Vibrational Spectroscopic Investigation of Blood Plasma and Serum by Drop Coating Deposition for Clinical Application
Abstract
:1. Introduction
2. Results and Discussions
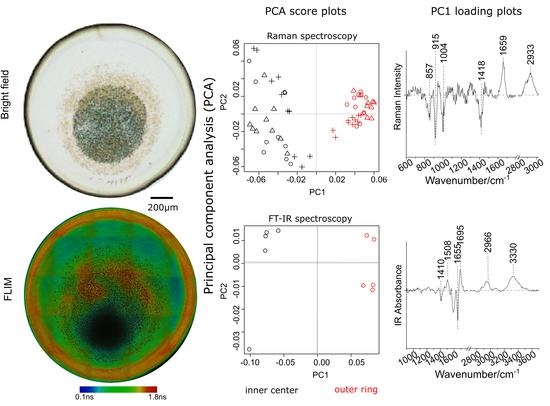
2.1. Microscopic Morphology and Measurement Schematic of Dried Sample Drop
2.2. Vibrational Spectra Comparison between the Inner and Outer-Ring Region of Dried Sample Drop
2.3. Distribution of Abundant Blood Protein in the Dried Droplet
2.4. PCA Analysis of Spectra between Inner and Outer-Ring Regions of Dried Sample Drop
2.5. Influence of Biomolecule Differences in the Inner and Outer-Ring Regions of Dried Sample Drop for Patient Characterization
2.5.1. A Heart Failure Patient
2.5.2. An ischemic Cardiomyopathy Patient
3. Experimental
3.1. Sample Collection and Preparation
3.2. Vibrational Spectroscopy Measurements
3.3. Fluorescence Lifetime Measurements
3.4. Data Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Larson, R.G. Twenty Years of Drying Droplets. Nature 2017, 550, 466–467. [Google Scholar] [CrossRef] [PubMed]
- Deegan, D.R.; Bakajin, O.; Witten, T.A. Capillary Flow as the Cause of Ring Stains from Dried Liquid Drops. Nature 1997, 389, 827. [Google Scholar] [CrossRef]
- Deegan, D.R.; Bakajin, O.; Dupont, T.F.; Huber, G.; Nagel, S.R.; Witten, T.A. Contact Line Deposits in an Evaporating Drop. Phys. Rev. 2000, 62, 756. [Google Scholar] [CrossRef] [Green Version]
- Hua, H.; Larson, R.G. Marangoni Effect Reverses Coffee-Ring Depositions. J. Phys. Chem. 2006, 110, 7090–7094. [Google Scholar] [CrossRef] [PubMed]
- Deegan, R.D. Pattern Formation in Drying Drops. Phys. Rev. 2000, 61, 475. [Google Scholar] [CrossRef] [Green Version]
- Hua, H.; Larson, R.G. Analysis of the Effects of Marangoni Stresses on the Microflow in an Evaporating Sessile Droplet. Langmuir 2005, 21, 3972–3980. [Google Scholar] [CrossRef]
- Singh, B.K.; Tirumkudulu, M.S. Cracking in Drying Colloidal Films. Phys. Rev. Lett. 2007, 98, 218302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, C.-S.; Shi, B.; Liu, C.; Gao, L.; Inyang, H.I. Experimental Investigation of the Desiccation Cracking Behavior of Soil Layers During Drying. J. Mater. Civ. Eng. 2010, 23, 873–878. [Google Scholar] [CrossRef]
- Tang, C.-S.; Shi, B.; Liu, C.; Suo, W.; Gao, L. Experimental Characterization of Shrinkage and Desiccation Cracking in Thin Clay Layer. Appl. Clay Sci. 2011, 52, 69–77. [Google Scholar] [CrossRef]
- Chen, R.; Zhang, L.; Zang, D.; Shen, W. Blood Drop Patterns: Formation and Applications. Adv. Colloid Interface Sci. 2016, 231, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Mohamed, G.J. Complex Protein Patterns Formation Via Salt-Induced Self-Assembly and Droplet Evaporation. Eur. Phys. J. 2010, 33, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Yakhno, T.A. Complex Pattern Formation in Sessile Droplets of Protein-Salt Solutions with Low Protein Content. What Substance Fabricates These Patterns? Phys. Chem. 2011, 1, 10–13. [Google Scholar] [CrossRef] [Green Version]
- Yakhno, T.A. Salt-Induced Protein Phase Transitions in Drying Drops. J. Colloid Interface Sci. 2008, 318, 225–230. [Google Scholar] [CrossRef] [PubMed]
- Zang, D.; Tarafdar, S.; Tarasevich, Y.Y.; Choudhury, M.D.; Dutta, T. Evaporation of a Droplet: From Physics to Applications. Phys. Rep. 2019, 804, 1–56. [Google Scholar] [CrossRef]
