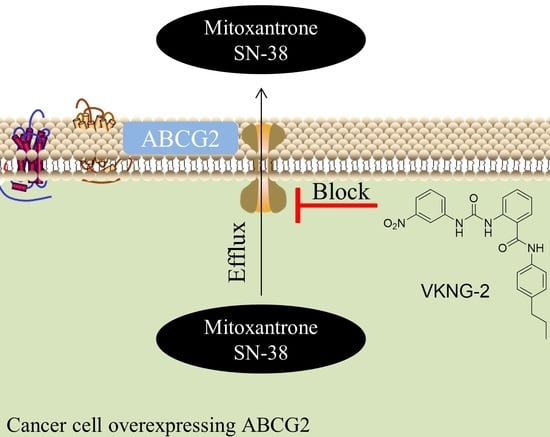
The Novel Benzamide Derivative, VKNG-2, Restores the Efficacy of Chemotherapeutic Drugs in Colon Cancer Cell Lines by Inhibiting the ABCG2 Transporter
Abstract
:1. Introduction
2. Results
2.1. VKNG-2 Sensitizes ABCG2 Overexpressing Cells to Mitoxantrone and SN-38
2.2. VKNG-2 Significantly Decreases the Efflux of [3H]-mitoxantrone in S1-M1-80 Colon Cancer Cells
2.3. The Effect of VKNG-2 on the Expression of the ABCG2 Transporter
2.4. The Effect of VKNG-2 on the ATPase Activity of the ABCG2 and ABCB1 Transporter
2.5. The Effect of VKNG-2 on the Cellular Localization of the ABCG2 Transporter Protein
2.6. The Docking Simulation of VKNG-2 in the Drug-binding Pocket of the Human ABCG2, ABCB1 and ABCC1 Protein
3. Discussion and Conclusions
4. Materials and Methods
4.1. Chemicals
4.2. Equipment
4.3. Cell Lines
4.4. MTT Assay for Cytotoxicity Determination and the Reversal Experiments
4.5. [3. H]- Mitoxantrone accumulation and efflux assay
4.6. Western Blot Analysis
4.7. ATPase Assay
4.8. Immunofluorescence
4.9. Molecular Docking of VKNG-2 with the Human ABCG2, ABCB1 and ABCC1 Model
4.10. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Narayanan, S.; Gupta, P.; Nazim, U.; Ali, M.; Karadkhelkar, N.; Ahmad, M.; Chen, Z.-S. Anti-cancer effect of Indanone-based thiazolyl hydrazone derivative on colon cancer cell lines. Int. J. Biochem. Cell Biol. 2019, 110, 21–28. [Google Scholar] [CrossRef]
- Barbuti, A.M.; Zhang, G.-N.; Gupta, P.; Narayanan, S.; Chen, Z.-S. EGFR and HER2 Inhibitors as Sensitizing Agents for Cancer Chemotherapy. In Protein Kinase Inhibitors as Sensitizing Agents for Chemotherapy; Elsevier: Amsterdam, The Netherlands, 2019; Volume 4, pp. 1–11. [Google Scholar]
- Gupta, P.; Narayanan, S.; Yang, D.-H. CDK Inhibitors as Sensitizing Agents for Cancer Chemotherapy. In Protein Kinase Inhibitors as Sensitizing Agents for Chemotherapy; Elsevier: Amsterdam, The Netherlands, 2019; Volume 4, pp. 125–149. [Google Scholar]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [Green Version]
- Narayanan, S.; Cai, C.-Y.; Assaraf, Y.G.; Guo, H.-Q.; Cui, Q.; Wei, L.; Huang, J.-J.; Ashby, C.R.; Chen, Z.-S. Targeting the ubiquitin-proteasome pathway to overcome anti-cancer drug resistance. Drug Resist. Updat. 2020, 48, 100663. [Google Scholar] [CrossRef] [PubMed]
- Silpa, N.; Koya, J.; Wang, J.; Assaraf, Y.G.; Ashby, C.R., Jr.; Chen, Z.-S. Poly (ADP-ribose) polymerase (PARP) inhibitors as chemosensitizing compounds for the treatment of drug resistant cancers. J. Mol. Clin. Med. 2019, 2, 55–67. [Google Scholar]
- Gupta, P.; Xie, M.; Narayanan, S.; Wang, Y.-J.; Wang, X.-Q.; Yuan, T.; Wang, Z.; Yang, N.-H.; Chen, Z.-S. GSK1904529A, a Potent IGF-IR Inhibitor, Reverses MRP1-Mediated Multidrug Resistance. J. Cell. Biochem. 2017, 118, 3260–3267. [Google Scholar] [CrossRef]
- Binkhathlan, Z. P-glycoprotein Inhibition as a Therapeutic Approach for Overcoming Multidrug Resistance in Cancer: Current Status and Future Perspectives. Curr. Cancer Drug Targets 2013, 13, 326–346. [Google Scholar] [CrossRef]
- Tiwari, A.K.; Sodani, K.; Dai, C.-L.; Ashby, C.R.; Chen, Z.-S. Revisiting the ABCs of Multidrug Resistance in Cancer Chemotherapy. Curr. Pharm. Biotechnol. 2011, 12, 570–594. [Google Scholar] [CrossRef]
