N-(1,3,4-Oxadiazol-2-yl)Benzamides as Antibacterial Agents against Neisseria gonorrhoeae
Abstract
:1. Introduction
2. Results and Discussion
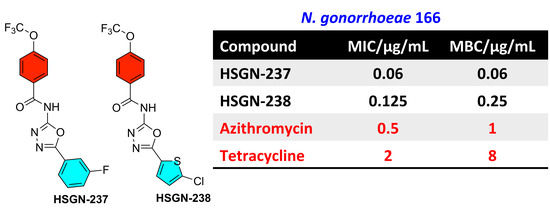
2.1. Synthesis and Antigonococcal Activity of N-(1,3,4-oxadiazol-2-yl)benzamides:
2.2. Antibacterial Activity of N-(1,3,4-oxadiazol-2-yl)benzamides against Other Bacterial Species:
2.3. Antibacterial Activity of N-(1,3,4-oxadiazol-2-yl)benzamides against N. gonorrhoeae in Presence of Serum:
2.4. N-(1,3,4-Oxadiazol-2-yl)benzamides Are Highly Tolerable to Human Cell Lines:
2.5. HSGN-238 Demonstrates High Intestinal Permeability:
3. Materials and Methods
3.1. Chemistry
3.2. Synthesis of 1,3,4-Oxadiazol-2-Amines [A.1-A.3]
3.3. Amide Coupling Procedure for Synthesis of Compounds
3.4. 3-Fluoro-4-(trifluoromethoxy)-N-(5-(4-(trifluoromethyl)phenyl)-1,3,4-oxadiazol-2-yl)benzamide (HSGN-235)
3.5. N-(5-(3-Fluorophenyl)-1,3,4-oxadiazol-2-yl)-4-(trifluoromethoxy)benzamide (HSGN-237)
3.6. N-(5-(5-Chlorothiophen-2-yl)-1,3,4-oxadiazol-2-yl)-4-(trifluoromethoxy)benzamide (HSGN-238)
3.7. Bacterial Strains, Media, Reagents and Cell Lines
3.8. Determination of the MICs of Compounds and Control Drugs against N. gonorrhoeae Strains
3.9. Determination of the MICs and MBCs of Compounds and Control Drugs against Clinically Important Gram-Positive and Gram-Negative Bacteria
3.10. In Vitro Cytotoxicity Analysis of HSGN-237 and -238 against Human Colorectal Cells
3.11. Caco-2 Permeability Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Unemo, M.; A Nicholas, R. Emergence of multidrug-resistant, extensively drug-resistant and untreatable gonorrhea. Futur. Microbiol. 2012, 7, 1401–1422. [Google Scholar] [CrossRef] [Green Version]
- Hill, S.A.; Masters, T.L.; Wachter, J. Gonorrhea—An evolving disease of the new millennium. Microb. Cell 2016, 3, 371–389. [Google Scholar] [CrossRef] [PubMed]
- Burnett, A.M.; Anderson, C.P.; Zwank, M.D. Laboratory-confirmed gonorrhea and/or chlamydia rates in clinically di-agnosed pelvic inflammatory disease and cervicitis. Am. J. Emerg. Med. 2012, 30, 1114–1117. [Google Scholar] [CrossRef]
- CDC. Antibiotic Resistance Threats in the United States. Available online: www.cdc.gov/DrugResistance/Biggest-Threats.html (accessed on 7 October 2020).
- Kirkcaldy, R.D.; Harvey, A.; Papp, J.R.; Del Rio, C.; Soge, O.O.; Holmes, K.K.; Torrone, E. Neisseria gonorrhoeae Antimicrobial Susceptibility Surveillance—The Gonococcal Isolate Surveillance Project, 27 Sites, United States, 2014. MMWR Surveill. Summ. 2016, 65, 1–19. [Google Scholar] [CrossRef] [Green Version]
- Newman, L.M.; Rowley, J.T.; Hoorn, S.V.; Wijesooriya, N.S.; Unemo, M.; Low, N.; Stevens, G.A.; Gottlieb, S.L.; Kiarie, J.; Temmerman, M. Global Estimates of the Prevalence and Incidence of Four Curable Sexually Transmitted Infections in 2012 Based on Systematic Review and Global Reporting. PLoS ONE 2015, 10, e0143304. [Google Scholar] [CrossRef] [Green Version]
- Kirkcaldy, R.D.; Weston, E.; Segurado, A.C.; Hughes, G. Epidemiology of gonorrhoea: A global perspective. Sex. Heal. 2019, 16, 401–411. [Google Scholar] [CrossRef] [PubMed]
- World Health Organisation. WHO Publishes List of Bacteria for which New Antibiotics are Urgently Needed. 27 February 2017, News Release, Geneva. Available online: https://www.who.int/news-room/detail/27-02-2017-who-publishes-list-of-bacteria-for-which-new-antibiotics-are-urgently-needed (accessed on 25 October 2018).
