporters [corrected] in cancer. 2. Ion channels and the control of cancer cell migration. Am. J. Physiol. Cell Physiol. 2011, 301, C541–C549. [Google Scholar] [CrossRef] [Green Version]
- Stroka, K.M.; Jiang, H.; Chen, S.H.; Tong, Z.; Wirtz, D.; Sun, S.X.; Konstantopoulos, K. Water permeation drives tumor cell migration in confined microenvironments. Cell 2014, 157, 611–623. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, E.S.; Ahn, E.H.; Dvir, T.; Kim, D.H. Emerging nanotechnology approaches in tissue engineering and regenerative medicine. Int. J. Nanomed. 2014, 9, 1–5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, J.; Votruba, A.R.; Farokhzad, O.C.; Langer, R. Nanotechnology in drug delivery and tissue engineering: From discovery to applications. Nano Lett. 2010, 10, 3223–3230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cunha, C.; Panseri, S.; Antonini, S. Emerging nanotechnology approaches in tissue engineering for peripheral nerve regeneration. Nanomedicine 2011, 7, 50–59. [Google Scholar] [CrossRef]
- Harrington, D.A.; Sharma, A.K.; Erickson, B.A.; Cheng, E.Y. Bladder tissue engineering through nanotechnology. World J. Urol. 2008, 26, 315–322. [Google Scholar] [CrossRef]
- Mironov, V.; Kasyanov, V.; Markwald, R.R. Nanotechnology in vascular tissue engineering: From nanoscaffolding towards rapid vessel biofabrication. Trends Biotechnol. 2008, 26, 338–344. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Xiao, Y.; Hsieh, A.; Thavandiran, N.; Radisic, M. Micro- and nanotechnology in cardiovascular tissue engineering. Nanotechnology 2011, 22, 494003. [Google Scholar] [CrossRef]
- Mohamed, A.; Xing, M.M. Nanomaterials and nanotechnology for skin tissue engineering. Int. J. Burns Trauma 2012, 2, 29–41. [Google Scholar]
- Walmsley, G.G.; McArdle, A.; Tevlin, R.; Momeni, A.; Atashroo, D.; Hu, M.S.; Feroze, A.H.; Wong, V.W.; Lorenz, P.H.; Longaker, M.T.; et al. Nanotechnology in bone tissue engineering. Nanomedicine 2015, 11, 1253–1263. [Google Scholar] [CrossRef] [Green Version]
- Griffin, M.F.; Kalaskar, D.M.; Seifalian, A.; Butler, P.E. An update on the Application of Nanotechnology in Bone Tissue Engineering. Open Orthop. J. 2016, 10, 836–848. [Google Scholar] [CrossRef]
- Orsini, G.; Putignano, A.; Mitsiadis, T.A. Editorial: Advances in Craniofacial and Dental Materials through Nanotechnology and Tissue Engineering. Front. Physiol. 2019, 10, 303. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gottlieb, S. Statement by FDA Commissioner Scott Gottlieb, M.D., on Efforts to Reduce Animal Testing through a Study Aimed at Eliminating the Use of Dogs in Certain Trials. Available online: https://www.fda.gov/news-events/press-announcements/statement-fda-commissioner-scott-gottlieb-md-efforts-reduce-animal-testing-through-study-aimed (accessed on 19 January 2021).
