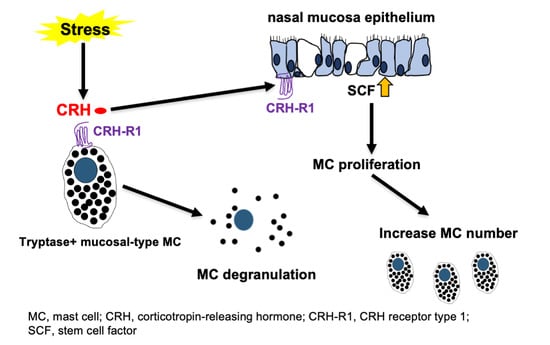
Stress and Nasal Allergy: Corticotropin-Releasing Hormone Stimulates Mast Cell Degranulation and Proliferation in Human Nasal Mucosa
Abstract
:1. Introduction
2. Results
2.1. c-Kit or Tryptase+ Human Nasal Mucosa MCs Expressed CRH-R1s In Situ
2.2. CRH Increased the Number and Degranulation of Tryptase+ hM-MCs In Situ
2.3. CRH Stimulated the Intramucosal Proliferation of Tryptase+ hM-MCs
2.4. CRH-Induced hM-MC Effects in Human Upper Airway Mucosa Depended on Signaling Through CRH-R1
2.5. CRH Increased SCF Expression in NP Epithelium In Situ
2.6. SCF Was Required for CRH-Induced hM-MC Proliferation and Partially for MC Degranulation
2.7. CRH Sensitized hM-MCs to Further CRH Stimulation and Promoted a Pro-Inflammatory hM-MC Phenotype
2.8. Perceived Stress Increased MC Number and Degranulation in Murine Nasal Mucosa In Vivo via CRH-R1-Mediated Signaling
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Human Nasal Polyp Organ Culture
4.3. Toluidine Blue Histochemistry
4.4. Immunohistochemistry/Immunofluorescence Microscopy
4.5. Quantitative (immuno)histomorphometry
4.6. CRH-R1 Gene Knockdown
4.7. Restraint Stress Mouse Model
4.8. Measurement of Plasma Corticosterone and CRH Levels
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, D.; Luo, W. Nasal diseases and psychological distress. Psychol. Health Med. 2016, 21, 67–73. [Google Scholar] [CrossRef]
- Davis, G.E.; Yueh, B.; Walker, E.; Katon, W.; Koepsell, T.D.; Weymuller, E.A. Psychiatric distress amplifies symptoms after surgery for chronic rhinosinusitis. Otolaryngol. Head Neck Surg. Off. J. Am. Acad. Otolaryngol. Head Neck Surg. 2005, 132, 189–196. [Google Scholar] [CrossRef]
- Philpott, C.; Erskine, S.; Hopkins, C.; Coombes, E.; Kara, N.; Sunkareneni, V.; Anari, S.; Salam, M.; Farboud, A.; Clark, A. A case-control study of medical, psychological and socio-economic factors influencing the severity of chronic rhinosinusitis. Rhinology 2016, 54, 134–140. [Google Scholar] [CrossRef] [Green Version]
- El Hennawi Del, D.; Ahmed, M.R.; Farid, A.M. Psychological stress and its relationship with persistent allergic rhinitis. Eur. Arch. Otorhinolaryngol. 2016, 273, 899–904. [Google Scholar] [CrossRef]
- Jarillo-Luna, R.A.; Gutiérrez-Meza, J.M.; Franco-Vadillo, A.; Rivera-Aguilar, V.; Toledo-Blas, M.; Cárdenas-Jaramillo, L.M. Restraint stress increased the permeability of the nasal epithelium in BALB/c mice. Psychoneuroendocrinology 2020, 117, 104700. [Google Scholar] [CrossRef]
- Conrad, L.A.; Rauh, V.A.; Hoepner, L.A.; Acosta, L.M.; Perera, F.P.; Rundle, A.G.; Arteaga-Solis, E.; Miller, R.L.; Perzanowski, M.S. Report of prenatal maternal demoralization and material hardship and infant rhinorrhea and watery eyes. Ann. Allergy Asthma Immunol. Off. Publ. Am. Coll. Allergy Asthma Immunol. 2020, 125, 399–404.e2. [Google Scholar] [CrossRef] [PubMed]
- Brunwasser, S.M.; Slavich, G.M.; Newcomb, D.C.; Gebretsadik, T.; Turi, K.N.; Stone, C., Jr.; Anderson, L.J.; Hartert, T.V. Sex-specific association between prenatal life stress exposure and infant pro-inflammatory cytokine levels during acute respiratory infection. Brain Behav. Immun. 2019, 76, 275–279. [Google Scholar] [CrossRef] [PubMed]