- Anderson, L.N.; Anderson, N.G. The Human Plasma Proteome: History, Character, and Diagnostic Prospects. Mol. Cell. Proteom. 2002, 1, 845–867. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Geyer, E.P.; Kulak, N.A.; Pichler, G.; Holdt, L.M.; Teupser, D.; Mann, M. Plasma Proteome Profiling to Assess Human Health and Disease. Cell Syst. 2016, 2, 185–195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Geyer, E.P.; Holdt, L.M.; Teupser, D.; Mann, M. Revisiting Biomarker Discovery by Plasma Proteomics. Mol. Syst. Biol. 2017, 13, 942. [Google Scholar] [CrossRef]
- Beck, C.H.; Overgaard, M.; Rasmussen, L.M. Plasma Proteomics to Identify Biomarkers–Application to Cardiovascular Diseases. Transl. Proteom. 2015, 7, 40–48. [Google Scholar] [CrossRef] [Green Version]
- Mateos-Cáceres, J.P.; García-Méndez, A.; Farré, A.L.; Macaya, C.; Núñez, A.; Gómez, J.; Alonso-Orgaz, S.; Carrasco, C.; Burgos, M.E.; de Andrés, R. Proteomic Analysis of Plasma from Patients During an Acute Coronary Syndrome. J. Am. Coll. Cardiol. 2004, 44, 1578–1583. [Google Scholar] [CrossRef] [Green Version]
- Coombes, R.K.; Morris, J.S.; Hu, J.; Edmonson, S.R.; Baggerly, K.A. Serum Proteomics Profiling—A Young Technology Begins to Mature. Nat. Biotechnol. 2005, 23, 291. [Google Scholar] [CrossRef]
- Liotta, A.L.; Ferrari, M.; Petricoin, E. Clinical Proteomics: Written in Blood. Nature 2003, 425, 905. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gowda, N.A.G.; Raftery, D. Analysis of Plasma, Serum, and Whole Blood Metabolites Using 1 h Nmr Spectroscopy. In Nmr-Based Metabolomics: Methods and Protocols; Nagana, G.A., Daniel, R., Eds.; Springer: New York, NY, USA, 2019; pp. 17–34. [Google Scholar]
- Suarez-Diez, M.; Chasapi, C.L.; Spyroulias, L.T.; Saccenti, E. Plasma and Serum Metabolite Association Networks: Comparability within and between Studies Using Nmr and Ms Profiling. J. Proteome Res. 2017, 16, 2547–2559. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Xie, Y.; Mrozek, M.F.; Ortiz, C.; Davisson, V.J.; Ben-Amotz, D. Raman Detection of Proteomic Analytes. Anal. Chem. 2003, 75, 5703–5709. [Google Scholar] [CrossRef] [PubMed]
- Neugebauer, U.; Trenkmann, S.; Bocklitz, T.; Schmerler, D.; Kiehntopf, M.; Popp, J. Fast Differentiation of Sirs and Sepsis from Blood Plasma of Icu Patients Using Raman Spectroscopy. J. Biophotonics 2014, 7, 232–240. [Google Scholar] [CrossRef]
- Barman, I.; Dingari, N.C.; Kang, J.W.; Horowitz, G.L.; Dasari, R.R.; Feld, M.S. Raman Spectroscopy-Based Sensitive and Specific Detection of Glycated Hemoglobin. Anal. Chem. 2012, 84, 2474–2482. [Google Scholar] [CrossRef] [Green Version]
- Owens, L.G.; Gajjar, K.; Trevisan, J.; Fogarty, S.W.; Taylor, S.E.; da Gama-Rose, B.; Martin-Hirsch, P.L.; Martin, F.L. Vibrational Biospectroscopy Coupled with Multivariate Analysis Extracts Potentially Diagnostic Features in Blood Plasma/Serum of Ovarian Cancer Patients. J. Biophotonics 2014, 7, 200–209. [Google Scholar] [CrossRef] [PubMed]
- Gajjar, K.; Trevisan, J.; Owens, G.; Keating, P.J.; Wood, N.J.; Stringfellow, H.F.; Martin-Hirsch, P.L.; Martin, F.L. Fourier-Transform Infrared Spectroscopy Coupled with a Classification Machine for the Analysis of Blood Plasma or Serum: A Novel Diagnostic Approach for Ovarian Cancer. Analyst 2013, 138, 3917–3926. [Google Scholar] [CrossRef]
