Dynamics of Cyclooxygenase-1 Positive Microglia/Macrophage in the Retina of Pathological Model Mice as a Biomarker of the Retinal Inflammatory Diseases
Abstract
:1. Introduction
2. Results
2.1. Expression of COX-1 in Normal Retina
2.2. Expression of COX-2 in Normal Retina
2.3. Expressions of COX-1 and COX-2 in Primary Cultured Retinal Microglia/Macrophage and Splenic Macrophage
2.4. Analysis of CD45 and COX-1 Expression in CD11b-Positive Cells Collected from the CNS
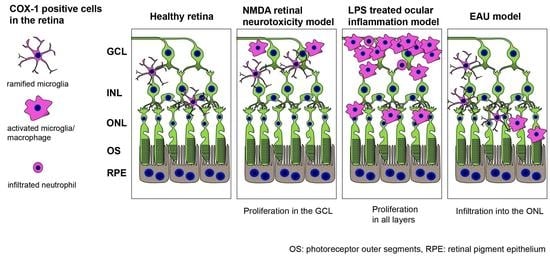
2.5. COX-1 in the Retina of N-Methyl-D-Aspartate (NMDA)-Administered Retinal Degeneration Model
2.6. COX-1 in the Retina of Lipopolysaccharide (LPS)-Administered Ocular Inflammation Model
2.7. Early Stage Inflammation in Experimental Autoimmune Uveoretinitis (EAU) Evaluated by COX-1+ Cells
3. Discussion
4. Material and Methods
4.1. Isolation and Culture of Retinal Microglia
4.2. Isolation and Preparation of Splenic Macrophage
4.3. Isolation of CNS Microglia/Macrophage
4.4. Flow Cytometry
4.5. Experimental Animals
4.6. NMDA-Administration Retinopathy Model
4.7. LPS-Administered Ocular Inflammation Induction Model
4.8. Experimental Autoimmune Uveoretinitis Model
4.9. Optical Coherence Tomography (OCT) Imaging
4.10. Immunohistochemistry
4.11. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| COX-1 | cyclooxygenase-1 |
| COX-2 | cyclooxygenase-2 |
| NMDA | N-methyl-D-aspartate |
| LPS | lipopolysaccharide |
| EAU | experimental autoimmune uveoretinitis |
| OCT | optical coherence tomography |
| IRBP | interphotoreceptor retinoid-binding protein |
| GCL | ganglion cell layer |
| ONL | outer nuclear layer |
| INL | inner nuclear layer |
References
- Augusto-Oliveira, M.; Arrifano, G.P.; Lopes-Araujo, A.; Santos-Sacramento, L.; Takeda, P.Y.; Anthony, D.C.; Malva, J.O.; Crespo-Lopez, M.E. What Do Microglia Really Do in Healthy Adult Brain? Cells 2019, 8, 1293. [Google Scholar] [CrossRef] [PubMed]
- Aguzzi, A.; Barres, B.A.; Bennett, M.L. Microglia: Scapegoat, saboteur, or something else? Science 2013, 339, 156–161. [Google Scholar] [CrossRef]
- Vowinckel, E.; Reutens, D.; Becher, B.; Verge, G.; Evans, A.; Owens, T.; Antel, J.P. PK11195 binding to the peripheral benzodiazepine receptor as a marker of microglia activation in multiple sclerosis and experimental autoimmune encephalomyelitis. J. Neurosci. Res. 1997, 50, 345–353. [Google Scholar] [CrossRef]
- Cagnin, A.; Brooks, D.J.; Kennedy, A.M.; Gunn, R.N.; Myers, R.; Turkheimer, F.E.; Jones, T.; Banati, R.B. In-vivo measurement of activated microglia in dementia. Lancet 2001, 358, 461–467. [Google Scholar] [CrossRef]
- Cagnin, A.; Myers, R.; Gunn, R.N.; Lawrence, A.D.; Stevens, T.; Kreutzberg, G.W.; Jones, T.; Banati, R.B. In vivo visualization of activated glia by [11C] (R)-PK11195-PET following herpes encephalitis reveals projected neuronal damage beyond the primary focal lesion. Brain 2001, 124 Pt 10, 2014–2027. [Google Scholar] [CrossRef]