- Melmed, S.; Koenig, R.; Rosen, C.; Auchus, R.; Goldfine, A. Williams Textbook of Endocrinology, 14th ed.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 135–145. [Google Scholar]
- Ramot, Y.; Böhm, M.; Paus, R. Translational Neuroendocrinology of Human Skin: Concepts and Perspectives. Trends Mol. Med. 2020, 27, 60–74. [Google Scholar] [CrossRef] [PubMed]
- Kempuraj, D.; Selvakumar, G.P.; Thangavel, R.; Ahmed, M.E.; Zaheer, S.; Raikwar, S.P.; Iyer, S.S.; Bhagavan, S.M.; Beladakere-Ramaswamy, S.; Zaheer, A. Mast Cell Activation in Brain Injury, Stress, and Post-traumatic Stress Disorder and Alzheimer’s Disease Pathogenesis. Front. Neurosci. 2017, 11, 703. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ito, N.; Sugawara, K.; Bodo, E.; Takigawa, M.; van Beek, N.; Ito, T.; Paus, R. Corticotropin-releasing hormone stimulates the in situ generation of mast cells from precursors in the human hair follicle mesenchyme. J. Investig. Dermatol. 2010, 130, 995–1004. [Google Scholar] [CrossRef]
- Theoharides, T.C. Neuroendocrinology of mast cells: Challenges and controversies. Exp. Dermatol. 2017, 26, 751–759. [Google Scholar] [CrossRef] [Green Version]
- Theoharides, T.C. The impact of psychological stress on mast cells. Ann. Allergy Asthma Immunol. Off. Publ. Am. Coll. Allergy Asthma Immunol. 2020, 125, 388–392. [Google Scholar] [CrossRef]
- Slominski, A.; Zbytek, B.; Pisarchik, A.; Slominski, R.M.; Zmijewski, M.A.; Wortsman, J. CRH functions as a growth factor/cytokine in the skin. J. Cell. Physiol. 2006, 206, 780–791. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, C.L.; Yu, X.J.; Chen, L.M.; Jiang, H.; Li, C.Y. Corticotropin-releasing hormone attenuates vascular endothelial growth factor release from human HaCaT keratinocytes. Regul. Pept. 2010, 160, 115–120. [Google Scholar] [CrossRef] [PubMed]
- Zbytek, B.; Mysliwski, A.; Slominski, A.; Wortsman, J.; Wei, E.T.; Mysliwska, J. Corticotropin-releasing hormone affects cytokine production in human HaCaT keratinocytes. Life Sci. 2002, 70, 1013–1021. [Google Scholar] [CrossRef]
- Pondeljak, N.; Lugović-Mihić, L. Stress-induced Interaction of Skin Immune Cells, Hormones, and Neurotransmitters. Clin. Ther. 2020, 42, 757–770. [Google Scholar] [CrossRef]
- Lee, E.Y.; Nam, Y.J.; Kang, S.; Choi, E.J.; Han, I.; Kim, J.; Kim, D.H.; An, J.H.; Lee, S.; Lee, M.H.; et al. The local hypothalamic-pituitary-adrenal axis in cultured human dermal papilla cells. BMC Mol. Cell. Biol. 2020, 21, 42. [Google Scholar] [CrossRef]
- Ayasse, M.T.; Buddenkotte, J.; Alam, M.; Steinhoff, M. Role of neuroimmune circuits and pruritus in psoriasis. Exp. Dermatol. 2020, 29, 414–426. [Google Scholar] [CrossRef] [PubMed]
- Ito, N.; Ito, T.; Kromminga, A.; Bettermann, A.; Takigawa, M.; Kees, F.; Straub, R.H.; Paus, R. Human hair follicles display a functional equivalent of the hypothalamic-pituitary-adrenal axis and synthesize cortisol. FASEB J. 2005, 19, 1332–1334. [Google Scholar] [CrossRef] [PubMed]
- Cuellar-Barboza, A.; Cardenas-de la Garza, J.A.; Cruz-Gómez, L.G.; Barboza-Quintana, O.; Flores-Gutiérrez, J.P.; Gómez-Flores, M.; Welsh, O.; Ocampo-Candiani, J.; Herz-Ruelas, M.E. Local secretion of stress hormones increases in alopecia areata lesions after treatment with UVA-1 phototherapy. Exp. Dermatol. 2020, 29, 259–264. [Google Scholar] [CrossRef] [Green Version]
