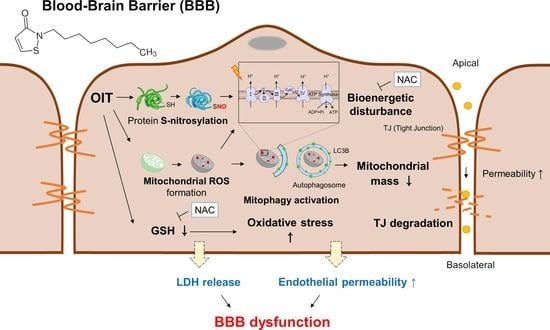
A Commonly Used Biocide 2-N-octyl-4-isothiazolin-3-oneInduces Blood–Brain Barrier Dysfunction via Cellular Thiol Modification and Mitochondrial Damage
Abstract
:1. Introduction
2. Results
2.1. Impact of OIT on bEnd.3 Cell Deaths
2.2. Impairment of Endothelial Barrier Function of bEnd.3 Cells by OIT
2.3. Tight Junction Protein Degradation and Modification of Intracellular Thiol Status Induced by OIT
2.4. OIT-Induced Mitochondrial Defects
2.5. OIT-Induced Mitophagy and Changes in Mitochondrial Mass and Morphology
2.6. NAC Effects on OIT-Induced Changes
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Cell Culture
4.3. Measurement of Cell Viability with MTT Reduction and LDH Assay
4.4. In Vitro Permeability Assay
4.5. Immunofluorescence Staining
4.6. Total Cellular and Mitochondrial ROS Detection Assays
4.7. GSH Assay
4.8. Cytosol/Mitochondria Fractionation
4.9. Measurement of Bioenergetic Function
4.10. Analysis of Mitochondrial Mass
4.11. Western Blot
4.12. Protein SNO Analysis
4.13. Flow Cytometry
4.14. Transmission Electron Microscopy (TEM)
4.15. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations:
| AJ | Adherens junctions |
| BBB | Blood–brain barrier |
| BCA | Bicinchoninic acid |
| CNS | Central nervous system |
| DAPI | 4,6-Diamidino-2-phenylindole |
| DCF-DA | Dichlorofluorescein diacetate |
| EC | Endothelial cell |
| ECHA | European Chemicals Agency |
| ETC | Electron transport chain |
| FCCP | Carbonyl cyanide-4-(trifluoromethoxy) phenylhydrazone |
| FITC | Fluorescein isothiocyanate |
| GSH | Glutathione |
| IT | Isothiazolinone |
| LDH | Lactate dehydrogenase |
| MMP | Mitochondrial membrane potential |
| MMTS | S-methyl methanethiosulfonate |
| MTT | Thiazolyl blue tetrazolium bromide |
| NAC | N-acetyl L-cysteine |
| NAO | Nonyl acridine orange |
| NO | Nitric oxide |
| OCR | Oxygen consumption rate |
| OIT | 2-N-octyl-4-isothiazolin-3-one |
| PS | Phosphatidylserine |
| ROS | Reactive oxygen species |
| Rot/AA | Rotenone/antimycin A |
| SNO | S-nitrosylation |
| TEER | Trans-endothelial electrical resistance |
| TEM | Transmission electron microscopy |
| TJ | Tight junction |
| US EPA | United States Environmental Protection Agency |
| VDAC | Voltage-dependent anion channel |
| VEGF | Vascular endothelial growth factors |
| VE-cadherin | Vascular endothelium cadherin |
| ZO-1 | Zonula occludens-1 |
References
- Silva, V.; Silva, C.; Soares, P.; Garrido, E.M.; Borges, F.; Garrido, J. Isothiazolinone Biocides: Chemistry, Biological, and Toxicity Profiles. Molecules 2020, 25, 991. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Herman, A.; Aerts, O.; de Montjoye, L.; Tromme, I.; Goossens, A.; Baeck, M. Isothiazolinone derivatives and allergic contact dermatitis: A review and update. J. Eur. Acad. Dermatol. Venereol. 2019, 33, 267–276. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.M.; Roh, T.H.; Lim, D.S.; Kacew, S.; Kim, H.S.; Lee, B.M. Risk assessment of benzalkonium chloride in cosmetic products. J. Toxicol. Environ. Health B Crit. Rev. 2018, 21, 8–23. [Google Scholar] [CrossRef] [PubMed]