- Ouchi, Y.; Yoshikawa, E.; Sekine, Y.; Futatsubashi, M.; Kanno, T.; Ogusu, T.; Torizuka, T. Microglial activation and dopamine terminal loss in early Parkinson’s disease. Ann. Neurol. 2005, 57, 168–175. [Google Scholar] [CrossRef] [PubMed]
- Anrather, J.; Gallo, E.F.; Kawano, T.; Orio, M.; Abe, T.; Gooden, C.; Zhou, P.; Iadecola, C. Purinergic signaling induces cyclooxygenase-1-dependent prostanoid synthesis in microglia: Roles in the outcome of excitotoxic brain injury. PLoS ONE 2011, 6, e25916. [Google Scholar] [CrossRef]
- Zhang, F.Y.; Wan, Y.; Zhang, Z.K.; Light, A.R.; Fu, K.Y. Peripheral formalin injection induces long-lasting increases in cyclooxygenase 1 expression by microglia in the spinal cord. J. Pain 2007, 8, 110–117. [Google Scholar] [CrossRef]
- Ju, W.K.; Neufeld, A.H. Cellular localization of cyclooxygenase-1 and cyclooxygenase-2 in the normal mouse, rat, and human retina. J. Comp. Neurol. 2002, 452, 392–399. [Google Scholar] [CrossRef]
- Smith, W.L.; Garavito, R.M.; DeWitt, D.L. Prostaglandin endoperoxide H synthases (cyclooxygenases)-1 and -2. J. Biol. Chem. 1996, 271, 33157–33160. [Google Scholar] [CrossRef]
- Smith, W.L.; Song, I. The enzymology of prostaglandin endoperoxide H synthases-1 and -2. Prostaglandins Other Lipid Mediat 2002, 68–69, 115–128. [Google Scholar] [CrossRef]
- Shukuri, M.; Takashima-Hirano, M.; Tokuda, K.; Takashima, T.; Matsumura, K.; Inoue, O.; Doi, H.; Suzuki, M.; Watanabe, Y.; Onoe, H. In vivo expression of cyclooxygenase-1 in activated microglia and macrophages during neuroinflammation visualized by PET with 11C-ketoprofen methyl ester. J. Nucl. Med. 2011, 52, 1094–1101. [Google Scholar] [CrossRef] [PubMed]
- Shukuri, M.; Mawatari, A.; Ohno, M.; Suzuki, M.; Doi, H.; Watanabe, Y.; Onoe, H. Detection of Cyclooxygenase-1 in Activated Microglia During Amyloid Plaque Progression: PET Studies in Alzheimer’s Disease Model Mice. J. Nucl. Med. 2016, 57, 291–296. [Google Scholar] [CrossRef]
- Singh, P.; Shrestha, S.; Cortes-Salva, M.Y.; Jenko, K.J.; Zoghbi, S.S.; Morse, C.L.; Innis, R.B.; Pike, V.W. 3-Substituted 1,5-Diaryl-1 H-1,2,4-triazoles as Prospective PET Radioligands for Imaging Brain COX-1 in Monkey. Part 1: Synthesis and Pharmacology. ACS Chem. Neurosci. 2018, 9, 2610–2619. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, S.; Singh, P.; Cortes-Salva, M.Y.; Jenko, K.J.; Ikawa, M.; Kim, M.J.; Kobayashi, M.; Morse, C.L.; Gladding, R.L.; Liow, J.S.; et al. 3-Substituted 1,5-Diaryl-1 H-1,2,4-triazoles as Prospective PET Radioligands for Imaging Brain COX-1 in Monkey. Part 2: Selection and Evaluation of [(11)C]PS13 for Quantitative Imaging. ACS Chem. Neurosci. 2018, 9, 2620–2627. [Google Scholar] [CrossRef] [PubMed]
- Hirasawa, T.; Ohsawa, K.; Imai, Y.; Ondo, Y.; Akazawa, C.; Uchino, S.; Kohsaka, S. Visualization of microglia in living tissues using Iba1-EGFP transgenic mice. J. Neurosci. Res. 2005, 81, 357–362. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Shi, F.D.; Jung, S.; Pien, G.C.; Wang, J.; Salazar-Mather, T.P.; He, T.T.; Weaver, J.T.; Ljunggren, H.G.; Biron, C.A.; et al. The neuronal chemokine CX3CL1/fractalkine selectively recruits NK cells that modify experimental autoimmune encephalomyelitis within the central nervous system. FASEB J. 2006, 20, 896–905. [Google Scholar] [CrossRef]