- Slominski, A.T.; Zmijewski, M.A.; Zbytek, B.; Tobin, D.J.; Theoharides, T.C.; Rivier, J. Key role of CRF in the skin stress response system. Endocr. Rev. 2013, 34, 827–884. [Google Scholar] [CrossRef] [PubMed]
- Theoharides, T.C.; Petra, A.I.; Stewart, J.M.; Tsilioni, I.; Panagiotidou, S.; Akin, C. High serum corticotropin-releasing hormone (CRH) and bone marrow mast cell CRH receptor expression in a mastocytosis patient. J. Allergy Clin. Immunol. 2014, 134, 1197–1199. [Google Scholar] [CrossRef]
- Jhang, J.F.; Birder, L.A.; Jiang, Y.H.; Hsu, Y.H.; Ho, H.C.; Kuo, H.C. Dysregulation of bladder corticotropin-releasing hormone receptor in the pathogenesis of human interstitial cystitis/bladder pain syndrome. Sci. Rep. 2019, 9, 19169. [Google Scholar] [CrossRef] [Green Version]
- Sagami, Y.; Shimada, Y.; Tayama, J.; Nomura, T.; Satake, M.; Endo, Y.; Shoji, T.; Karahashi, K.; Hongo, M.; Fukudo, S. Effect of a corticotropin releasing hormone receptor antagonist on colonic sensory and motor function in patients with irritable bowel syndrome. Gut 2004, 53, 958–964. [Google Scholar] [CrossRef]
- Wallon, C.; Yang, P.C.; Keita, A.V.; Ericson, A.C.; McKay, D.M.; Sherman, P.M.; Perdue, M.H.; Söderholm, J.D. Corticotropin-releasing hormone (CRH) regulates macromolecular permeability via mast cells in normal human colonic biopsies in vitro. Gut 2008, 57, 50–58. [Google Scholar] [CrossRef]
- Zheng, P.Y.; Feng, B.S.; Oluwole, C.; Struiksma, S.; Chen, X.; Li, P.; Tang, S.G.; Yang, P.C. Psychological stress induces eosinophils to produce corticotrophin releasing hormone in the intestine. Gut 2009, 58, 1473–1479. [Google Scholar] [CrossRef] [PubMed]
- Kawahito, Y.; Sano, H.; Mukai, S.; Asai, K.; Kimura, S.; Yamamura, Y.; Kato, H.; Chrousos, G.P.; Wilder, R.L.; Kondo, M. Corticotropin releasing hormone in colonic mucosa in patients with ulcerative colitis. Gut 1995, 37, 544–551. [Google Scholar] [CrossRef] [Green Version]
- Kawahito, Y.; Sano, H.; Kawata, M.; Yuri, K.; Mukai, S.; Yamamura, Y.; Kato, H.; Chrousos, G.P.; Wilder, R.L.; Kondo, M. Local secretion of corticotropin-releasing hormone by enterochromaffin cells in human colon. Gastroenterology 1994, 106, 859–865. [Google Scholar] [CrossRef]
- Kim, T.H.; Lee, S.H.; Lee, H.M.; Lee, S.H.; Lee, S.W.; Kim, W.J.; Park, S.J.; Kim, Y.S.; Choe, H.; Hwang, H.Y.; et al. Over-expression of neuropeptide urocortin and its receptors in human allergic nasal mucosa. Laryngoscope 2007, 117, 1513–1518. [Google Scholar] [CrossRef]
- Theoharides, T.C.; Enakuaa, S.; Sismanopoulos, N.; Asadi, S.; Papadimas, E.C.; Angelidou, A.; Alysandratos, K.D. Contribution of stress to asthma worsening through mast cell activation. Ann. Allergy Asthma Immunol. Off. Publ. Am. Coll. Allergy Asthma Immunol. 2012, 109, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, P.; Zhang, L.; Fu, J.; Di, T.; Li, N.; Meng, Y.; Guo, J.; Zhao, J. Stress aggravates and prolongs imiquimod-induced psoriasis-like epidermal hyperplasis and IL-1β/IL-23p40 production. J. Leukoc. Biol. 2020, 108, 267–281. [Google Scholar] [CrossRef] [PubMed]