- Masroor, N.; Doll, M.; Stevens, M.; Bearman, G. Approaches to hand hygiene monitoring: From low to high technology approaches. Int. J. Infect. Dis. 2017, 65, 101–104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marrero-Aleman, G.; Borrego, L.; Antuna, A.G.; Montes, A.M.; Luzardo, O.P. Isothiazolinones in cleaning products: Analysis with liquid chromatography tandem mass spectrometry of samples from sensitized patients and market. Contact Dermat. 2020, 82, 94–100. [Google Scholar] [CrossRef] [PubMed]
- Zhong, H.; Li, Z.; Chen, S.; Zeng, Y.; Zheng, J.; Zeng, Y.; Li, D. Simultaneous Quantitative Analysis of Six Isothiazolinones in Water-Based Adhesive Used for Food Contact Materials by High-Performance Liquid Chromatography-Tandem Mass Spectrometry (HPLC-MS/MS). Molecules 2019, 24, 3894. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garcia-Hidalgo, E.; Schneider, D.; von Goetz, N.; Delmaar, C.; Siegrist, M.; Hungerbuhler, K. Aggregate consumer exposure to isothiazolinones via household care and personal care products: Probabilistic modelling and benzisothiazolinone risk assessment. Environ. Int. 2018, 118, 245–256. [Google Scholar] [CrossRef]
- Park, S.K.; Seol, H.S.; Park, H.J.; Kim, Y.S.; Ryu, S.H.; Kim, J.; Kim, S.; Lee, J.H.; Kwon, J.H. Experimental determination of indoor air concentration of 5-chloro-2-methylisothiazol-3(2H)-one/2-methylisothiazol-3(2H)-one (CMIT/MIT) emitted by the use of humidifier disinfectant. Environ. Anal. Health Toxicol. 2020, 35, e2020008. [Google Scholar] [CrossRef]
- Castro, G.; Rodriguez, I.; Ramil, M.; Cela, R. Assessment of gas chromatography time-of-flight mass spectrometry for the screening of semi-volatile compounds in indoor dust. Sci. Total Environ. 2019, 688, 162–173. [Google Scholar] [CrossRef] [PubMed]
- Friis, U.F.; Menne, T.; Flyvholm, M.A.; Bonde, J.P.; Lepoittevin, J.P.; Coz, C.J.L.; Johansen, J.D. Isothiazolinones in commercial products at Danish workplaces. Contact Dermat. 2014, 71, 65–74. [Google Scholar] [CrossRef]
- Reeder, M.; Atwater, A.R. Methylisothiazolinone and isothiazolinone allergy. Cutis 2019, 104, 94–96. [Google Scholar] [PubMed]
- Aerts, O.; Goossens, A.; Lambert, J.; Lepoittevin, J.P. Contact allergy caused by isothiazolinone derivatives: An overview of non-cosmetic and unusual cosmetic sources. Eur. J. Dermatol. 2017, 27, 115–122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balaguer, J.R.; Herrera, A.M.; de la Oyanguren, J.C.; de Rojas, D.H.F. Occupational allergic contact dermatitis to 2-N-octyl-4-isothiazolin-3-one. J. Investig. Allergol. Clin. Immunol. 2008, 18, 76–77. [Google Scholar]
- Aalto-Korte, K.; Alanko, K.; Henriks-Eckerman, M.L.; Kuuliala, O.; Jolanki, R. Occupational allergic contact dermatitis from 2-N-octyl-4-isothiazolin-3-one. Contact Dermat. 2007, 56, 160–163. [Google Scholar] [CrossRef] [PubMed]