- De Vera, A.A.; Gupta, P.; Lei, Z.; Liao, D.; Narayanan, S.; Teng, Q.; Reznik, S.E.; Chen, Z.-S. Immuno-oncology agent IPI-549 is a modulator of P-glycoprotein (P-gp, MDR1, ABCB1)-mediated multidrug resistance (MDR) in cancer: In vitro and in vivo. Cancer Lett. 2019, 442, 91–103. [Google Scholar] [CrossRef] [PubMed]
- Robey, R.W.; Pluchino, K.M.; Hall, M.D.; Fojo, A.T.; Bates, S.E.; Gottesman, M.M. Revisiting the role of ABC transporters in multidrug-resistant cancer. Nat. Rev. Cancer 2018, 18, 452–464. [Google Scholar] [CrossRef]
- Gottesman, M.M.; Lavi, O.; Hall, M.D.; Gillet, J.-P. Toward a Better Understanding of the Complexity of Cancer Drug Resistance. Annu. Rev. Pharmacol. Toxicol. 2016, 56, 85–102. [Google Scholar] [CrossRef] [PubMed]
- Favoriti, P.; Carbone, G.; Greco, M.; Pirozzi, F.; Pirozzi, R.E.M.; Corcione, F. Worldwide burden of colorectal cancer: A review. Updat. Surg. 2016, 68, 7–11. [Google Scholar] [CrossRef]
- Gustavsson, B.; Carlsson, G.; Machover, D.; Petrelli, N.; Roth, A.; Schmoll, H.-J.; Tveit, K.-M.; Gibson, F. A Review of the Evolution of Systemic Chemotherapy in the Management of Colorectal Cancer. Clin. Color. Cancer 2015, 14, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Fakih, M.G. Metastatic Colorectal Cancer: Current State and Future Directions. J. Clin. Oncol. 2015, 33, 1809–1824. [Google Scholar] [CrossRef] [PubMed]
- Hammond, W.A.; Swaika, A.; Mody, K. Pharmacologic resistance in colorectal cancer: A review. Ther. Adv. Med Oncol. 2015, 8, 57–84. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fletcher, J.I.; Williams, R.T.; Henderson, M.J.; Norris, M.D.; Haber, M. ABC transporters as mediators of drug resistance and contributors to cancer cell biology. Drug Resist. Updat. 2016, 26, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Zhang, H.; Assaraf, Y.G.; Zhao, K.; Xu, X.; Xie, J.; Yang, D.-H.; Chen, Z.-S. Overcoming ABC transporter-mediated multidrug resistance: Molecular mechanisms and novel therapeutic drug strategies. Drug Resist. Updat. 2016, 27, 14–29. [Google Scholar] [CrossRef]
- Chen, Z.; Shi, T.; Zhang, L.; Zhu, P.; Deng, M.; Huang, C.; Hu, T.; Jiang, L.; Li, J. Mammalian drug efflux transporters of the ATP binding cassette (ABC) family in multidrug resistance: A review of the past decade. Cancer Lett. 2016, 370, 153–164. [Google Scholar] [CrossRef]
- Kathawala, R.J.; Li, T.; Yang, D.; Guo, H.-Q.; Yang, N.-H.; Chen, X.; Cheng, C.; Chen, Z.-S. 2-Trifluoromethyl-2-Hydroxypropionamide Derivatives as Novel Reversal Agents of ABCG2 (BCRP)-Mediated Multidrug Resistance: Synthesis and Biological Evaluations. J. Cell. Biochem. 2017, 118, 2420–2429. [Google Scholar] [CrossRef]
- Noguchi, K.; Katayama, K.; Sugimoto, Y. Human ABC transporter ABCG2/BCRP expression in chemoresistance: Basic and clinical perspectives for molecular cancer therapeutics. Pharmacogenomics Pers. Med. 2014, 7, 53–64. [Google Scholar] [CrossRef] [Green Version]
- Doyle, L.A.; Ross, D.D. Multidrug resistance mediated by the breast cancer resistance protein BCRP (ABCG2). Oncogene 2003, 22, 7340–7358. [Google Scholar] [CrossRef] [Green Version]