- Mochizuki, H.; Lavery, M.J.; Nattkemper, L.A.; Albornoz, C.; Valdes Rodriguez, R.; Stull, C.; Weaver, L.; Hamsher, J.; Sanders, K.M.; Chan, Y.H.; et al. Impact of acute stress on itch sensation and scratching behaviour in patients with atopic dermatitis and healthy controls. Br. J. Dermatol. 2019, 180, 821–827. [Google Scholar] [CrossRef] [PubMed]
- Vasiadi, M.; Therianou, A.; Sideri, K.; Smyrnioti, M.; Sismanopoulos, N.; Delivanis, D.A.; Asadi, S.; Katsarou-Katsari, A.; Petrakopoulou, T.; Theoharides, A.; et al. Increased serum CRH levels with decreased skin CRHR-1 gene expression in psoriasis and atopic dermatitis. J. Allergy Clin. Immunol. 2012, 129, 1410–1413. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, J.E.; Cho, D.H.; Kim, H.S.; Kim, H.J.; Lee, J.Y.; Cho, B.K.; Park, H.J. Expression of the corticotropin-releasing hormone-proopiomelanocortin axis in the various clinical types of psoriasis. Exp. Dermatol. 2007, 16, 104–109. [Google Scholar] [CrossRef] [PubMed]
- Loite, U.; Kingo, K.; Reimann, E.; Reemann, P.; Vasar, E.; Silm, H.; Kõks, S. Gene expression analysis of the corticotrophin-releasing hormone-proopiomelanocortin system in psoriasis skin biopsies. Acta Derm. Venereol. 2013, 93, 400–405. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, L.K.; Pang, X.; Alexacos, N.; Letourneau, R.; Theoharides, T.C. Acute immobilization stress triggers skin mast cell degranulation via corticotropin releasing hormone, neurotensin, and substance P: A link to neurogenic skin disorders. Brain Behav. Immun. 1999, 13, 225–239. [Google Scholar] [CrossRef] [Green Version]
- Arck, P.C.; Handjiski, B.; Kuhlmei, A.; Peters, E.M.; Knackstedt, M.; Peter, A.; Hunt, S.P.; Klapp, B.F.; Paus, R. Mast cell deficient and neurokinin-1 receptor knockout mice are protected from stress-induced hair growth inhibition. J. Mol. Med. 2005, 83, 386–396. [Google Scholar] [CrossRef]
- Theoharides, T.C.; Donelan, J.M.; Papadopoulou, N.; Cao, J.; Kempuraj, D.; Conti, P. Mast cells as targets of corticotropin-releasing factor and related peptides. Trends Pharmacol. Sci. 2004, 25, 563–568. [Google Scholar] [CrossRef]
- Lytinas, M.; Kempuraj, D.; Huang, M.; Boucher, W.; Esposito, P.; Theoharides, T.C. Acute stress results in skin corticotropin-releasing hormone secretion, mast cell activation and vascular permeability, an effect mimicked by intradermal corticotropin-releasing hormone and inhibited by histamine-1 receptor antagonists. Int. Arch. Allergy Immunol. 2003, 130, 224–231. [Google Scholar] [CrossRef]
- Theoharides, T.C.; Singh, L.K.; Boucher, W.; Pang, X.; Letourneau, R.; Webster, E.; Chrousos, G. Corticotropin-releasing hormone induces skin mast cell degranulation and increased vascular permeability, a possible explanation for its proinflammatory effects. Endocrinology 1998, 139, 403–413. [Google Scholar] [CrossRef] [PubMed]
- Theoharides, T.C.; Stewart, J.M.; Taracanova, A.; Conti, P.; Zouboulis, C.C. Neuroendocrinology of the skin. Rev. Endocr. Metab. Disord. 2016, 17, 287–294. [Google Scholar] [CrossRef] [PubMed]