- Naclerio, G.A.; Sintim, H.O. Multiple ways to kill bacteria via inhibiting novel cell wall or membrane targets. Futur. Med. Chem. 2020, 12, 1253–1279. [Google Scholar] [CrossRef]
- Unemo, M.; Shafer, W.M. Antimicrobial Resistance in Neisseria gonorrhoeae in the 21st Century: Past, Evolution, and Future. Clin. Microbiol. Rev. 2014, 27, 587–613. [Google Scholar] [CrossRef] [Green Version]
- Workowski, K.A.; Bolan, G.A.; Prevention, C.F.D.C.A. Sexually transmitted diseases treatment guidelines, 2015. MMWR. Recomm. Rep. 2015, 64, 1–137. [Google Scholar] [PubMed]
- Wise, R.; BSAC Working Party on The Urgent Need: Regenerating Antibacterial Drug Discovery and Development; Blaser, M.; Carrs, O.; Cassell, G.; Fishman, N.; White, T. The urgent need for new antibacterial agents. J. Antimicrobe Chemother. 2011, 66, 1939–1940. [Google Scholar] [CrossRef] [Green Version]
- Blomquist, P.B.; Miari, V.F.; Biddulph, J.P.; Charalambous, B.M. Is gonorrhea becoming untreatable? Futur. Microbiol. 2014, 9, 189–201. [Google Scholar] [CrossRef]
- Barbee, L.A. Preparing for an era of untreatable gonorrhea. Curr. Opin. Infect. Dis. 2014, 27, 282–287. [Google Scholar] [CrossRef] [Green Version]
- Naclerio, G.A.; Karanja, C.W.; Opoku-Temeng, C.; Sintim, H.O. Antibacterial Small Molecules That Potently Inhibit Staphylococcus aureus Lipoteichoic Acid Biosynthesis. ChemMedChem 2019, 14, 1000–1004. [Google Scholar] [CrossRef]
- Opoku-Temeng, C.; Naclerio, G.A.; Mohammad, H.; Dayal, N.; Abutaleb, N.S.; Seleem, M.N.; Sintim, H.O. N-(1,3,4-oxadiazol-2-yl)benzamide analogs, bacteriostatic agents against methicillin- and vancomycin-resistant bacteria. Eur. J. Med. Chem. 2018, 155, 797–805. [Google Scholar] [CrossRef] [PubMed]
- Naclerio, G.A.; Abutaleb, N.S.; Onyedibe, K.I.; Seleem, M.N.; Sintim, H.O. Potent trifluoromethoxy, trifluoromethylsulfonyl, trifluoromethylthio and pentafluorosulfanyl containing (1,3,4-oxadiazol-2-yl)benzamides against drug-resistant Gram-positive bacteria. RSC Med. Chem. 2020, 11, 102–110. [Google Scholar] [CrossRef] [PubMed]
- Dansette, P.M.; Bertho, G.; Mansuy, D. First evidence that cytochrome P450 may catalyze both S-oxidation and epoxidation of thiophene derivatives. Biochem. Biophys. Res. Commun. 2005, 338, 450–455. [Google Scholar] [CrossRef]
- Valadon, P.; Dansette, P.M.; Girault, J.P.; Amar, C.; Mansuy, D. Thiophene sulfoxides as reactive metabolites: Formation upon microsomal oxidation of a 3-aroylthiophene and fate in the presence of nucleophiles in vitro and in vivo. Chem. Res. Toxicol. 1996, 9, 1403–1413. [Google Scholar] [CrossRef]
- Mansuy, D.; Dansette, P.M. Sulfenic acids as reactive intermediates in xenobiotic metabolism. Arch. Biochem. Biophys. 2011, 507, 174–185. [Google Scholar] [CrossRef] [Green Version]
- Gramec, D.; Mašič, L.P.; Dolenc, M.S. Bioactivation Potential of Thiophene-Containing Drugs. Chem. Res. Toxicol. 2014, 27, 1344–1358. [Google Scholar] [CrossRef] [PubMed]