- Sasmono, R.T.; Williams, E. Generation and characterization of MacGreen mice, the Cfs1r-EGFP transgenic mice. Methods Mol. Biol. 2012, 844, 157–176. [Google Scholar]
- Zeng, H.Y.; Green, W.R.; Tso, M.O. Microglial activation in human diabetic retinopathy. Arch. Ophthalmol. 2008, 126, 227–232. [Google Scholar] [CrossRef]
- Neufeld, A.H. Microglia in the optic nerve head and the region of parapapillary chorioretinal atrophy in glaucoma. Arch. Ophthalmol. 1999, 117, 1050–1056. [Google Scholar] [CrossRef]
- Bosco, A.; Romero, C.O.; Breen, K.T.; Chagovetz, A.A.; Steele, M.R.; Ambati, B.K.; Vetter, M.L. Neurodegeneration severity can be predicted from early microglia alterations monitored in vivo in a mouse model of chronic glaucoma. Dis. Model. Mech. 2015, 8, 443–455. [Google Scholar] [CrossRef]
- Gupta, N.; Brown, K.E.; Milam, A.H. Activated microglia in human retinitis pigmentosa, late-onset retinal degeneration, and age-related macular degeneration. Exp. Eye Res. 2003, 76, 463–471. [Google Scholar] [CrossRef]
- Zhao, L.; Zabel, M.K.; Wang, X.; Ma, W.; Shah, P.; Fariss, R.N.; Qian, H.; Parkhurst, C.N.; Gan, W.B.; Wong, W.T. Microglial phagocytosis of living photoreceptors contributes to inherited retinal degeneration. EMBO Mol. Med. 2015, 7, 1179–1197. [Google Scholar] [CrossRef]
- Peng, B.; Xiao, J.; Wang, K.; So, K.F.; Tipoe, G.L.; Lin, B. Suppression of microglial activation is neuroprotective in a mouse model of human retinitis pigmentosa. J. Neurosci. 2014, 34, 8139–8150. [Google Scholar] [CrossRef] [PubMed]
- Ramadan, E.; Basselin, M.; Rao, J.S.; Chang, L.; Chen, M.; Ma, K.; Rapoport, S.I. Lamotrigine blocks NMDA receptor-initiated arachidonic acid signalling in rat brain: Implications for its efficacy in bipolar disorder. Int. J. Neuropsychopharmacol. 2012, 15, 931–943. [Google Scholar] [CrossRef] [PubMed]
- Taha, A.Y.; Blanchard, H.C.; Cheon, Y.; Ramadan, E.; Chen, M.; Chang, L.; Rapoport, S.I. Dietary Linoleic Acid Lowering Reduces Lipopolysaccharide-Induced Increase in Brain Arachidonic Acid Metabolism. Mol. Neurobiol. 2017, 54, 4303–4315. [Google Scholar] [CrossRef] [PubMed]
- Ayoub, S.S.; Wood, E.G.; Hassan, S.U.; Bolton, C. Cyclooxygenase expression and prostaglandin levels in central nervous system tissues during the course of chronic relapsing experimental autoimmune encephalomyelitis (EAE). Inflamm. Res. 2011, 60, 919–928. [Google Scholar] [CrossRef] [PubMed]
- Martin, E.; El-Behi, M.; Fontaine, B.; Delarasse, C. Analysis of Microglia and Monocyte-derived Macrophages from the Central Nervous System by Flow Cytometry. J. Vis. Exp. 2017, 124, e55781. [Google Scholar] [CrossRef]
- Li, Y.; Schlamp, C.L.; Nickells, R.W. Experimental induction of retinal ganglion cell death in adult mice. Investig. Ophthalmol. Vis. Sci. 1999, 40, 1004–1008. [Google Scholar]
- Caspi, R.R. Experimental autoimmune uveoretinitis in the rat and mouse. Curr. Protoc. Immunol. 2003, 53, 15.6.1–15.6.20. [Google Scholar] [CrossRef]