- Ayyadurai, S.; Gibson, A.J.; D’Costa, S.; Overman, E.L.; Sommerville, L.J.; Poopal, A.C.; Mackey, E.; Li, Y.; Moeser, A.J. Frontline Science: Corticotropin-releasing factor receptor subtype 1 is a critical modulator of mast cell degranulation and stress-induced pathophysiology. J. Leukoc. Biol. 2017, 102, 1299–1312. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stassen, M.; Hartmann, A.K.; Delgado, S.J.; Dehmel, S.; Braun, A. Mast cells within cellular networks. J. Allergy Clin. Immunol. 2019, 144, S46–s54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Steelant, B.; Seys, S.F.; Van Gerven, L.; Van Woensel, M.; Farré, R.; Wawrzyniak, P.; Kortekaas Krohn, I.; Bullens, D.M.; Talavera, K.; Raap, U.; et al. Histamine and T helper cytokine-driven epithelial barrier dysfunction in allergic rhinitis. J. Allergy Clin. Immunol. 2018, 141, 951–963.e8. [Google Scholar] [CrossRef] [Green Version]
- Hayes, S.M.; Biggs, T.C.; Goldie, S.P.; Harries, P.G.; Walls, A.F.; Allan, R.N.; Pender, S.L.F.; Salib, R.J. Staphylococcus aureus internalization in mast cells in nasal polyps: Characterization of interactions and potential mechanisms. J. Allergy Clin. Immunol. 2020, 145, 147–159. [Google Scholar] [CrossRef] [Green Version]
- Biggs, T.C.; Abadalkareem, R.S.; Hayes, S.M.; Holding, R.E.; Lau, L.C.; Harries, P.G.; Allan, R.N.; Pender, S.L.F.; Walls, A.F.; Salib, R.J. Staphylococcus aureus internalisation enhances bacterial survival through modulation of host immune responses and mast cell activation. Allergy 2020. [Google Scholar] [CrossRef]
- Silverman, E.S.; Breault, D.T.; Vallone, J.; Subramanian, S.; Yilmaz, A.D.; Mathew, S.; Subramaniam, V.; Tantisira, K.; Pacák, K.; Weiss, S.T.; et al. Corticotropin-releasing hormone deficiency increases allergen-induced airway inflammation in a mouse model of asthma. J. Allergy Clin. Immunol. 2004, 114, 747–754. [Google Scholar] [CrossRef] [PubMed]
- D’Costa, S.; Ayyadurai, S.; Gibson, A.J.; Mackey, E.; Rajput, M.; Sommerville, L.J.; Wilson, N.; Li, Y.; Kubat, E.; Kumar, A.; et al. Mast cell corticotropin-releasing factor subtype 2 suppresses mast cell degranulation and limits the severity of anaphylaxis and stress-induced intestinal permeability. J. Allergy Clin. Immunol. 2019, 143, 1865–1877.e4. [Google Scholar] [CrossRef] [Green Version]
- Sugawara, K.; Zakany, N.; Hundt, T.; Emelianov, V.; Tsuruta, D.; Schafer, C.; Kloepper, J.E.; Biro, T.; Paus, R. Cannabinoid receptor 1 controls human mucosal-type mast cell degranulation and maturation in situ. J. Allergy Clin. Immunol. 2013, 132, 182–193. [Google Scholar] [CrossRef] [Green Version]
- Jiang, S.Z.; Eiden, L.E. Activation of the HPA axis and depression of feeding behavior induced by restraint stress are separately regulated by PACAPergic neurotransmission in the mouse. Stress 2016, 19, 374–382. [Google Scholar] [CrossRef] [Green Version]
- Seki, M.; Zha, X.M.; Inamura, S.; Taga, M.; Matsuta, Y.; Aoki, Y.; Ito, H.; Yokoyama, O. Role of corticotropin-releasing factor on bladder function in rats with psychological stress. Sci. Rep. 2019, 9, 9828. [Google Scholar] [CrossRef] [Green Version]
- Sugawara, K.; Biro, T.; Tsuruta, D.; Toth, B.I.; Kromminga, A.; Zakany, N.; Zimmer, A.; Funk, W.; Gibbs, B.F.; Zimmer, A.; et al. Endocannabinoids limit excessive mast cell maturation and activation in human skin. J. Allergy Clin. Immunol. 2012, 129, 726–738.e8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schierhorn, K.; Brunnee, T.; Paus, R.; Schultz, K.D.; Niehus, J.; Agha-Mir-Salim, P.; Kunkel, G. Gelatin sponge-supported histoculture of human nasal mucosa. In Vitro Cell. Dev. Biol. Anim. 1995, 31, 215–220. [Google Scholar] [CrossRef]