- Goler-Baron, V.; Assaraf, Y.G. Structure and Function of ABCG2-Rich Extracellular Vesicles Mediating Multidrug Resistance. PLoS ONE 2011, 6, e16007. [Google Scholar] [CrossRef] [Green Version]
- Nie, S.; Huang, Y.; Shi, M.; Qian, X.; Li, H.; Peng, C.; Kong, B.; Zou, X.; Shen, S. Protective role of ABCG2 against oxidative stress in colorectal cancer and its potential underlying mechanism. Oncol. Rep. 2018, 40, 2137–2146. [Google Scholar] [CrossRef]
- Sissung, T.M.; Goey, A.K.L.; Ley, A.M.; Strope, J.D.; Figg, W.D. Pharmacogenetics of Membrane Transporters: A Review of Current Approaches. Methods Mol. Biol. 2014, 1175, 91–120. [Google Scholar] [CrossRef]
- Candeil, L.; Gourdier, I.; Peyron, D.; Vezzio, N.; Copois, V.; Bibeau, F.; Orsetti, B.; Scheffer, G.L.; Ychou, M.; Khan, Q.A.; et al. ABCG2 overexpression in colon cancer cells resistant to SN38 and in irinotecan-treated metastases. Int. J. Cancer 2004, 109, 848–854. [Google Scholar] [CrossRef] [PubMed]
- Hsu, H.-H.; Chen, M.-C.; Baskaran, R.; Lin, Y.-M.; Day, C.H.; Lin, Y.-J.; Tu, C.-C.; Padma, V.V.; Kuo, W.-W.; Huang, C.-Y. Oxaliplatin resistance in colorectal cancer cells is mediated via activation of ABCG2 to alleviate ER stress induced apoptosis. J. Cell. Physiol. 2017, 233, 5458–5467. [Google Scholar] [CrossRef]
- Zinzi, L.; Capparelli, E.; Cantore, M.; Contino, M.; Leopoldo, M.; Colabufo, N.A. Small and Innovative Molecules as New Strategy to Revert MDR. Front. Oncol. 2014, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Christensen, S.; Van Der Roest, B.; Besselink, N.; Janssen, R.; Boymans, S.; Martens, J.W.M.; Yaspo, M.-L.; Priestley, P.; Kuijk, E.; Cuppen, E.; et al. 5-Fluorouracil treatment induces characteristic T>G mutations in human cancer. Nat. Commun. 2019, 10, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Fujita, K.-I.; Kubota, Y.; Ishida, H.; Sasaki, Y. Irinotecan, a key chemotherapeutic drug for metastatic colorectal cancer. World J. Gastroenterol. 2015, 21, 12234–12248. [Google Scholar] [CrossRef]
- Taflin, H.; Wettergren, Y.; Odin, E.; Derwinger, K. Folate levels measured by LC–MS/MS in patients with colorectal cancer treated with different leucovorin dosages. Cancer Chemother. Pharmacol. 2014, 74, 1167–1174. [Google Scholar] [CrossRef] [Green Version]
- Alcindor, T.; Beauger, N. Oxaliplatin: A Review in the Era of Molecularly Targeted Therapy. Curr. Oncol. 2011, 18, 18–25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Q.; Yan, H.; Zhao, P.; Yang, Y.; Cao, B. Efficacy and Safety of Bevacizumab Combined with Chemotherapy for Managing Metastatic Breast Cancer: A Meta-Analysis of Randomized Controlled Trials. Sci. Rep. 2015, 5, 15746. [Google Scholar] [CrossRef] [Green Version]
- Sato, J.D.; Kawamoto, T.; Le, A.D.; Mendelsohn, J.; Polikoff, J.; Sato, G.H. Biological effects in vitro of monoclonal antibodies to human epidermal growth factor receptors. Mol. Boil. Med. 1983, 1, 511–529. [Google Scholar]
- Cardinale, D.; Iacopo, F.; Cipolla, C.M. Cardiotoxicity of Anthracyclines. Front. Cardiovasc. Med. 2020, 7, 26. [Google Scholar] [CrossRef] [Green Version]