- Feng, P.; Lee, K.N.; Lee, J.W.; Zhan, C.; Ngai, M.Y. Access to a new class of synthetic building blocks via trifluorometh-oxylation of pyridines and pyrimidines. Chem. Sci. 2016, 7, 424–429. [Google Scholar] [CrossRef] [Green Version]
- Leroux, F.R.; Manteau, B.; Vors, J.P.; Pazenok, S. Trifluoromethyl ethers--synthesis and properties of an unusual sub-stituent. Beilstein J. Org. Chem. 2008, 4, 13. [Google Scholar] [CrossRef] [Green Version]
- van de Waterbeemd, H.; Testa, B.; Mannhold, R.; Kubinyi, H.; Folkers, G. Drug Bioavailability: Estimation of Solubility, Permeability, Absorption and Bioavailability, 2nd ed.; Wiley: Hoboken, NJ, USA, 2008; p. 649. [Google Scholar]
- Gentry, C.L.; Egleton, R.D.; Gillespie, T.; Abbruscato, T.J.; Bechowski, H.B.; Hruby, V.J.; Davis, T.P. The effect of halo-genation on blood-brain barrier permeability of a novel peptide drug. Peptides 1999, 20, 1229–1238. [Google Scholar] [CrossRef]
- Hernandes, M.Z.; Cavalcanti, S.M.T.; Moreira, D.R.M.; Junior, W.F.D.A.; Leite, A.C.L. Halogen Atoms in the Modern Medicinal Chemistry: Hints for the Drug Design. Curr. Drug Targets 2010, 11, 303–314. [Google Scholar] [CrossRef]
- Gerebtzoff, G.; Li-Blatter, X.; Fischer, H.; Frentzel, A.; Seelig, A. Halogenation of Drugs Enhances Membrane Binding and Permeation. ChemBioChem 2004, 5, 676–684. [Google Scholar] [CrossRef]
- Mendez, L.; Henriquez, G.; Sirimulla, S.; Narayan, M. Looking Back, Looking Forward at Halogen Bonding in Drug Discovery. Molecules 2017, 22, 1397. [Google Scholar] [CrossRef] [PubMed]
- Wilcken, R.; Zimmermann, M.O.; Lange, A.; Joerger, A.C.; Boeckler, F.M. Principles and Applications of Halogen Bonding in Medicinal Chemistry and Chemical Biology. J. Med. Chem. 2013, 56, 1363–1388. [Google Scholar] [CrossRef]
- Unemo, M.; Golparian, D.; Sanchez-Buso, L.; Grad, Y.; Jacobsson, S.; Ohnishi, M.; Lahra, M.M.; Limnios, A.; Sikora, A.E.; Wi, T.; et al. The novel 2016 WHO Neisseria gonorrhoeae reference strains for global quality assurance of laboratory investigations: Phenotypic, genetic and reference genome characterization. J. Antimicrob. Chemother. 2016, 71, 3096–3108. [Google Scholar] [CrossRef] [Green Version]
- Piddock, L.J.V. Multidrug-resistance efflux pumps? not just for resistance. Nat. Rev. Genet. 2006, 4, 629–636. [Google Scholar] [CrossRef]
- Opperman, T.J.; Nguyen, S.T. Recent advances toward a molecular mechanism of efflux pump inhibition. Front. Microbiol. 2015, 6, 421. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.; Xie, S.; Ahmed, S.; Wang, F.; Gu, Y.; Zhang, C.; Chai, X.; Wu, Y.; Cai, J.; Cheng, G. Antimicrobial Activity and Re-sistance: Influencing Factors. Front Pharmacol. 2017, 8, 364. [Google Scholar] [CrossRef] [Green Version]
- Kaplan, N.; Awrey, D.; Bardouniotis, E.; Berman, J.; Yethon, J.; Pauls, H.W.; Hafkin, B. In vitroactivity (MICs and rate of kill) of AFN-1252, a novel FabI inhibitor, in the presence of serum and in combination with other antibiotics. J. Chemother. 2013, 25, 18–25. [Google Scholar] [CrossRef] [Green Version]