- Elieh Ali Komi, D.; Wöhrl, S.; Bielory, L. Mast Cell Biology at Molecular Level: A Comprehensive Review. Clin. Rev. Allergy Immunol. 2020, 58, 342–365. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Liu, Z.; Li, Z.; Wu, Y. Molecular regulation of mast cell development and maturation. Mol. Biol. Rep. 2010, 37, 1993–2001. [Google Scholar] [CrossRef]
- Dahlin, J.S.; Hallgren, J. Mast cell progenitors: Origin, development and migration to tissues. Mol. Immunol. 2015, 63, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Baritaki, S.; de Bree, E.; Chatzaki, E.; Pothoulakis, C. Chronic Stress, Inflammation, and Colon Cancer: A CRH System-Driven Molecular Crosstalk. J. Clin. Med. 2019, 8, 1669. [Google Scholar] [CrossRef] [Green Version]
- Combs, J.W. Maturation of rat mast cells. An electron microscope study. J. Cell. Biol. 1966, 31, 563–575. [Google Scholar] [CrossRef]
- Wernersson, S.; Pejler, G. Mast cell secretory granules: Armed for battle. Nat. Rev. Immunol. 2014, 14, 478–494. [Google Scholar] [CrossRef] [PubMed]
- Popov, A.A. Ultrastructural changes in the size of mast cell granules and in the granular matrix during an experimental anaphylactic shock. Anat. Anz. 1988, 166, 267–273. [Google Scholar]
- Miyachi, K.; Fritzler, M.J.; Tan, E.M. Autoantibody to a nuclear antigen in proliferating cells. J. Immunol. 1978, 121, 2228–2234. [Google Scholar]
- Bertolini, M.; Zilio, F.; Rossi, A.; Kleditzsch, P.; Emelianov, V.E.; Gilhar, A.; Keren, A.; Meyer, K.C.; Wang, E.; Funk, W.; et al. Abnormal interactions between perifollicular mast cells and CD8+ T-cells may contribute to the pathogenesis of alopecia areata. PLoS ONE 2014, 9, e94260. [Google Scholar] [CrossRef]
- Wu, C.C.; Kim, J.N.; Wang, Z.; Chang, Y.L.; Zengler, K.; Di Nardo, A. Mast cell recruitment is modulated by the hairless skin microbiome. J. Allergy Clin. Immunol. 2019, 144, 330–333.e6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, S.; Anderson, D.F.; Bradding, P.; Coward, W.R.; Baddeley, S.M.; MacLeod, J.D.; McGill, J.I.; Church, M.K.; Holgate, S.T.; Roche, W.R. Human mast cells express stem cell factor. J. Pathol. 1998, 186, 59–66. [Google Scholar] [CrossRef]
- Tomljenovic, D.; Pinter, D.; Kalogjera, L. Perceived stress and severity of chronic rhinosinusitis in allergic and nonallergic patients. Allergy Asthma Proc. 2014, 35, 398–403. [Google Scholar] [CrossRef] [PubMed]
- Trueba, A.; Ryan, M.W.; Vogel, P.D.; Ritz, T. Effects of academic exam stress on nasal leukotriene B4 and vascular endothelial growth factor in asthma and health. Biol. Psychol. 2016, 118, 44–51. [Google Scholar] [CrossRef]
- Bagher, M.; Larsson-Callerfelt, A.K.; Rosmark, O.; Hallgren, O.; Bjermer, L.; Westergren-Thorsson, G. Mast cells and mast cell tryptase enhance migration of human lung fibroblasts through protease-activated receptor 2. Cell Commun. Signal. 2018, 16, 59. [Google Scholar] [CrossRef]
- Pejler, G.; Ronnberg, E.; Waern, I.; Wernersson, S. Mast cell proteases: Multifaceted regulators of inflammatory disease. Blood 2010, 115, 4981–4990. [Google Scholar] [CrossRef] [Green Version]