- Hands, R.J.; Dorling, K.M.; Abel, P.; Ashton, K.M.; Brodbelt, A.; Davis, C.; Dawson, T.; Jenkinson, M.D.; Lea, R.W.; Walker, C. Attenuated Total Reflection Fourier Transform Infrared (Atr-Ftir) Spectral Discrimination of Brain Tumour Severity from Serum Samples. J. Biophotonics 2014, 7, 189–199. [Google Scholar] [CrossRef] [PubMed]
- Thumanu, K.; Sangrajrang, S.; Khuhaprema, T.; Kalalak, A.; Tanthanuch, W.; Pongpiachan, S.; Heraud, P. Diagnosis of Liver Cancer from Blood Sera Using Ftir Microspectroscopy: A Preliminary Study. J. Biophotonics 2014, 7, 222–231. [Google Scholar] [CrossRef]
- Zhang, D.; Mrozek, M.F.; Xie, Y.; Ben-Amotz, D. Chemical Segregation and Reduction of Raman Background Interference Using Drop Coating Deposition. Appl. Spectrosc. 2004, 58, 929–933. [Google Scholar] [CrossRef]
- Bahmani, L. The Study of Drying and Pattern Formation of Whole Human Blood Drops and the Effect of Thalassaemia and Neonatal Jaundice on the Patterns a Physicochemical and Engineering Aspects. Colloids Surf. 2017, 513, 10–75. [Google Scholar] [CrossRef]
- Cameron, J.; Butler, D.; Matthew, J.B. Biofluid Spectroscopic Disease Diagnostics: A Review on the Processes and Spectral Impact of Drying. J. Biophotonics 2018, 11, e201700299. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, J.; Ramoji, A.; Guo, S.; Bocklitz, T.; Boivin-Jahns, V.; Möller, J.; Kiehntopf, M.; Noutsias, M.; Popp, J.; Neugebauer, U. Vibrational Spectroscopy as a Powerful Tool for Follow-up Immunoadsorption Therapy Treatment of Dilated Cardiomyopathy—A Case Report. Analyst 2020, 145, 486–496. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2013. [Google Scholar]
- Wold, S.; Esbensen, K.; Geladi, P. Principal Component Analysis. Chemom. Intell. Lab. Syst. 1987, 2, 37–52. [Google Scholar] [CrossRef]
- Jansen, J.; Hoefsloot, J.; Timmerman, J.; Age, K.S. Asca: Analysis of Multivariate Data Obtained from an Experimental Design. J. Chemom. 2005, 19, 469–481. [Google Scholar] [CrossRef]
- Smilde, A.; Jansen, H.; Hoefsloot, R.; Lamers, J.; Marieke, E.T. Anova-Simultaneous Component Analysis (Asca): A New Tool for Analyzing Designed Metabolomics Data. Bioinformatics 2005, 21, 3043–3048. [Google Scholar] [CrossRef]
- Bocklitz, T.; Walter, A.; Hartmann, K.; Rösch, P.; Popp, J. How to Pre-Process Raman Spectra for Reliable and Stable Models? Anal. Chim. Acta 2011, 704, 47–56. [Google Scholar] [CrossRef]
- Eilers, C.P.H.; Boelens, H.F.M. Baseline Correction with Asymmetric Least Squares Smoothing; Royal Society of Chemistry: London, UK, 2005. [Google Scholar]
- Guo, S.; Heinke, R.; Stöckel, S.; Rösch, P.; Popp, J.; Bocklitz, T. Model Transfer for Raman-Spectroscopy-Based Bacterial Classification. J. Raman Spectrosc. 2018, 49, 627–637. [Google Scholar] [CrossRef]
- Iqbal, R.; Amy, S.Q.; Sen, A.K. Understanding of the Role of Dilution on Evaporative Deposition Patterns of Blood Droplets over Hydrophilic and Hydrophobic Substrates. J. Colloid Interface Sci. 2020, 579, 541–550. [Google Scholar] [CrossRef] [PubMed]
- Eales, D.A.; Dartnell, N.; Goddard, S.; Routh, A.F. The Impact of Trough Geometry on Film Shape. A Theoretical Study of Droplets Containing Polymer, for P-Oled Display Applications. J. Colloid Interface Sci. 2015, 458, 53–61. [Google Scholar] [CrossRef]