- Zeitlinger, M.A.; Derendorf, H.; Mouton, J.W.; Cars, O.; Craig, W.A.; Andes, D.; Theuretzbacher, U. Protein Binding: Do We Ever Learn? Antimicrob. Agents Chemother. 2011, 55, 3067–3074. [Google Scholar] [CrossRef] [Green Version]
- Tang, W.-H.; Wang, C.-F.; Liao, Y.-D. Fetal bovine serum albumin inhibits antimicrobial peptide activity and binds drug only in complex with α1-antitrypsin. Sci. Rep. 2021, 11, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Schneider, E.K.; Huang, J.X.; Carbone, V.; Han, M.; Zhu, Y.; Nang, S.; Khoo, K.K.; Mak, J.; Cooper, M.A.; Li, J.; et al. Plasma Protein Binding Structure–Activity Relationships Related to the N-Terminus of Daptomycin. ACS Infect. Dis. 2017, 3, 249–258. [Google Scholar] [CrossRef] [PubMed]
- Hou, T.J.; Zhang, W.; Xia, K.; Qiao, X.B.; Xu, X.J. ADME evaluation in drug discovery. 5. Correlation of Caco-2 permeation with simple molecular properties. J. Chem. Inf. Comput. Sci. 2004, 44, 1585–1600. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grès, M.; Julian, B.; Bourrié, M.; Meunier, V.; Roques, C.; Berger, M.; Boulenc, X.; Berger, Y.; Fabre, G. Correlation Between Oral Drug Absorption in Humans, and Apparent Drug Permeability in TC-7 Cells, A Human Epithelial Intestinal Cell Line: Comparison with the Parental Caco-2 Cell Line. Pharm. Res. 1998, 15, 726–733. [Google Scholar] [CrossRef] [PubMed]
- Artursson, P. Cell cultures as models for drug absorption across the intestinal mucosa. Crit. Rev. Ther. Drug Carr. Syst. 1991, 8, 305–330. [Google Scholar]
- Kaur, J.; Soto-Velasquez, M.; Ding, Z.; Ghanbarpour, A.; Lill, M.A.; Van Rijn, R.M.; Watts, V.J.; Flaherty, D.P. Optimization of a 1,3,4-oxadiazole series for inhibition of Ca2+/calmodulin-stimulated activity of adenylyl cyclases 1 and 8 for the treatment of chronic pain. Eur. J. Med. Chem. 2019, 162, 568–585. [Google Scholar] [CrossRef]
- Alhashimi, M.; Mayhoub, A.; Seleem, M.N. Repurposing Salicylamide for Combating Multidrug-Resistant Neisseria gonorrhoeae. Antimicrob. Agents Chemother. 2019, 63. [Google Scholar] [CrossRef]
- Elkashif, A.; Seleem, M.N. Investigation of auranofin and gold-containing analogues antibacterial activity against mul-tidrug-resistant Neisseria gonorrhoeae. Sci. Rep. 2020, 10, 5602. [Google Scholar] [CrossRef] [PubMed]
- Seong, Y.J.; Alhashimi, M.; Mayhoub, A.; Mohammad, H.; Seleem, M.N. Repurposing Fenamic Acid Drugs to Combat Multidrug-Resistant Neisseria gonorrhoeae. Antimicrob. Agents Chemother. 2020, 64, 7. [Google Scholar] [CrossRef]
- Abutaleb, N.S.; Seleem, M.N. Repurposing the Antiamoebic Drug Diiodohydroxyquinoline for Treatment of Clostridi-oides difficile Infections. Antimicrob. Agents Chemother. 2020, 64. [Google Scholar] [CrossRef]
- Elsebaei, M.M.; Mohammad, H.; Samir, A.; Abutaleb, N.S.; Norvil, A.B.; Michie, A.R.; Moustafa, M.M.; Samy, H.; Gowher, H.; Seleem, M.N.; et al. Lipophilic efficient phenylthiazoles with potent undecaprenyl pyrophosphatase in-hibitory activity. Eur. J. Med. Chem. 2019, 175, 49–62. [Google Scholar] [CrossRef]