- Gibbons, M.C.; Foley, M.A.; Cardinal, K.O. Thinking inside the box: Keeping tissue-engineered constructs in vitro for use as preclinical models. Tissue Eng. Part B Rev. 2013, 19, 14–30. [Google Scholar] [CrossRef] [PubMed]
- Beissner, N.; Bolea Albero, A.; Fuller, J.; Kellner, T.; Lauterboeck, L.; Liang, J.; Bol, M.; Glasmacher, B.; Muller-Goymann, C.C.; Reichl, S. Improved in vitro models for preclinical drug and formulation screening focusing on 2D and 3D skin and cornea constructs. Eur. J. Pharm. Biopharm. 2018, 126, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Hahne, M.; Reichl, S. Development of a serum-free human cornea construct for in vitro drug absorption studies: The influence of varying cultivation parameters on barrier characteristics. Int. J. Pharm. 2011, 416, 268–279. [Google Scholar] [CrossRef]
- Bilal, M.; Iqbal, H.M.N. An insight into toxicity and human-health-related adverse consequences of cosmeceuticals—A review. Sci. Total Environ. 2019, 670, 555–568. [Google Scholar] [CrossRef]
- Suuronen, E.J.; McLaughlin, C.R.; Stys, P.K.; Nakamura, M.; Munger, R.; Griffith, M. Functional innervation in tissue engineered models for in vitro study and testing purposes. Toxicol. Sci. 2004, 82, 525–533. [Google Scholar] [CrossRef]
- Van Goethem, F.; Adriaens, E.; Alepee, N.; Straube, F.; De Wever, B.; Cappadoro, M.; Catoire, S.; Hansen, E.; Wolf, A.; Vanparys, P. Prevalidation of a new in vitro reconstituted human cornea model to assess the eye irritating potential of chemicals. Toxicol. In Vitro 2006, 20, 1–17. [Google Scholar] [CrossRef]
- Tegtmeyer, S.; Papantoniou, I.; Muller-Goymann, C.C. Reconstruction of an in vitro cornea and its use for drug permeation studies from different formulations containing pilocarpine hydrochloride. Eur. J. Pharm. Biopharm. 2001, 51, 119–125. [Google Scholar] [CrossRef]
- Reichl, S.; Dohring, S.; Bednarz, J.; Muller-Goymann, C.C. Human cornea construct HCC-an alternative for in vitro permeation studies? A comparison with human donor corneas. Eur. J. Pharm. Biopharm. 2005, 60, 305–308. [Google Scholar] [CrossRef]
- Sharma, A.; Tandon, A.; Tovey, J.C.; Gupta, R.; Robertson, J.D.; Fortune, J.A.; Klibanov, A.M.; Cowden, J.W.; Rieger, F.G.; Mohan, R.R. Polyethylenimine-conjugated gold nanoparticles: Gene transfer potential and low toxicity in the cornea. Nanomedicine 2011, 7, 505–513. [Google Scholar] [CrossRef] [Green Version]
- Tandon, A.; Sharma, A.; Rodier, J.T.; Klibanov, A.M.; Rieger, F.G.; Mohan, R.R. BMP7 gene transfer via gold nanoparticles into stroma inhibits corneal fibrosis in vivo. PLoS ONE 2013, 8, e66434. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ouellette, M.; Masse, F.; Lefebvre-Demers, M.; Maestracci, Q.; Grenier, P.; Millar, R.; Bertrand, N.; Prieto, M.; Boisselier, E. Insights into gold nanoparticles as a mucoadhesive system. Sci. Rep. 2018, 8, 14357. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Masse, F.; Desjardins, P.; Ouellette, M.; Couture, C.; Omar, M.M.; Pernet, V.; Guerin, S.; Boisselier, E. Synthesis of Ultrastable Gold Nanoparticles as a New Drug Delivery System. Molecules 2019, 24, 2929. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Masse, F.; Ouellette, M.; Lamoureux, G.; Boisselier, E. Gold nanoparticles in ophthalmology. Med. Res. Rev. 2019, 39, 302–327. [Google Scholar] [CrossRef] [Green Version]