- Brutin, D.; Sobac, B.L.; Sampol, J. Pattern Formation in Drying Drops of Blood. J. Fluid Mech. 2011, 667, 85–95. [Google Scholar] [CrossRef] [Green Version]
- Wong, T.-S.; Chen, T.; Shen, X.; Ho, C. Nanochromatography Driven by the Coffee Ring Effect. Anal. Chem. 2011, 83, 1871–1873. [Google Scholar] [CrossRef] [PubMed]
- Schaller, J.; Gerber, S.; Kämpfer, U.; Lejon, S.; Trachsel, C. Blood Components. In Human Blood Plasma Proteins: Structure and Function; John Wiley & Sons Ltd.: West Sussex, UK, 2008; pp. 7–16. [Google Scholar]
- Fanali, G.; Trezza, M.; Marino, M.F.; Ascenzi, P. Human Serum Albumin: From Bench to Bedside. Mol. Asp. Med. 2012, 33, 209–290. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Xiang, X.; Wu, K.; He, H.; Chen, H.; Ma, C. A Novel Detection Method of Human Serum Albumin Based on the Poly(Thymine)-Templated Copper Nanoparticles. Sensors 2017, 17, 2684. [Google Scholar] [CrossRef] [Green Version]
- Sett, A. Colloids Dasgupta, Surfaces A: Rapid Estimation of the β-Sheet Content of Human Serum Albumin from the Drying Patterns of Hsa-Nanoparticle Droplets. Physicochem. Eng. Asp. 2018, 540, 177–185. [Google Scholar] [CrossRef]
- Katan, B.M.; Zock, P.L.; Mensink, R.P. Effects of Fats and Fatty Acids on Blood Lipids in Humans: An Overview. Am. J. Clin. Nutr. 1994, 60, 1017S–1022S. [Google Scholar] [CrossRef] [Green Version]





| Factors | Batches | Time | Position | Residuals | |
|---|---|---|---|---|---|
| Variance | |||||
| Raman (in %) | 23.74 | 23.69 | 4.22 | 48.36 | |
| FT-IR (in %) | 0.77 | 3.53 | 78.84 | 16.86 | |
| Factors | Batches | Time | Position | Residuals | |
|---|---|---|---|---|---|
| Variance | |||||
| Raman (in %) | 25.80 | 2.91 | 16.06 | 55.23 | |
| FT-IR (in %) | 1.42 | 3.21 | 69.82 | 25.55 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, J.; Ali, N.; Quansah, E.; Guo, S.; Noutsias, M.; Meyer-Zedler, T.; Bocklitz, T.; Popp, J.; Neugebauer, U.; Ramoji, A. Vibrational Spectroscopic Investigation of Blood Plasma and Serum by Drop Coating Deposition for Clinical Application. Int. J. Mol. Sci. 2021, 22, 2191. https://doi.org/10.3390/ijms22042191
Huang J, Ali N, Quansah E, Guo S, Noutsias M, Meyer-Zedler T, Bocklitz T, Popp J, Neugebauer U, Ramoji A. Vibrational Spectroscopic Investigation of Blood Plasma and Serum by Drop Coating Deposition for Clinical Application. International Journal of Molecular Sciences. 2021; 22(4):2191. https://doi.org/10.3390/ijms22042191
Chicago/Turabian StyleHuang, Jing, Nairveen Ali, Elsie Quansah, Shuxia Guo, Michel Noutsias, Tobias Meyer-Zedler, Thomas Bocklitz, Jürgen Popp, Ute Neugebauer, and Anuradha Ramoji. 2021. "Vibrational Spectroscopic Investigation of Blood Plasma and Serum by Drop Coating Deposition for Clinical Application" International Journal of Molecular Sciences 22, no. 4: 2191. https://doi.org/10.3390/ijms22042191
APA StyleHuang, J., Ali, N., Quansah, E., Guo, S., Noutsias, M., Meyer-Zedler, T., Bocklitz, T., Popp, J., Neugebauer, U., & Ramoji, A. (2021). Vibrational Spectroscopic Investigation of Blood Plasma and Serum by Drop Coating Deposition for Clinical Application. International Journal of Molecular Sciences, 22(4), 2191. https://doi.org/10.3390/ijms22042191