- Devarajan, G.; Chen, M.; Muckersie, E.; Xu, H. Culture and characterization of microglia from the adult murine retina. Sci. World J. 2014, 2014, 894368. [Google Scholar] [CrossRef]
- Lin, Y.S.; Kuo, K.T.; Chen, S.K.; Huang, H.S. RBFOX3/NeuN is dispensable for visual function. PLoS ONE 2018, 13, e0192355. [Google Scholar] [CrossRef]
- Nakazawa, T.; Shimura, M.; Endo, S.; Takahashi, H.; Mori, N.; Tamai, M. N-Methyl-D-Aspartic acid suppresses Akt activity through protein phosphatase in retinal ganglion cells. Mol. Vis. 2005, 11, 1173–1182. [Google Scholar]
- Nickells, R.W. Retinal ganglion cell death in glaucoma: The how, the why, and the maybe. J. Glaucoma 1996, 5, 345–356. [Google Scholar] [CrossRef] [PubMed]
- Gu, L.; Xu, H.; Wang, F.; Xu, G.; Sinha, D.; Wang, J.; Xu, J.Y.; Tian, H.; Gao, F.; Li, W.; et al. Erythropoietin exerts a neuroprotective function against glutamate neurotoxicity in experimental diabetic retina. Investig. Ophthalmol. Vis. Sci. 2014, 55, 8208–8222. [Google Scholar] [CrossRef] [PubMed]
- Nakano, N.; Ikeda, H.O.; Hangai, M.; Muraoka, Y.; Toda, Y.; Kakizuka, A.; Yoshimura, N. Longitudinal and simultaneous imaging of retinal ganglion cells and inner retinal layers in a mouse model of glaucoma induced by N-methyl-D-aspartate. Investig. Ophthalmol. Vis. Sci. 2011, 52, 8754–8762. [Google Scholar] [CrossRef] [PubMed]
- Tikka, T.M.; Koistinaho, J.E. Minocycline provides neuroprotection against N-methyl-D-aspartate neurotoxicity by inhibiting microglia. J. Immunol. 2001, 166, 7527–7533. [Google Scholar] [CrossRef]
- Monif, M.; Burnstock, G.; Williams, D.A. Microglia: Proliferation and activation driven by the P2X7 receptor. Int. J. Biochem. Cell Biol. 2010, 42, 1753–1756. [Google Scholar] [CrossRef]
- Hines, D.J.; Choi, H.B.; Hines, R.M.; Phillips, A.G.; MacVicar, B.A. Prevention of LPS-induced microglia activation, cytokine production and sickness behavior with TLR4 receptor interfering peptides. PLoS ONE 2013, 8, e60388. [Google Scholar] [CrossRef] [PubMed]
- Jose, S.; Tan, S.W.; Ooi, Y.Y.; Ramasamy, R.; Vidyadaran, S. Mesenchymal stem cells exert anti-proliferative effect on lipopolysaccharide-stimulated BV2 microglia by reducing tumour necrosis factor-alpha levels. J. Neuroinflamm. 2014, 11, 149. [Google Scholar] [CrossRef]
- Yuan, Z.; Chen, X.; Yang, W.; Lou, B.; Ye, N.; Liu, Y. The anti-inflammatory effect of minocycline on endotoxin-induced uveitis and retinal inflammation in rats. Mol. Vis. 2019, 25, 359–372. [Google Scholar] [PubMed]
- Chu, C.J.; Gardner, P.J.; Copland, D.A.; Liyanage, S.E.; Gonzalez-Cordero, A.; Kleine Holthaus, S.M.; Luhmann, U.F.; Smith, A.J.; Ali, R.R.; Dick, A.D. Multimodal analysis of ocular inflammation using the endotoxin-induced uveitis mouse model. Dis. Model. Mech. 2016, 9, 473–481. [Google Scholar] [CrossRef] [PubMed]