- Fukunaga, A.; Nishiyama, S.; Shimizu, H.; Nagai, H.; Horikawa, T.; Mori, A.; Inoue, N.; Sasaki, K.; Nishigori, C. Non-occupational allergic contact dermatitis from 2-N-octyl-4-isothiazolin-3-one in a Japanese mattress gel-sheet used for cooling. Contact Dermat. 2010, 62, 317–318. [Google Scholar] [CrossRef] [PubMed]
- Denyer, S.P. Mechanisms of action of antibacterial biocides. Int. Biodeterior. Biodegrad. 1995, 36, 227–245. [Google Scholar] [CrossRef]
- Di Stefano, A.; Frosali, S.; Leonini, A.; Ettorre, A.; Priora, R.; Di Simplicio, F.C.; Di Simplicio, P. GSH depletion, protein S-glutathionylation and mitochondrial transmembrane potential hyperpolarization are early events in initiation of cell death induced by a mixture of isothiazolinones in HL60 cells. Biochim. Biophys. Acta 2006, 1763, 214–225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frosali, S.; Leonini, A.; Ettorre, A.; Di Maio, G.; Nuti, S.; Tavarini, S.; Di Simplicio, P.; Di Stefano, A. Role of intracellular calcium and S-glutathionylation in cell death induced by a mixture of isothiazolinones in HL60 cells. Biochim. Biophys. Acta 2009, 1793, 572–583. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, K.A.; Shin, D.; Kim, J.H.; Shin, Y.J.; Rajanikant, G.K.; Majid, A.; Baek, S.H.; Bae, O.N. Role of Autophagy in Endothelial Damage and Blood-Brain Barrier Disruption in Ischemic Stroke. Stroke 2018, 49, 1571–1579. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.A.; Kim, D.; Kim, J.H.; Shin, Y.J.; Kim, E.S.; Akram, M.; Kim, E.H.; Majid, A.; Baek, S.H.; Bae, O.N. Autophagy-mediated occludin degradation contributes to blood-brain barrier disruption during ischemia in bEnd.3 brain endothelial cells and rat ischemic stroke models. Fluids Barriers CNS 2020, 17, 21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, D.; Kim, K.A.; Kim, J.H.; Kim, E.H.; Bae, O.N. Methylglyoxal-Induced Dysfunction in Brain Endothelial Cells via the Suppression of Akt/HIF-1alpha Pathway and Activation of Mitophagy Associated with Increased Reactive Oxygen Species. Antioxidants 2020, 9, 820. [Google Scholar] [CrossRef] [PubMed]
- Wnuk, A.; Kajta, M. Steroid and Xenobiotic Receptor Signalling in Apoptosis and Autophagy of the Nervous System. Int. J. Mol. Sci. 2017, 18, 2394. [Google Scholar] [CrossRef] [Green Version]
- Yuan, S.; Wang, J.; Jiang, Q.; He, Z.; Huang, Y.; Li, Z.; Cai, L.; Cao, S. Long-term exposure to PM2.5 and stroke: A systematic review and meta-analysis of cohort studies. Environ. Res. 2019, 177, 108587. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.L.; Mukda, S.; Chen, S.D. Diverse roles of mitochondria in ischemic stroke. Redox Biol. 2018, 16, 263–275. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Lu, J.; Manaenko, A.; Tang, J.; Hu, Q. Mitochondria in Ischemic Stroke: New Insight and Implications. Aging Dis. 2018, 9, 924–937. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andrabi, S.S.; Parvez, S.; Tabassum, H. Ischemic stroke and mitochondria: Mechanisms and targets. Protoplasma 2020, 257, 335–343. [Google Scholar] [CrossRef] [PubMed]