- Bedoui, Y.; Guillot, X.; Sélambarom, J.; Guiraud, P.; Giry, C.; Jaffar-Bandjee, M.C.; Ralandison, S.; Gasque, P. Methotrexate an Old Drug with New Tricks. Int. J. Mol. Sci. 2019, 20, 5023. [Google Scholar] [CrossRef] [Green Version]
- Gujarati, N.A.; Zeng, L.; Gupta, P.; Chen, Z.-S.; Korlipara, V.L. Design, synthesis and biological evaluation of benzamide and phenyltetrazole derivatives with amide and urea linkers as BCRP inhibitors. Bioorganic Med. Chem. Lett. 2017, 27, 4698–4704. [Google Scholar] [CrossRef]
- Pampaloni, F.; Reynaud, E.G.; Stelzer, E.H.K. The third dimension bridges the gap between cell culture and live tissue. Nat. Rev. Mol. Cell Biol. 2007, 8, 839–845. [Google Scholar] [CrossRef] [PubMed]
- Vesel, M.; Rapp, J.; Feller, D.; Kiss, E.; Jaromi, L.; Meggyes, M.; Miskei, G.; Duga, B.; Smuk, G.; Laszlo, T.; et al. ABCB1 and ABCG2 drug transporters are differentially expressed in non-small cell lung cancers (NSCLC) and expression is modified by cisplatin treatment via altered Wnt signaling. Respir. Res. 2017, 18, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Lin, N.; Li, Y. The PI3K/AKT signaling pathway regulates ABCG2 expression and confers resistance to chemotherapy in human multiple myeloma. Oncol. Rep. 2019, 41, 1678–1690. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Li, Y.; Wang, Q.; Chen, Z.; Li, X.; Wu, Z.; Hu, C.; Liao, D.; Zhang, W.; Chen, Z.-S. The PI3K subunits, P110α and P110β are potential targets for overcoming P-gp and BCRP-mediated MDR in cancer. Mol. Cancer 2020, 19, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, Y.H.; Yu, Y.H.C.A.A.-M. ABC Transporters in Multidrug Resistance and Pharmacokinetics, and Strategies for Drug Development. Curr. Pharm. Des. 2014, 20, 793–807. [Google Scholar] [CrossRef] [PubMed]
- Murakami, M.; Ohnuma, S.; Fukuda, M.; Chufan, E.E.; Kudoh, K.; Kanehara, K.; Sugisawa, N.; Ishida, M.; Naitoh, T.; Shibata, H.; et al. Synthetic Analogs of Curcumin Modulate the Function of Multidrug Resistance–Linked ATP-Binding Cassette Transporter ABCG2. Drug Metab. Dispos. 2017, 45, 1166–1177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.-K.; Wang, Y.-J.; Lei, Z.-N.; Zhang, G.-N.; Zhang, X.-Y.; Wang, D.-S.; Al Rihani, S.B.; Shukla, S.; Ambudkar, S.V.; Kaddoumi, A.; et al. Regorafenib antagonizes BCRP-mediated multidrug resistance in colon cancer. Cancer Lett. 2019, 442, 104–112. [Google Scholar] [CrossRef]
- Guo, X.; To, K.K.W.; Chen, Z.; Wang, X.; Zhang, J.; Luo, M.; Wang, F.; Yan, S.; Fu, L. Dacomitinib potentiates the efficacy of conventional chemotherapeutic agents via inhibiting the drug efflux function of ABCG2 in vitro and in vivo. J. Exp. Clin. Cancer Res. 2018, 37, 31. [Google Scholar] [CrossRef] [Green Version]
- Wu, C.-P.; Hsiao, S.-H.; Huang, Y.-H.; Hung, L.-C.; Yu, Y.-J.; Chang, Y.-T.; Hung, T.-H.; Wu, Y.-S. Sitravatinib Sensitizes ABCB1- and ABCG2-Overexpressing Multidrug-Resistant Cancer Cells to Chemotherapeutic Drugs. Cancers 2020, 12, 195. [Google Scholar] [CrossRef] [Green Version]