- Rao, N.A.; Kimoto, T.; Zamir, E.; Giri, R.; Wang, R.; Ito, S.; Pararajasegaram, G.; Read, R.W.; Wu, G.S. Pathogenic role of retinal microglia in experimental uveoretinitis. Investig. Ophthalmol. Vis. Sci. 2003, 44, 22–31. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.S.; Sevanian, A.; Rao, N.A. Detection of retinal lipid hydroperoxides in experimental uveitis. Free Radic. Biol. Med. 1992, 12, 19–27. [Google Scholar] [CrossRef]
- Chi, Y.; Guo, C.; Peng, Y.; Qiao, L.; Yang, L. A prospective, observational study on the application of ultra-wide-field angiography in the evaluation and management of patients with anterior uveitis. PLoS ONE 2015, 10, e0122749. [Google Scholar] [CrossRef]
- Aggarwal, K.; Mulkutkar, S.; Mahajan, S.; Singh, R.; Sharma, A.; Bansal, R.; Gupta, V.; Gupta, A. Role of Ultra-Wide Field Imaging in the Management of Tubercular Posterior Uveitis. Ocul. Immunol. Inflamm. 2016, 24, 631–636. [Google Scholar] [CrossRef]
- Sugita, S.; Iwasaki, Y.; Makabe, K.; Kamao, H.; Mandai, M.; Shiina, T.; Ogasawara, K.; Hirami, Y.; Kurimoto, Y.; Takahashi, M. Successful Transplantation of Retinal Pigment Epithelial Cells from MHC Homozygote iPSCs in MHC-Matched Models. Stem. Cell Rep. 2016, 7, 635–648. [Google Scholar] [CrossRef]
- Sugita, S.; Makabe, K.; Fujii, S.; Iwasaki, Y.; Kamao, H.; Shiina, T.; Ogasawara, K.; Takahashi, M. Detection of Retinal Pigment Epithelium-Specific Antibody in iPSC-Derived Retinal Pigment Epithelium Transplantation Models. Stem. Cell Rep. 2017, 9, 1501–1515. [Google Scholar] [CrossRef] [PubMed]
- Fujii, S.; Sugita, S.; Futatsugi, Y.; Ishida, M.; Edo, A.; Makabe, K.; Kamao, H.; Iwasaki, Y.; Sakaguchi, H.; Hirami, Y.; et al. A Strategy for Personalized Treatment of iPS-Retinal Immune Rejections Assessed in Cynomolgus Monkey Models. Int J. Mol. Sci 2020, 21, 3077. [Google Scholar] [CrossRef]
- Sugita, S.; Mandai, M.; Hirami, Y.; Takagi, S.; Maeda, T.; Fujihara, M.; Matsuzaki, M.; Yamamoto, M.; Iseki, K.; Hayashi, N.; et al. HLA-Matched Allogeneic iPS Cells-Derived RPE Transplantation for Macular Degeneration. J. Clin. Med. 2020, 9, 2217. [Google Scholar] [CrossRef] [PubMed]
- Makabe, K.; Sugita, S.; Mandai, M.; Futatsugi, Y.; Takahashi, M. Microglia dynamics in retinitis pigmentosa model: Formation of fundus whitening and autofluorescence as an indicator of activity of retinal degeneration. Sci. Rep. 2020, 10, 14700. [Google Scholar] [CrossRef]
- Garcia Hernandez, T.; Vicedo Gonzalez, A.; Ferrer Rebolleda, J.; Sanchez Jurado, R.; Rosello Ferrando, J.; Brualla Gonzalez, L.; Granero Cabanero, D.; Del Puig Cozar Santiago, M. Performance evaluation of a high resolution dedicated breast PET scanner. Med. Phys. 2016, 43, 2261. [Google Scholar] [CrossRef]
- Caravaggio, F.; Scifo, E.; Sibille, E.L.; Hernandez-Da Mota, S.E.; Gerretsen, P.; Remington, G.; Graff-Guerrero, A. Expression of dopamine D2 and D3 receptors in the human retina revealed by positron emission tomography and targeted mass spectrometry. Exp. Eye Res. 2018, 175, 32–41. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Han, Z.; Tao, J.; Zhang, W.; Li, P.; Tang, L.; Gu, Y. A novel near-infrared fluorescent probe for monitoring cyclooxygenase-2 in inflammation and tumor. J. Biophotonics 2018, 11, e201700339. [Google Scholar] [CrossRef] [PubMed]