- Gupta, S.; Fink, M.K.; Ghosh, A.; Tripathi, R.; Sinha, P.R.; Sharma, A.; Hesemann, N.P.; Chaurasia, S.S.; Giuliano, E.A.; Mohan, R.R. Novel Combination BMP7 and HGF Gene Therapy Instigates Selective Myofibroblast Apoptosis and Reduces Corneal Haze In Vivo. Investig. Ophthalmol. Vis. Sci. 2018, 59, 1045–1057. [Google Scholar] [CrossRef] [Green Version]
- Barrangou, R.; Birmingham, A.; Wiemann, S.; Beijersbergen, R.L.; Hornung, V.; Smith, A. Advances in CRISPR-Cas9 genome engineering: Lessons learned from RNA interference. Nucleic Acids Res. 2015, 43, 3407–3419. [Google Scholar] [CrossRef] [Green Version]
- Jasin, M.; Haber, J.E. The democratization of gene editing: Insights from site-specific cleavage and double-strand break repair. DNA Repair 2016, 44, 6–16. [Google Scholar] [CrossRef] [Green Version]
- Xu, X.; Gao, J.; Dai, W.; Wang, D.; Wu, J.; Wang, J. Gene activation by a CRISPR-assisted trans enhancer. ELife 2019, 8. [Google Scholar] [CrossRef]
- Thakore, P.I.; D’Ippolito, A.M.; Song, L.; Safi, A.; Shivakumar, N.K.; Kabadi, A.M.; Reddy, T.E.; Crawford, G.E.; Gersbach, C.A. Highly specific epigenome editing by CRISPR-Cas9 repressors for silencing of distal regulatory elements. Nat. Methods 2015, 12, 1143–1149. [Google Scholar] [CrossRef] [Green Version]
- van Diemen, F.R.; Lebbink, R.J. CRISPR/Cas9, a powerful tool to target human herpesviruses. Cell. Microbial. 2017, 19. [Google Scholar] [CrossRef]
- Usui, T. To Protect Corneal Transparency against Diseases. Nippon. Ganka Gakkai Zasshi 2016, 120, 246–262, discussion 263. [Google Scholar] [PubMed]
- Dong, F.; Jin, X.; Boettler, M.A.; Sciulli, H.; Abu-Asab, M.; Del Greco, C.; Wang, S.; Hu, Y.C.; Campos, M.M.; Jackson, S.N.; et al. A Mouse Model of Schnyder Corneal Dystrophy with the N100S Point Mutation. Sci. Rep. 2018, 8, 10219. [Google Scholar] [CrossRef] [PubMed]
- Cursiefen, C. Immune privilege and angiogenic privilege of the cornea. Chem. Immunol. Allergy 2007, 92, 50–57. [Google Scholar] [PubMed]
- Charlesworth, C.T.; Deshpande, P.S.; Dever, D.P.; Camarena, J.; Lemgart, V.T.; Cromer, M.K.; Vakulskas, C.A.; Collingwood, M.A.; Zhang, L.; Bode, N.M.; et al. Identification of preexisting adaptive immunity to Cas9 proteins in humans. Nat. Med. 2019, 25, 249–254. [Google Scholar] [CrossRef]








Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guérin, L.-P.; Le-Bel, G.; Desjardins, P.; Couture, C.; Gillard, E.; Boisselier, É.; Bazin, R.; Germain, L.; Guérin, S.L. The Human Tissue-Engineered Cornea (hTEC): Recent Progress. Int. J. Mol. Sci. 2021, 22, 1291. https://doi.org/10.3390/ijms22031291
Guérin L-P, Le-Bel G, Desjardins P, Couture C, Gillard E, Boisselier É, Bazin R, Germain L, Guérin SL. The Human Tissue-Engineered Cornea (hTEC): Recent Progress. International Journal of Molecular Sciences. 2021; 22(3):1291. https://doi.org/10.3390/ijms22031291
Chicago/Turabian StyleGuérin, Louis-Philippe, Gaëtan Le-Bel, Pascale Desjardins, Camille Couture, Elodie Gillard, Élodie Boisselier, Richard Bazin, Lucie Germain, and Sylvain L. Guérin. 2021. "The Human Tissue-Engineered Cornea (hTEC): Recent Progress" International Journal of Molecular Sciences 22, no. 3: 1291. https://doi.org/10.3390/ijms22031291
APA StyleGuérin, L. -P., Le-Bel, G., Desjardins, P., Couture, C., Gillard, E., Boisselier, É., Bazin, R., Germain, L., & Guérin, S. L. (2021). The Human Tissue-Engineered Cornea (hTEC): Recent Progress. International Journal of Molecular Sciences, 22(3), 1291. https://doi.org/10.3390/ijms22031291