- Xu, L.; Huang, J.; Liu, J.; Xi, Y.; Zheng, Z.; To, K.K.; Chen, Z.; Wang, F.; Zhang, Y.; Fu, L. CM082 Enhances the Efficacy of Chemotherapeutic Drugs by Inhibiting the Drug Efflux Function of ABCG2. Mol. Ther. Oncolytics 2020, 16, 100–110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Toyoda, Y.; Takada, T.; Suzuki, H. Inhibitors of Human ABCG2: From Technical Background to Recent Updates with Clinical Implications. Front. Pharmacol. 2019, 10, 208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Narayanan, S.; Gujarati, N.A.; Teng, Q.-X.; Wang, J.-Q.; Cai, C.-Y.; Yang, Y.; Chintalapati, A.J.; Lei, Y.; Korlipara, V.L. VKNG-1 reverses multidrug resistance by inhibiting ABCG2-mediated drug transport via p-AKT pathway: In vitro and in vivo study. Eur. J. Cancer 2020. under review. [Google Scholar]
- Heyes, N.; Kapoor, P.; Kerr, I.D. Polymorphisms of the Multidrug Pump ABCG2: A Systematic Review of Their Effect on Protein Expression, Function, and Drug Pharmacokinetics. Drug Metab. Dispos. 2018, 46, 1886–1899. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kathawala, R.J.; Chen, J.-J.; Zhang, Y.-K.; Wang, Y.-J.; Patel, A.; Wang, D.-S.; Talele, T.T.; Ashby, C.R.; Chen, Z.-S. Masitinib antagonizes ATP-binding cassette subfamily G member 2-mediated multidrug resistance. Int. J. Oncol. 2014, 44, 1634–1642. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huo, Q.; Yuan, J.; Zhu, T.; Li, Z.; Xie, N. A Combined Bioinformatic and Nanoparticle-Based Study Reveal the Role of ABCG2 in the Resistance of Breast Cancer. Recent Patents Anti Cancer Drug Discov. 2021, 16, 1. [Google Scholar] [CrossRef]
- Ota, S.; Ishii, G.; Goto, K.; Kubota, K.; Kim, Y.H.; Kojika, M.; Murata, Y.; Yamazaki, M.; Nishiwaki, Y.; Eguchi, K.; et al. Immunohistochemical expression of BCRP and ERCC1 in biopsy specimen predicts survival in advanced non-small-cell lung cancer treated with cisplatin-based chemotherapy. Lung Cancer 2009, 64, 98–104. [Google Scholar] [CrossRef]
- De Lima, L.T.; Vivona, D.; Bueno, C.T.; Hirata, R.D.C.; Hirata, M.H.; Luchessi, A.D.; De Castro, F.A.; Chauffaille, M.D.L.F.; Zanichelli, M.A.; Chiattone, C.S.; et al. Reduced ABCG2 and increased SLC22A1 mRNA expression are associated with imatinib response in chronic myeloid leukemia. Med. Oncol. 2014, 31, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.S. Chemotherapy Resistance in Lung Cancer. Adv. Exp. Med. Biol. 2016, 893, 189–209. [Google Scholar] [CrossRef] [PubMed]
- Sodani, K.; Patel, A.; Anreddy, N.; Singh, S.; Yang, N.-H.; Kathawala, R.J.; Kumar, P.; Talele, T.T.; Chen, Z.-S. Telatinib reverses chemotherapeutic multidrug resistance mediated by ABCG2 efflux transporter in vitro and in vivo. Biochem. Pharmacol. 2014, 89, 52–61. [Google Scholar] [CrossRef] [Green Version]
- Westover, D.; Li, F. New trends for overcoming ABCG2/BCRP-mediated resistance to cancer therapies. J. Exp. Clin. Cancer Res. 2015, 34, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Ji, N.; Yang, Y.; Lei, Z.-N.; Cai, C.-Y.; Wang, J.-Q.; Gupta, P.; Xian, X.; Yang, D.-H.; Kong, D.; Chen, Z.-S. Ulixertinib (BVD-523) antagonizes ABCB1- and ABCG2-mediated chemotherapeutic drug resistance. Biochem. Pharmacol. 2018, 158, 274–285. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.-F.; Zhang, W.; Zeng, L.; Lei, Z.-N.; Cai, C.-Y.; Gupta, P.; Yang, D.-H.; Cui, Q.; Qin, Z.-D.; Chen, Z.-S.; et al. Dacomitinib antagonizes multidrug resistance (MDR) in cancer cells by inhibiting the efflux activity of ABCB1 and ABCG2 transporters. Cancer Lett. 2018, 421, 186–198. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.-K.; Zhang, X.-Y.; Zhang, G.-N.; Wang, Y.-J.; Xu, H.; Zhang, D.; Shukla, S.; Liu, L.; Yang, D.-H.; Ambudkar, S.V.; et al. Selective reversal of BCRP-mediated MDR by VEGFR-2 inhibitor ZM323881. Biochem. Pharmacol. 2017, 132, 29–37. [Google Scholar] [CrossRef]