- Takefuji, M.; Murohara, T. Corticotropin-Releasing Hormone Family and Their Receptors in the Cardiovascular System. Circ. J. 2019, 83, 261–266. [Google Scholar] [CrossRef] [Green Version]
- Modena, B.D.; Dazy, K.; White, A.A. Emerging concepts: Mast cell involvement in allergic diseases. Transl. Res. 2016, 174, 98–121. [Google Scholar] [CrossRef]
- Kim, H.S.; Cho, D.H.; Kim, H.J.; Lee, J.Y.; Cho, B.K.; Park, H.J. Immunoreactivity of corticotropin-releasing hormone, adrenocorticotropic hormone and alpha-melanocyte-stimulating hormone in alopecia areata. Exp. Dermatol. 2006, 15, 515–522. [Google Scholar] [CrossRef]
- Nakashima, C.; Ishida, Y.; Kitoh, A.; Otsuka, A.; Kabashima, K. Interaction of peripheral nerves and mast cells, eosinophils, and basophils in the development of pruritus. Exp. Dermatol. 2019, 28, 1405–1411. [Google Scholar] [CrossRef] [Green Version]
- Babina, M.; Guhl, S.; Artuc, M.; Zuberbier, T. Allergic FcεRI- and pseudo-allergic MRGPRX2-triggered mast cell activation routes are independent and inversely regulated by SCF. Allergy 2018, 73, 256–260. [Google Scholar] [CrossRef]
- Kawabori, S.; Kanai, N.; Tosho, T. Proliferative activity of mast cells in allergic nasal mucosa. Clin. Exp. Allergy J. Br. Soc. Allergy Clin. Immunol. 1995, 25, 173–178. [Google Scholar] [CrossRef]
- Balseiro-Gomez, S.; Flores, J.A.; Acosta, J.; Ramirez-Ponce, M.P.; Ales, E. Identification of a New Exo-Endocytic Mechanism Triggered by Corticotropin-Releasing Hormone in Mast Cells. J. Immunol. 2015, 195, 2046–2056. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elieh Ali Komi, D.; Shafaghat, F.; Kovanen, P.T.; Meri, S. Mast cells and complement system: Ancient interactions between components of innate immunity. Allergy 2020, 75, 2818–2828. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, L.B.; Irani, A.M.; Roller, K.; Castells, M.C.; Schechter, N.M. Quantitation of histamine, tryptase, and chymase in dispersed human T and TC mast cells. J. Immunol. 1987, 138, 2611–2615. [Google Scholar] [PubMed]
- Gotovina, J.; Bianchini, R.; Fazekas-Singer, J.; Herrmann, I.; Pellizzari, G.; Haidl, I.D.; Hufnagl, K.; Karagiannis, S.N.; Marshall, J.S.; Jensen-Jarolim, E. Epinephrine drives human M2a allergic macrophages to a regulatory phenotype reducing mast cell degranulation in vitro. Allergy 2020, 75, 2939–2942. [Google Scholar] [CrossRef]
- Mendez-Enriquez, E.; Alvarado-Vazquez, P.A.; Abma, W.; Simonson, O.E.; Rodin, S.; Feyerabend, T.B.; Rodewald, H.R.; Malinovschi, A.; Janson, C.; Adner, M.; et al. Mast cell-derived serotonin enhances methacholine-induced airway hyperresponsiveness in house dust mite-induced experimental asthma. Allergy 2021. [Google Scholar] [CrossRef]
- Reber, L.L.; Sibilano, R.; Mukai, K.; Galli, S.J. Potential effector and immunoregulatory functions of mast cells in mucosal immunity. Mucosal Immunol. 2015, 8, 444–463. [Google Scholar] [CrossRef] [Green Version]
- Fukudo, S. Role of corticotropin-releasing hormone in irritable bowel syndrome and intestinal inflammation. J. Gastroenterol. 2007, 42, 48–51. [Google Scholar] [CrossRef]