- Kotb, A.; Abutaleb, N.S.; Hagras, M.; Bayoumi, A.; Moustafa, M.M.; Ghiaty, A.; Seleem, M.N.; Mayhoub, A.S. tert-Butylphenylthiazoles with an oxadiazole linker: A novel orally bioavailable class of antibiotics exhibiting antibiofilm activity. RSC Adv. 2019, 9, 6770–6778. [Google Scholar] [CrossRef] [Green Version]
- Cockerill, F.R.; Hindler, J.A. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically, 9th ed.; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2012. [Google Scholar]
- Dokla, E.M.; Abutaleb, N.S.; Milik, S.N.; Li, D.; El-Baz, K.; Shalaby, M.-A.W.; Al-Karaki, R.; Nasr, M.; Klein, C.D.; Abouzid, K.A.; et al. Development of benzimidazole-based derivatives as antimicrobial agents and their synergistic effect with colistin against gram-negative bacteria. Eur. J. Med. Chem. 2020, 186, 111850. [Google Scholar] [CrossRef] [PubMed]
- Hammad, S.G.; El-Gazzar, M.G.; Abutaleb, N.S.; Li, D.; Ramming, I.; Shekhar, A.; Abdel-Halim, M.; Elrazaz, E.Z.; Seleem, M.N.; Bilitewski, U.; et al. Synthesis and antimicrobial evaluation of new halogenated 1,3-Thiazolidin-4-ones. Bioorganic Chem. 2020, 95, 103517. [Google Scholar] [CrossRef] [PubMed]
- Obach, R.S.; Baxter, J.G.; E Liston, T.; Silber, B.M.; Jones, B.C.; MacIntyre, F.; Rance, D.J.; Wastall, P. The prediction of human pharmacokinetic parameters from preclinical and in vitro metabolism data. J. Pharmacol. Exp. Ther. 1997, 283, 46–58. [Google Scholar] [PubMed]
- Hammad, A.; Abutaleb, N.S.; Elsebaei, M.M.; Norvil, A.B.; Alswah, M.; Ali, A.O.; Abdel-Aleem, J.A.; Alattar, A.; Bayoumi, S.A.; Gowher, H.; et al. From Phenylthiazoles to Phenylpyrazoles: Broadening the Antibacterial Spectrum toward Carbapenem-Resistant Bacteria. J. Med. Chem. 2019, 62, 7998–8010. [Google Scholar] [CrossRef] [PubMed]
- Opoku-Temeng, C.; Dayal, N.; Miller, J.; Sintim, H.O. Hydroxybenzylidene-indolinones, c-di-AMP synthase inhibitors, have antibacterial and anti-biofilm activities and also re-sensitize resistant bacteria to methicillin and vancomycin. RSC Adv. 2017, 7, 8288–8294. [Google Scholar] [CrossRef] [Green Version]
- Opoku-Temeng, C.; Onyedibe, K.I.; Aryal, U.K.; Sintim, H.O. Proteomic analysis of bacterial response to a 4-hydroxybenzylidene indolinone compound, which re-sensitizes bacteria to traditional antibiotics. J. Proteom. 2019, 202, 103368. [Google Scholar] [CrossRef]
- Dayal, N.; Opoku-Temeng, C.; Mohammad, H.; Abutaleb, N.S.; Hernandez, D.; Onyedibe, K.I.; Wang, M.; Zeller, M.; Seleem, M.N.; Sintim, H.O. Inhibitors of Intracellular Gram-Positive Bacterial Growth Synthesized via Povarov–Doebner Reactions. ACS Infect. Dis. 2019, 5, 1820–1830. [Google Scholar] [CrossRef] [PubMed]
- Naclerio, G.A.; Abutaleb, N.S.; Li, D.; Seleem, M.N.; Sintim, H.O. Ultrapotent Inhibitor of Clostridioides difficile Growth, Which Suppresses Recurrence In Vivo. J. Med. Chem. 2020, 63, 11934–11944. [Google Scholar] [CrossRef] [PubMed]



| Compound/Control Drug | N. gonorrhoeae Strain 181 |
| Compound 6 | 0.5 |
| Compound 12 | 0.06 |
| Compound 13 | 0.06 |
| HSGN-235 | 16 |
| HSGN-237 | 0.125 |
| HSGN-238 | 0.125 |
| Azithromycin | 256 |
| Tetracycline | 2 |