- Pun, P.B.; Lu, J.; Moochhala, S. Involvement of ROS in BBB dysfunction. Free Radic. Res. 2009, 43, 348–364. [Google Scholar] [CrossRef] [PubMed]
- Qi, Z.; Shi, W.; Zhao, Y.; Ji, X.; Liu, K.J. Zinc accumulation in mitochondria promotes ischemia-induced BBB disruption through Drp1-dependent mitochondria fission. Toxicol. Appl. Pharmacol. 2019, 377, 114601. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Luo, Y.X.; Chen, H.Z.; Liu, D.P. Mitochondria, endothelial cell function, and vascular diseases. Front. Physiol. 2014, 5, 175. [Google Scholar] [CrossRef]
- Supinski, G.S.; Schroder, E.A.; Callahan, L.A. Mitochondria and Critical Illness. Chest 2020, 157, 310–322. [Google Scholar] [CrossRef]
- Nunnari, J.; Suomalainen, A. Mitochondria: In sickness and in health. Cell 2012, 148, 1145–1159. [Google Scholar] [CrossRef] [Green Version]
- Benedet, P.O.; Menegatti, A.C.O.; Goncalves, M.C.; Terenzi, H.; Assreuy, J. The therapeutic value of protein (de)nitrosylation in experimental septic shock. Biochim. Biophys. Acta Mol. Basis Dis. 2018, 1864, 307–316. [Google Scholar] [CrossRef]
- Yang, Y.; Loscalzo, J. S-nitrosoprotein formation and localization in endothelial cells. Proc. Natl. Acad. Sci. USA 2005, 102, 117–122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakamura, T.; Lipton, S.A. ‘SNO’-Storms Compromise Protein Activity and Mitochondrial Metabolism in Neurodegenerative Disorders. Trends Endocrinol. Metab. 2017, 28, 879–892. [Google Scholar] [CrossRef] [PubMed]
- Hsu, M.; Srinivas, B.; Kumar, J.; Subramanian, R.; Andersen, J. Glutathione depletion resulting in selective mitochondrial complex I inhibition in dopaminergic cells is via an NO-mediated pathway not involving peroxynitrite: Implications for Parkinson’s disease. J. Neurochem. 2005, 92, 1091–1103. [Google Scholar] [CrossRef]
- Peillex, C.; Kerever, A.; Lachhab, A.; Pelletier, M. Bisphenol A, bisphenol S and their glucuronidated metabolites modulate glycolysis and functional responses of human neutrophils. Environ. Res. 2020, 110336. [Google Scholar] [CrossRef]
- Shi, Y. Mechanisms of caspase activation and inhibition during apoptosis. Mol. Cell 2002, 9, 459–470. [Google Scholar] [CrossRef]
- Sweeney, M.D.; Zhao, Z.; Montagne, A.; Nelson, A.R.; Zlokovic, B.V. Blood-Brain Barrier: From Physiology to Disease and Back. Physiol. Rev. 2019, 99, 21–78. [Google Scholar] [CrossRef]
- Lochhead, J.J.; Yang, J.; Ronaldson, P.T.; Davis, T.P. Structure, Function, and Regulation of the Blood-Brain Barrier Tight Junction in Central Nervous System Disorders. Front. Physiol. 2020, 11, 914. [Google Scholar] [CrossRef] [PubMed]
- Pan, L.; Lin, Z.; Tang, X.; Tian, J.; Zheng, Q.; Jing, J.; Xie, L.; Chen, H.; Lu, Q.; Wang, H.; et al. S-Nitrosylation of Plastin-3 Exacerbates Thoracic Aortic Dissection Formation via Endothelial Barrier Dysfunction. Arter. Thromb. Vasc. Biol. 2020, 40, 175–188. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Qiu, H.; Shi, F.; Wang, Z.; Wang, X.; Jin, L.; Chi, L.; Zhang, Q. The protein S-nitrosylation of splicing and translational machinery in vascular endothelial cells is susceptible to oxidative stress induced by oxidized low-density lipoprotein. J. Proteom. 2019, 195, 11–22. [Google Scholar] [CrossRef]