- Cheng, G.M.Y.; To, K.K.W. Adverse Cell Culture Conditions Mimicking the Tumor Microenvironment Upregulate ABCG2 to Mediate Multidrug Resistance and a More Malignant Phenotype. ISRN Oncol. 2012, 2012, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, C.-P.; Lusvarghi, S.; Hsiao, S.-H.; Liu, T.-C.; Li, Y.-Q.; Huang, Y.-H.; Hung, T.-H.; Ambudkar, S.V. Licochalcone A Selectively Resensitizes ABCG2-Overexpressing Multidrug-Resistant Cancer Cells to Chemotherapeutic Drugs. J. Nat. Prod. 2020, 83, 1461–1472. [Google Scholar] [CrossRef]
- Robey, R.W.; Honjo, Y.; Morisaki, K.; A Nadjem, T.; Runge, S.; Risbood, M.; Poruchynsky, M.S.; E Bates, S. Mutations at amino-acid 482 in the ABCG2 gene affect substrate and antagonist specificity. Br. J. Cancer 2003, 89, 1971–1978. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Liu, Y.; Dong, Z.; Xu, J.; Peng, H.; Liu, Z.; Zhang, J.-T. Regulation of Function by Dimerization through the Amino-terminal Membrane-spanning Domain of Human ABCC1/MRP1. J. Biol. Chem. 2007, 282, 8821–8830. [Google Scholar] [CrossRef] [Green Version]
- Wang, D.-S.; Patel, A.; Sim, H.-M.; Zhang, Y.-K.; Wang, Y.-J.; Kathawala, R.J.; Zhang, H.; Talele, T.T.; Ambudkar, S.V.; Xu, R.-H.; et al. ARRY-334543 Reverses Multidrug Resistance by Antagonizing the Activity of ATP-Binding Cassette Subfamily G Member 2. J. Cell. Biochem. 2014, 115, 1381–1391. [Google Scholar] [CrossRef] [Green Version]
- Wong, T.; Narayanan, S.; Brown, D.P.; Chen, Z.-S. Synthesis and Cytotoxicity Studies of Stilbene Long-Chain Fatty Acid Conjugates. J. Nat. Prod. 2020, 83, 1563–1570. [Google Scholar] [CrossRef] [PubMed]
- Carmichael, J.; DeGraff, W.G.; Gazdar, A.F.; Minna, J.D.; Mitchell, J.B. Evaluation of a Tetrazolium-based Semiautomated Colorimetric Assay: Assessment of Chemosensitivity Testing. Cancer Res. 1987, 47, 936–942. [Google Scholar]
- Bahuguna, A.; Khan, I.; Bajpai, V.K.; Kang, S.C. MTT assay to evaluate the cytotoxic potential of a drug. Bangladesh J. Pharmacol. 2017, 12, 8. [Google Scholar] [CrossRef]
- Nanayakkara, A.K.; Follit, C.A.; Chen, G.; Williams, N.S.; Vogel, P.D.; Wise, J.G. Targeted inhibitors of P-glycoprotein increase chemotherapeutic-induced mortality of multidrug resistant tumor cells. Sci. Rep. 2018, 8, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.-K.; Zhang, G.-N.; Wang, Y.-J.; Patel, B.A.; Talele, T.T.; Yang, D.-H.; Chen, Z.-S. Bafetinib (INNO-406) reverses multidrug resistance by inhibiting the efflux function of ABCB1 and ABCG2 transporters. Sci. Rep. 2016, 6, 25694. [Google Scholar] [CrossRef] [Green Version]