- Ra, H.; Gonzalez-Gonzalez, E.; Uddin, M.J.; King, B.L.; Lee, A.; Ali-Khan, I.; Marnett, L.J.; Tang, J.Y.; Contag, C.H. Detection of non-melanoma skin cancer by in vivo fluorescence imaging with fluorocoxib A. Neoplasia 2015, 17, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Uddin, M.J.; Moore, C.E.; Crews, B.C.; Daniel, C.K.; Ghebreselasie, K.; McIntyre, J.O.; Marnett, L.J.; Jayagopal, A. Fluorocoxib A enables targeted detection of cyclooxygenase-2 in laser-induced choroidal neovascularization. J. Biomed. Opt. 2016, 21, 90503. [Google Scholar] [CrossRef]
- Scilimati, A.; Ferorelli, S.; Iaselli, M.C.; Miciaccia, M.; Pati, M.L.; Fortuna, C.G.; Aleem, A.M.; Marnett, L.J.; Perrone, M.G. Targeting COX-1 by mofezolac-based fluorescent probes for ovarian cancer detection. Eur. J. Med. Chem. 2019, 179, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Wahl, R.L.; Jacene, H.; Kasamon, Y.; Lodge, M.A. From RECIST to PERCIST: Evolving Considerations for PET response criteria in solid tumors. J. Nucl. Med. 2009, 50 (Suppl. S1), 122S–150S. [Google Scholar] [CrossRef]
- Sugita, S.; Shimizu, J.; Makabe, K.; Keino, H.; Watanabe, T.; Takahashi, M. Inhibition of T cell-mediated inflammation in uveitis by a novel anti-CD3 antibody. Arthritis Res. Ther. 2017, 19, 176. [Google Scholar] [CrossRef]
- Alatery, A.; Basta, S. An efficient culture method for generating large quantities of mature mouse splenic macrophages. J. Immunol. Methods 2008, 338, 47–57. [Google Scholar] [CrossRef]
- Harimoto, K.; Ito, M.; Karasawa, Y.; Sakurai, Y.; Takeuchi, M. Evaluation of mouse experimental autoimmune uveoretinitis by spectral domain optical coherence tomography. Br. J. Ophthalmol. 2014, 98, 808–812. [Google Scholar] [CrossRef] [PubMed]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Makabe, K.; Sugita, S.; Futatsugi, Y.; Takahashi, M. Dynamics of Cyclooxygenase-1 Positive Microglia/Macrophage in the Retina of Pathological Model Mice as a Biomarker of the Retinal Inflammatory Diseases. Int. J. Mol. Sci. 2021, 22, 3396. https://doi.org/10.3390/ijms22073396
Makabe K, Sugita S, Futatsugi Y, Takahashi M. Dynamics of Cyclooxygenase-1 Positive Microglia/Macrophage in the Retina of Pathological Model Mice as a Biomarker of the Retinal Inflammatory Diseases. International Journal of Molecular Sciences. 2021; 22(7):3396. https://doi.org/10.3390/ijms22073396
Chicago/Turabian StyleMakabe, Kenichi, Sunao Sugita, Yoko Futatsugi, and Masayo Takahashi. 2021. "Dynamics of Cyclooxygenase-1 Positive Microglia/Macrophage in the Retina of Pathological Model Mice as a Biomarker of the Retinal Inflammatory Diseases" International Journal of Molecular Sciences 22, no. 7: 3396. https://doi.org/10.3390/ijms22073396
APA StyleMakabe, K., Sugita, S., Futatsugi, Y., & Takahashi, M. (2021). Dynamics of Cyclooxygenase-1 Positive Microglia/Macrophage in the Retina of Pathological Model Mice as a Biomarker of the Retinal Inflammatory Diseases. International Journal of Molecular Sciences, 22(7), 3396. https://doi.org/10.3390/ijms22073396