- Zhou, S.N.; Lu, J.X.; Wang, X.Q.; Shan, M.R.; Miao, Z.; Pan, G.P.; Jian, X.; Li, P.; Ping, S.; Pang, X.Y.; et al. S-Nitrosylation of Prostacyclin Synthase Instigates Nitrate Cross-Tolerance In Vivo. Clin. Pharmacol. Ther. 2019, 105, 201–209. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, W.; Busu, C.; Circu, M.L.; Aw, T.Y. Glutathione in cerebral microvascular endothelial biology and pathobiology: Implications for brain homeostasis. Int. J. Cell Biol. 2012, 2012, 434971. [Google Scholar] [CrossRef] [PubMed]
- Vyas, S.; Zaganjor, E.; Haigis, M.C. Mitochondria and Cancer. Cell 2016, 166, 555–566. [Google Scholar] [CrossRef] [PubMed]
- Schofield, J.H.; Schafer, Z.T. Mitochondrial Reactive Oxygen Species and Mitophagy: A Complex and Nuanced Relationship. Antioxid. Redox Signal 2020, 34, 517–530. [Google Scholar] [CrossRef]
- Yoo, S.M.; Jung, Y.K. A Molecular Approach to Mitophagy and Mitochondrial Dynamics. Mol. Cells 2018, 41, 18–26. [Google Scholar] [PubMed]
- Aldini, G.; Altomare, A.; Baron, G.; Vistoli, G.; Carini, M.; Borsani, L.; Sergio, F. N-Acetylcysteine as an antioxidant and disulphide breaking agent: The reasons why. Free Radic. Res. 2018, 52, 751–762. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, S.; Alhooshani, K.; Karanfil, T. Disinfection byproducts in swimming pool: Occurrences, implications and future needs. Water Res. 2014, 53, 68–109. [Google Scholar] [CrossRef] [PubMed]
- Rutala, W.A.; Weber, D.J. Disinfection and Sterilization in Health Care Facilities: An Overview and Current Issues. Infect. Dis. Clin. N. Am. 2016, 30, 609–637. [Google Scholar] [CrossRef] [PubMed]
- Pradhan, D.; Biswasroy, P.; Naik, P.K.; Ghosh, G.; Rath, G. A Review of Current Interventions for COVID-19 Prevention. Arch. Med. Res. 2020, 51, 363–374. [Google Scholar] [CrossRef]
- Nowak, K.; Jablonska, E.; Ratajczak-Wrona, W. Controversy around parabens: Alternative strategies for preservative use in cosmetics and personal care products. Environ. Res. 2020, 110488. [Google Scholar] [CrossRef] [PubMed]
- Arriaga-Gomez, E.; Kline, J.; Emanuel, E.; Neamonitaki, N.; Yangdon, T.; Zacheis, H.; Pasha, D.; Lim, J.; Bush, S.; Boo, B.; et al. Repeated Vaginal Exposures to the Common Cosmetic and Household Preservative Methylisothiazolinone Induce Persistent, Mast Cell-Dependent Genital Pain in ND4 Mice. Int. J. Mol. Sci. 2019, 20, 5361. [Google Scholar] [CrossRef] [Green Version]
- European Chemicals Agency, Opinion Proposing Harmonised Classification and Labelling at EU Level of Octhilinone (ISO); 2-octyl-2H-isothiazol-3-one; [OIT]; ECHA: Helsinki, Finland, 2018.
- United States Environmental Protection Agency. Reregistration Eligibility Decision for 2-Octyl-3 (2H)-isothiazolone (OIT); EPA: Washington, DC, USA, 2007.