- Khunweeraphong, N.; Szöllősi, D.; Stockner, T.; Kuchler, K. The ABCG2 multidrug transporter is a pump gated by a valve and an extracellular lid. Nat. Commun. 2019, 10, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Cai, C.-Y.; Zhai, H.; Lei, Z.-N.; Tan, C.-P.; Chen, B.-L.; Du, Z.-Y.; Wang, J.-Q.; Zhang, Y.-K.; Wang, Y.-J.; Gupta, P.; et al. Benzoyl indoles with metabolic stability as reversal agents for ABCG2-mediated multidrug resistance. Eur. J. Med. Chem. 2019, 179, 849–862. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.-Q.; Li, J.Y.; Teng, Q.-X.; Lei, Z.-N.; Ji, N.; Cui, Q.; Zeng, L.; Pan, Y.; Yang, D.-H.; Chen, Z.-S. Venetoclax, a BCL-2 Inhibitor, Enhances the Efficacy of Chemotherapeutic Agents in Wild-Type ABCG2-Overexpression-Mediated MDR Cancer Cells. Cancers 2020, 12, 466. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Orlando, B.J.; Liao, M. ABCG2 transports anticancer drugs via a closed-to-open switch. Nat. Commun. 2020, 11, 1–11. [Google Scholar] [CrossRef]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef] [Green Version]
- Patel, B.A.; Abel, B.; Barbuti, A.M.; Velagapudi, U.K.; Chen, Z.-S.; Ambudkar, S.V.; Talele, T.T. Comprehensive Synthesis of Amino Acid-Derived Thiazole Peptidomimetic Analogues to Understand the Enigmatic Drug/Substrate-Binding Site of P-Glycoprotein. J. Med. Chem. 2018, 61, 834–864. [Google Scholar] [CrossRef] [PubMed]
- Friesner, R.A.; Murphy, R.B.; Repasky, M.P.; Frye, L.L.; Greenwood, J.R.; Halgren, T.A.; Sanschagrin, P.C.; Mainz, D.T. Extra Precision Glide: Docking and Scoring Incorporating a Model of Hydrophobic Enclosure for Protein−Ligand Complexes. J. Med. Chem. 2006, 49, 6177–6196. [Google Scholar] [CrossRef] [PubMed] [Green Version]











| Drugs | Class | Target | References |
|---|---|---|---|
| 5-fluorouracil | Fluoropyrimidine | Thymidylate synthase | [29] |
| Irinotecan | Camptothecin analogue | Topoisomerase I | [30] |
| Leucovorin | 5-formyl derivative of tetrahydro folic acid | Decrease the toxic effects of methotrexate | [31] |
| Oxaliplatin | Platinum-based compound | Arrests DNA synthesis | [32] |
| Bevacizumab | Angiogenesis inhibitor | Anti-vascular endothelial growth factor (VEGF) | [33] |
| Cetuximab | Chimeric human- murine immunoglobulin G1 (IgG1) monoclonal antibody | Epidermal growth factor receptor (EGFR) | [34] |
| Anthracyclines | Cytostatic antibiotics | Generation of reactive oxygen species (ROS) | [35] |
| Methotrexate | Folic acid antagonist | Dihydrofolate reductase | [36] |
| Cell Lines | S1 | S1-M1-80 | ||
|---|---|---|---|---|
| Compounds | IC50 ± SD (µM) | FR | IC50 ± SD (µM) | FR |
| Mitoxantrone | 0.31 ± 0.03 | (1.0) | 21.57 ± 3.23 | [71.9] |
| +VKNG-2 (1 µM) | 0.22 ± 0.03 | (0.7) | 2.13 ± 0.38 | [7.1] |
| +VKNG-2 (5 µM) | 0.25 ± 0.04 | (0.7) | 0.19 ± 0.04 | [0.6] |
| +FTC (5 µM) | 0.33 ± 0.03 | (1.0) | 0.15 ± 0.03 | [0.5] |
| SN-38 | 0.22 ± 0.03 | (1.0) | 22.82 ± 3.95 | [114.1] |
| +VKNG-2 (1 µM) | 0.27 ± 0.05 | (1.0) | 4.73 ± 0.91 | [23.5] |
| +VKNG-2 (5 µM) | 0.19 ± 0.02 | (1.0) | 0.10 ± 0.01 | [0.5] |
| +FTC (5 µM) | 0.37 ± 0.06 | (1.7) | 0.09 ± 0.01 | [0.5] |
| Cisplatin | 99.37 ± 17.99 | (1.0) | 103.37 ± 14.37 | [1.0] |
| +VKNG-2 (1 µM) | 95.86 ± 10.55 | (1.0) | 107.13 ± 14.99 | [1.0] |
| +VKNG-2 (5 µM) | 115.27 ± 17.49 | (1.3) | 103.39 ± 12.51 | [1.0] |