- Lu, Z.; Hasse, S.; Bodo, E.; Rose, C.; Funk, W.; Paus, R. Towards the development of a simplified long-term organ culture method for human scalp skin and its appendages under serum-free conditions. Exp. Dermatol. 2007, 16, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Papadopoulou, N.; Kempuraj, D.; Boucher, W.S.; Sugimoto, K.; Cetrulo, C.L.; Theoharides, T.C. Human mast cells express corticotropin-releasing hormone (CRH) receptors and CRH leads to selective secretion of vascular endothelial growth factor. J. Immunol. 2005, 174, 7665–7675. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wierzbicka, J.M.; Żmijewski, M.A.; Antoniewicz, J.; Sobjanek, M.; Slominski, A.T. Differentiation of Keratinocytes Modulates Skin HPA Analog. J. Cell. Physiol. 2017, 232, 154–166. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.D.; Liao, X.M.; Uribe-Marino, A.; Liu, R.; Xie, X.M.; Jia, J.; Su, Y.A.; Li, J.T.; Schmidt, M.V.; Wang, X.D.; et al. Stress during a critical postnatal period induces region-specific structural abnormalities and dysfunction of the prefrontal cortex via CRF1. Neuropsychopharmacology 2015, 40, 1203–1215. [Google Scholar] [CrossRef] [PubMed]






| Antigen | Isotype | Dilution | Supplier | Cat.# | RRID |
|---|---|---|---|---|---|
| CRH | rabbit | 1:50 | Prointech | 10944 | AB_2084279 |
| CRH-R1 | goat | 1:200 | Abcam | ab77686 | AB_1566096 |
| CRH-R2 | rabbit | 1:200 | Invitrogen | 720291 | AB_2633243 |
| Tryptase | mouse | 1:500 | Abcam | ab2378 | AB_303023 |
| C-Kit | rabbit | 1:200 | Cell Marque | 117R-16 | AB_1159085 |
| Chymase | goat | 1:500 | Abcam | ab111239 | AB_10863662 |
| PCNA | rabbit | 1:200 | Abcam | ab92552 | AB_10561973 |
| Ki-67 | rabbit | 1:20 | Abcam | ab16667 | AB_302459 |
| SCF | rabbit | 1:100 | Abcam | ab64677 | AB_1861346 |
| AE1/3 | goat | 1:400 | Abcam | ab86734 | AB_10674321 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yamanaka-Takaichi, M.; Mizukami, Y.; Sugawara, K.; Sunami, K.; Teranishi, Y.; Kira, Y.; Paus, R.; Tsuruta, D. Stress and Nasal Allergy: Corticotropin-Releasing Hormone Stimulates Mast Cell Degranulation and Proliferation in Human Nasal Mucosa. Int. J. Mol. Sci. 2021, 22, 2773. https://doi.org/10.3390/ijms22052773
Yamanaka-Takaichi M, Mizukami Y, Sugawara K, Sunami K, Teranishi Y, Kira Y, Paus R, Tsuruta D. Stress and Nasal Allergy: Corticotropin-Releasing Hormone Stimulates Mast Cell Degranulation and Proliferation in Human Nasal Mucosa. International Journal of Molecular Sciences. 2021; 22(5):2773. https://doi.org/10.3390/ijms22052773
Chicago/Turabian StyleYamanaka-Takaichi, Mika, Yukari Mizukami, Koji Sugawara, Kishiko Sunami, Yuichi Teranishi, Yukimi Kira, Ralf Paus, and Daisuke Tsuruta. 2021. "Stress and Nasal Allergy: Corticotropin-Releasing Hormone Stimulates Mast Cell Degranulation and Proliferation in Human Nasal Mucosa" International Journal of Molecular Sciences 22, no. 5: 2773. https://doi.org/10.3390/ijms22052773
APA StyleYamanaka-Takaichi, M., Mizukami, Y., Sugawara, K., Sunami, K., Teranishi, Y., Kira, Y., Paus, R., & Tsuruta, D. (2021). Stress and Nasal Allergy: Corticotropin-Releasing Hormone Stimulates Mast Cell Degranulation and Proliferation in Human Nasal Mucosa. International Journal of Molecular Sciences, 22(5), 2773. https://doi.org/10.3390/ijms22052773