- Abdullahi, W.; Tripathi, D.; Ronaldson, P.T. Blood-brain barrier dysfunction in ischemic stroke: Targeting tight junctions and transporters for vascular protection. Am. J. Physiol. Cell Physiol. 2018, 315, C343–C356. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.L.; Yue, Z.; Arnold, D.M.; Artiushin, G.; Sehgal, A. A Circadian Clock in the Blood-Brain Barrier Regulates Xenobiotic Efflux. Cell 2018, 173, 130–139.e10. [Google Scholar] [CrossRef] [Green Version]
- Terasaki, T.; Ohtsuki, S. Brain-to-blood transporters for endogenous substrates and xenobiotics at the blood-brain barrier: An overview of biology and methodology. NeuroRx 2005, 2, 63–72. [Google Scholar] [CrossRef]
- Muller, S.M.; Ebert, F.; Raber, G.; Meyer, S.; Bornhorst, J.; Huwel, S.; Galla, H.J.; Francesconi, K.A.; Schwerdtle, T. Effects of arsenolipids on in vitro blood-brain barrier model. Arch. Toxicol. 2018, 92, 823–832. [Google Scholar] [CrossRef] [PubMed]
- Banks, W.A.; Gray, A.M.; Erickson, M.A.; Salameh, T.S.; Damodarasamy, M.; Sheibani, N.; Meabon, J.S.; Wing, E.E.; Morofuji, Y.; Cook, D.G.; et al. Lipopolysaccharide-induced blood-brain barrier disruption: Roles of cyclooxygenase, oxidative stress, neuroinflammation, and elements of the neurovascular unit. J. Neuroinflammation 2015, 12, 223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, Y.; Wang, C.; Zhang, X.; Zhu, J.; Wang, L.; Ji, M.; Zhang, Z.; Ji, X.M.; Wang, S.L. Perfluorooctane sulfonate disrupts the blood brain barrier through the crosstalk between endothelial cells and astrocytes in mice. Environ. Pollut. 2020, 256, 113429. [Google Scholar] [CrossRef]
- Sweeney, M.D.; Sagare, A.P.; Zlokovic, B.V. Blood-brain barrier breakdown in Alzheimer disease and other neurodegenerative disorders. Nat. Rev. Neurol. 2018, 14, 133–150. [Google Scholar] [CrossRef] [PubMed]
- Zlokovic, B.V. The blood-brain barrier in health and chronic neurodegenerative disorders. Neuron 2008, 57, 178–201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosenberg, G.A. Neurological diseases in relation to the blood-brain barrier. J. Cereb. Blood Flow. Metab. 2012, 32, 1139–1151. [Google Scholar] [CrossRef] [Green Version]
- Busija, D.W.; Rutkai, I.; Dutta, S.; Katakam, P.V. Role of Mitochondria in Cerebral Vascular Function: Energy Production, Cellular Protection, and Regulation of Vascular Tone. Compr. Physiol. 2016, 6, 1529–1548. [Google Scholar]
- Dobi, A.; Rosanaly, S.; Devin, A.; Baret, P.; Meilhac, O.; Harry, G.J.; D’Hellencourt, C.L.; Rondeau, P. Advanced glycation end-products disrupt brain microvascular endothelial cell barrier: The role of mitochondria and oxidative stress. Microvasc. Res. 2021, 133, 104098. [Google Scholar] [CrossRef] [PubMed]
- Alelwani, W.; Elmorsy, E.; Kattan, S.W.; Babteen, N.A.; Alnajeebi, A.M.; Al-Ghafari, A.; Carter, W.G. Carbamazepine induces a bioenergetics disruption to microvascular endothelial cells from the blood-brain barrier. Toxicol. Lett. 2020, 333, 184–191. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, T.; Prikhodko, O.A.; Pirie, E.; Nagar, S.; Akhtar, M.W.; Oh, C.K.; McKercher, S.R.; Ambasudhan, R.; Okamoto, S.; Lipton, S.A. Aberrant protein S-nitrosylation contributes to the pathophysiology of neurodegenerative diseases. Neurobiol. Dis. 2015, 84, 99–108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakamura, T.; Tu, S.; Akhtar, M.W.; Sunico, C.R.; Okamoto, S.; Lipton, S.A. Aberrant protein S-nitrosylation in neurodegenerative diseases. Neuron 2013, 78, 596–614. [Google Scholar] [CrossRef] [Green Version]