| Bacterial Strains | HSGN-235 | HSGN-237 | HSGN-238 | Azithromycin | Tetracycline | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | |
| N. gonorrhoeae 165 | 2 | 2 | 0.06 | 0.125 | 0.125 | 0.25 | 1 | 4 | 4 | 8 |
| N. gonorrhoeae 166 | 2 | 2 | 0.06 | 0.06 | 0.125 | 0.25 | 0.5 | 1 | 2 | 8 |
| N. gonorrhoeae 194 | 1 | 1 | 0.03 | 0.06 | 0.125 | 0.125 | 0.25 | 0.5 | 1 | 4 |
| N. gonorrhoeae 197 | 1 | 2 | 0.03 | 0.06 | 0.125 | 0.125 | 0.5 | 2 | 2 | 4 |
| N. gonorrhoeae 200 | 2 | 2 | 0.06 | 0.06 | 0.125 | 0.125 | 0.5 | 0.5 | 2 | 8 |
| N. gonorrhoeae WHO L | 1 | 2 | 0.06 | 0.06 | 0.06 | 0.125 | 0.5 | 1 | 0.5 | 2 |
| HSGN-235 | HSGN-237 | HSGN-238 | Vancomycin | Linezolid | Gentamicin | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bacterial Strains | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC |
| S. aureus ATCC 25923 | 1 | >64 | 0.25 | >64 | 0.25 | >64 | 1 | 1 | 2 | 64 | NT | NT |
| MRSA USA300 | 0.5 | 64 | 0.25 | 32 | 0.25 | 16 | 1 | 2 | 1 | 16 | NT | NT |
| E. faecalis ATCC 29212 | 4 | 32 | 1 | >64 | 1 | 32 | 1 | 1 | 2 | 64 | NT | NT |
| VRE. faecalisATCC 51575 | 2 | >64 | 1 | >64 | 1 | 32 | >64 | >64 | 2 | 64 | NT | NT |
| VRE. faecalis ATCC 51299 | 1 | 64 | 0.5 | 16 | 0.25 | 8 | >64 | >64 | 1 | 32 | NT | NT |
| VRE. faecium ATCC 700221 | 1 | 32 | 0.5 | 8 | 0.25 | 8 | 32 | 32 | 2 | 64 | NT | NT |
| L. monocytogenes ATCC 19115 | 1 | 64 | 0.5 | 64 | 0.5 | 32 | 1 | 1 | 2 | 64 | NT | NT |
| E. coli BW25113 (wild-type strain) | >8 | >8 | >8 | >8 | >8 | >8 | >64 | >64 | >64 | >64 | 0.25 | 0.25 |
| E. coli JW55031 (TolC Mutant) | 4 | >64 | 0.25 | 16 | 0.06 | 32 | >64 | >64 | 8 | >64 | 0.25 | 0.25 |
| Compound/Control Drug | Mean A → B Papp (cm s−1) | Mean B → A Papp (cm s−1) | Notes |
| HSGN-238 | 82.3 × 10−6 | 32.9 × 10−6 | High Permeability |
| Ranitidine | 0.5 × 10−6 | 1.3 × 10−6 | Low Permeability Control |
| Propranolol | 37.2 × 10−6 | 22.7 × 10−6 | High Permeability Control |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Naclerio, G.A.; Abutaleb, N.S.; Alhashimi, M.; Seleem, M.N.; Sintim, H.O. N-(1,3,4-Oxadiazol-2-yl)Benzamides as Antibacterial Agents against Neisseria gonorrhoeae. Int. J. Mol. Sci. 2021, 22, 2427. https://doi.org/10.3390/ijms22052427
Naclerio GA, Abutaleb NS, Alhashimi M, Seleem MN, Sintim HO. N-(1,3,4-Oxadiazol-2-yl)Benzamides as Antibacterial Agents against Neisseria gonorrhoeae. International Journal of Molecular Sciences. 2021; 22(5):2427. https://doi.org/10.3390/ijms22052427
Chicago/Turabian StyleNaclerio, George A., Nader S. Abutaleb, Marwa Alhashimi, Mohamed N. Seleem, and Herman O. Sintim. 2021. "N-(1,3,4-Oxadiazol-2-yl)Benzamides as Antibacterial Agents against Neisseria gonorrhoeae" International Journal of Molecular Sciences 22, no. 5: 2427. https://doi.org/10.3390/ijms22052427
APA StyleNaclerio, G. A., Abutaleb, N. S., Alhashimi, M., Seleem, M. N., & Sintim, H. O. (2021). N-(1,3,4-Oxadiazol-2-yl)Benzamides as Antibacterial Agents against Neisseria gonorrhoeae. International Journal of Molecular Sciences, 22(5), 2427. https://doi.org/10.3390/ijms22052427