| +FTC (5 µM) | 111.07 ± 17.33 | (1.3) | 109.37 ± 17.59 | [1.1] |
| Cell Lines | HEK293/pcDNA3.1 | HEK293/R2 | HEK293/G2 | HEK293/T7 | ||||
|---|---|---|---|---|---|---|---|---|
| Compounds | IC50 ±SD (µM) | FR | IC50 ±SD (µM) | FR | IC50 ±SD (µM) | FR | IC50 ±SD (µM) | FR |
| Mitoxantrone | 0.025 ± 0.002 | [1.0] | 1.181 ± 0.081 | [47.3] | 1.432± 0.139 | [56.5] | 1.233 ± 0.189 | [49.3] |
| + VKNG-2 (1µM) | 0.031 ± 0.006 | [1.25] | 0.178 ± 0.023 | [7.1] | 0.146 ± 0.022 | [5.1] | 0.857 ± 0.009 | [34.0] |
| +VKNG-2 (5 µM) | 0.023 ± 0.002 | [0.94] | 0.064 ± 0.023 | [2.5] | 0.055 ± 0.010 | [2.2] | 0.067 ± 0.013 | [2.5] |
| +FTC (5 µM) | 0.031 ± 0.006 | [1.26] | 0.059± 0.01 | [2.3] | 0.066 ± 0.019 | [2.7] | 0.053 ± 0.007 | [2.1] |
| SN-38 | 0.065± 0.005 | [1.0] | 2.379 ± 0.53 | [35.3] | 2.76 ± 0.52 | [42.2] | 2.665 ± 0.243 | [34.3] |
| +VKNG-2 (1 µM) | 0.057 ± 0.027 | [0.8] | 0.398 ± 0.157 | [6.1] | 0.86 ± 0.199 | [13.0] | 1.566 ± 0.277 | [23.1] |
| +VKNG-2 (5 µM) | 0.059 ± 0.003 | [0.9] | 0.198 ± 0.01 | [3.0] | 0.159 ± 0.020 | [2.3] | 0.173 ± 0.027 | [2.7] |
| +FTC (5 µM) | 0.075 ± 0.002 | [1.0] | 0.179 ± 0.02 | [3.8] | 0.123 ± 0.019 | [1.9] | 0.157 ± 0.035 | [2.3] |
| Cisplatin | 12.759 ± 2.33 | [1.0] | 10.56 ± 1.359 | [0.9] | 10.348 ± 1.579 | [0.9] | 14.323 ± 1.343 | [1.1] |
| +VKNG-2 (1 µM) | 13.985 ± 2.319 | [1.1] | 11.607 ± 1.464 | [0.9] | 11.997 ± 1.71 | [1.1] | 14.009 ± 1.317 | [1.1] |
| +VKNG-2 (5 µM) | 13.835 ± 1.295 | [1.1] | 12.156 ± 1.596 | [1.0] | 10.695 ± 1.913 | [0.9] | 12.964 ± 1.127 | [1.0] |
| +FTC (5 µM) | 14.950 ± 1.245 | [1.1] | 11.523 ± 1.525 | [1.1] | 12.541 ± 1.731 | [1.0] | 12.589 ± 1.311 | [1.0] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Narayanan, S.; Gujarati, N.A.; Wang, J.-Q.; Wu, Z.-X.; Koya, J.; Cui, Q.; Korlipara, V.L.; Ashby, C.R., Jr.; Chen, Z.-S. The Novel Benzamide Derivative, VKNG-2, Restores the Efficacy of Chemotherapeutic Drugs in Colon Cancer Cell Lines by Inhibiting the ABCG2 Transporter. Int. J. Mol. Sci. 2021, 22, 2463. https://doi.org/10.3390/ijms22052463
Narayanan S, Gujarati NA, Wang J-Q, Wu Z-X, Koya J, Cui Q, Korlipara VL, Ashby CR Jr., Chen Z-S. The Novel Benzamide Derivative, VKNG-2, Restores the Efficacy of Chemotherapeutic Drugs in Colon Cancer Cell Lines by Inhibiting the ABCG2 Transporter. International Journal of Molecular Sciences. 2021; 22(5):2463. https://doi.org/10.3390/ijms22052463
Chicago/Turabian StyleNarayanan, Silpa, Nehaben A. Gujarati, Jing-Quan Wang, Zhuo-Xun Wu, Jagadish Koya, Qingbin Cui, Vijaya L. Korlipara, Charles R. Ashby, Jr., and Zhe-Sheng Chen. 2021. "The Novel Benzamide Derivative, VKNG-2, Restores the Efficacy of Chemotherapeutic Drugs in Colon Cancer Cell Lines by Inhibiting the ABCG2 Transporter" International Journal of Molecular Sciences 22, no. 5: 2463. https://doi.org/10.3390/ijms22052463
APA StyleNarayanan, S., Gujarati, N. A., Wang, J. -Q., Wu, Z. -X., Koya, J., Cui, Q., Korlipara, V. L., Ashby, C. R., Jr., & Chen, Z. -S. (2021). The Novel Benzamide Derivative, VKNG-2, Restores the Efficacy of Chemotherapeutic Drugs in Colon Cancer Cell Lines by Inhibiting the ABCG2 Transporter. International Journal of Molecular Sciences, 22(5), 2463. https://doi.org/10.3390/ijms22052463