- Doulias, P.T.; Tenopoulou, M.; Greene, J.L.; Raju, K.; Ischiropoulos, H. Nitric oxide regulates mitochondrial fatty acid metabolism through reversible protein S-nitrosylation. Sci. Signal 2013, 6, rs1. [Google Scholar] [CrossRef] [Green Version]
- Mohr, S.; Stamler, J.S.; Brune, B. Mechanism of covalent modification of glyceraldehyde-3-phosphate dehydrogenase at its active site thiol by nitric oxide, peroxynitrite and related nitrosating agents. FEBS Lett. 1994, 348, 223–227. [Google Scholar] [CrossRef] [Green Version]
- Murray, C.I.; Uhrigshardt, H. O’Meally, R.N.; Cole, R.N.; van Eyk, J.E. Identification and quantification of S-nitrosylation by cysteine reactive tandem mass tag switch assay. Mol. Cell Proteomics 2012, 11, M111.013441. [Google Scholar] [CrossRef] [Green Version]
- Galkin, A.; Moncada, S. S-nitrosation of mitochondrial complex I depends on its structural conformation. J. Biol. Chem. 2007, 282, 37448–37453. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prime, T.A.; Blaikie, F.H.; Evans, C.; Nadtochiy, S.M.; James, A.M.; Dahm, C.C.; Vitturi, D.A.; Patel, R.P.; Hiley, C.R.; Abakumova, I.; et al. A mitochondria-targeted S-nitrosothiol modulates respiration, nitrosates thiols, and protects against ischemia-reperfusion injury. Proc. Natl. Acad. Sci. USA 2009, 106, 10764–10769. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chouchani, E.T.; Hurd, T.R.; Nadtochiy, S.M.; Brookes, P.S.; Fearnley, I.M.; Lilley, K.S.; Smith, R.A.; Murphy, M.P. Identification of S-nitrosated mitochondrial proteins by S-nitrosothiol difference in gel electrophoresis (SNO-DIGE): Implications for the regulation of mitochondrial function by reversible S-nitrosation. Biochem. J. 2010, 430, 49–59. [Google Scholar] [CrossRef] [Green Version]
- Chouchani, E.T.; Methner, C.; Nadtochiy, S.M.; Logan, A.; Pell, V.R.; Ding, S.; James, A.M.; Cocheme, H.M.; Reinhold, J.; Lilley, K.S.; et al. Cardioprotection by S-nitrosation of a cysteine switch on mitochondrial complex I. Nat. Med. 2013, 19, 753–759. [Google Scholar] [CrossRef] [Green Version]
- Zhdanov, A.V.; Waters, A.H.; Golubeva, A.V.; Dmitriev, R.I.; Papkovsky, D.B. Availability of the key metabolic substrates dictates the respiratory response of cancer cells to the mitochondrial uncoupling. Biochim. Biophys. Acta 2014, 1837, 51–62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martínez-Reyes, I.; Diebold, L.P.; Kong, H.; Schieber, M.; Huang, H.; Hensley, C.T.; Mehta, M.M.; Wang, T.; Santos, J.H.; Woychik, R.; et al. TCA Cycle and mitochondrial membrane potential are necessary for diverse biological functions. Mol. Cell 2016, 61, 199–209. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, J.; Lee, J.; Choi, C. Mitochondrial network determines intracellular ROS dynamics and sensitivity to oxidative stress through switching inter-mitochondrial messengers. PLoS ONE 2011, 6, e23211. [Google Scholar] [CrossRef] [PubMed]
- Natarajan, V.; Mah, T.; Peishi, C.; Tan, S.Y.; Chawla, R.; Arumugam, T.V.; Ramasamy, A.; Mallilankaraman, K. Oxygen Glucose Deprivation Induced Prosurvival Autophagy Is Insufficient to Rescue Endothelial Function. Front. Physiol. 2020, 11. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, J.; Namba, T.; Takabatake, Y.; Kimura, T.; Takahashi, A.; Yamamoto, T.; Minami, S.; Sakai, S.; Fujimura, R.; Kaimori, J.; et al. Antioxidant role of autophagy in maintaining the integrity of glomerular capillaries. Autophagy 2018, 14, 53–65. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hara, T.; Nakamura, K.; Matsui, M.; Yamamoto, A.; Nakahara, Y.; Suzuki-Migishima, R.; Yokoyama, M.; Mishima, K.; Saito, I.; Okano, H.; et al. Suppression of basal autophagy in neural cells causes neurodegenerative disease in mice. Nature 2006, 441, 885–889. [Google Scholar] [CrossRef]
- Levine, B.; Kroemer, G. Biological Functions of Autophagy Genes: A Disease Perspective. Cell 2019, 176, 11–42. [Google Scholar] [CrossRef] [Green Version]
- Li, W.; Chen, Z.; Chin, I.; Chen, Z.; Dai, H. The Role of VE-cadherin in blood-brain barrier integrity under central nervous system pathological conditions. Curr. Neuropharmacol. 2018, 16, 1375–1384. [Google Scholar] [CrossRef]
- Tietz, S.; Engelhardt, B. Brain barriers: Crosstalk between complex tight junctions and adherens junctions. J. Cell Biol. 2015, 209, 493–506. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suzuki, Y.; Nagai, N.; Umemura, K. A Review of the Mechanisms of blood-brain barrier permeability by tissue-type plasminogen activator treatment for cerebral ischemia. Front. Cell Neurosci. 2016, 10, 2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Argaw, A.T.; Gurfein, B.T.; Zhang, Y.; Zameer, A.; John, G.R. VEGF-mediated disruption of endothelial CLN-5 promotes blood-brain barrier breakdown. Proc. Natl. Acad. Sci. USA 2009, 106, 1977–1982. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nag, S.; Manias, J.; Eubanks, J.H.; Stewart, D.J. Increased expression of vascular endothelial growth factor-D following brain injury. Int. J. Mol. Sci. 2019, 20, 1594. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brown, R.C.; Morris, A.P.; O’Neil, R.G. Tight junction protein expression and barrier properties of immortalized mouse brain microvessel endothelial cells. Brain Res. 2007, 1130, 17–30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van der Stel, W.; Carta, G.; Eakins, J.; Darici, S.; Delp, J.; Forsby, A.; Bennekou, S.H.; Gardner, I.; Leist, M.; Danen, E.H.J.; et al. Multiparametric assessment of mitochondrial respiratory inhibition in HepG2 and RPTEC/TERT1 cells using a panel of mitochondrial targeting agrochemicals. Arch. Toxicol. 2020, 94, 2707–2729. [Google Scholar] [CrossRef]
- Jacobson, J.; Duchen, M.R.; Heales, S.J. Intracellular distribution of the fluorescent dye nonyl acridine orange responds to the mitochondrial membrane potential: Implications for assays of cardiolipin and mitochondrial mass. J. Neurochem. 2002, 82, 224–233. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, D.; Kim, E.-H.; Choi, S.; Lim, K.-M.; Tie, L.; Majid, A.; Bae, O.-N. A Commonly Used Biocide 2-N-octyl-4-isothiazolin-3-oneInduces Blood–Brain Barrier Dysfunction via Cellular Thiol Modification and Mitochondrial Damage. Int. J. Mol. Sci. 2021, 22, 2563. https://doi.org/10.3390/ijms22052563
Kim D, Kim E-H, Choi S, Lim K-M, Tie L, Majid A, Bae O-N. A Commonly Used Biocide 2-N-octyl-4-isothiazolin-3-oneInduces Blood–Brain Barrier Dysfunction via Cellular Thiol Modification and Mitochondrial Damage. International Journal of Molecular Sciences. 2021; 22(5):2563. https://doi.org/10.3390/ijms22052563
Chicago/Turabian StyleKim, Donghyun, Eun-Hye Kim, Sungbin Choi, Kyung-Min Lim, Lu Tie, Arshad Majid, and Ok-Nam Bae. 2021. "A Commonly Used Biocide 2-N-octyl-4-isothiazolin-3-oneInduces Blood–Brain Barrier Dysfunction via Cellular Thiol Modification and Mitochondrial Damage" International Journal of Molecular Sciences 22, no. 5: 2563. https://doi.org/10.3390/ijms22052563
APA StyleKim, D., Kim, E. -H., Choi, S., Lim, K. -M., Tie, L., Majid, A., & Bae, O. -N. (2021). A Commonly Used Biocide 2-N-octyl-4-isothiazolin-3-oneInduces Blood–Brain Barrier Dysfunction via Cellular Thiol Modification and Mitochondrial Damage. International Journal of Molecular Sciences, 22(5), 2563. https://doi.org/10.3390/ijms22052563