The Role of CXCL16 in the Pathogenesis of Cancer and Other Diseases
Abstract
:1. Introduction
2. CXC Motif Chemokine Ligand 16 (CXCL16): Background Information
3. CXCR6: Background Information
4. The Importance of CXCL16 in Non-Neoplastic Diseases
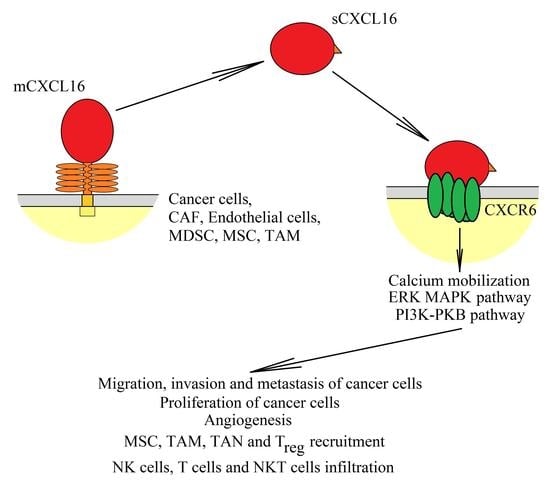
5. Effect of the CXCL16→CXCR6 Axis on Tumor Cells
5.1. Regulation of CXCL16 Expression in Tumors
5.2. Effect of CXCL16 on Cancer Cell Proliferation
5.3. Effect of CXCL16 on Cancer Cell Migration
5.4. Effect of CXCL16 on Metastasis
6. Role of CXCL16→CXCR6 Crosstalk on the Tumor Microenvironment
6.1. Effect of CXCL16 on Angiogenesis and the Role of Hypoxia on CXCL16 Function
6.2. CXCL16→CXCR6 and Tumor-Associated Cell Crosstalk
6.2.1. Cancer-Associated Fibroblasts
6.2.2. Endothelial Cells
6.2.3. Tumor-Associated Macrophages
6.2.4. Myeloid-Derived Suppressor Cells
6.2.5. Tumor-Associated Neutrophils
6.2.6. Mesenchymal Stem Cells
6.2.7. Astrocytes
6.2.8. Regulatory T Cells
6.2.9. Anti-Cancer Tumor-Infiltrating Lymphocytes
7. The CXCL16→CXCR6 Axis in Tumors
7.1. CXCL16
7.2. CXCR6
8. CXCL16→CXCR6 Axis and Anti-Cancer Therapy
9. Conclusions: Perspectives for Future Research
Author Contributions
Funding
Conflicts of Interest
References
- Hinshaw, D.C.; Shevde, L.A. The Tumor Microenvironment Innately Modulates Cancer Progression. Cancer Res. 2019, 79, 4557–4566. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hughes, C.E.; Nibbs, R.J.B. A guide to chemokines and their receptors. FEBS J. 2018, 285, 2944–2971. [Google Scholar] [CrossRef] [PubMed]
- Schall, T.J.; Jongstra, J.; Dyer, B.J.; Jorgensen, J.; Clayberger, C.; Davis, M.M.; Krensky, A.M. A human T cell-specific molecule is a member of a new gene family. J. Immunol. 1988, 141, 1018–1025. [Google Scholar] [PubMed]
- Kodelja, V.; Müller, C.; Politz, O.; Hakij, N.; Orfanos, C.E.; Goerdt, S. Alternative macrophage activation-associated CC-chemokine-1, a novel structural homologue of macrophage inflammatory protein-1 alpha with a Th2-associated expression pattern. J. Immunol. 1998, 160, 1411–1418. [Google Scholar] [PubMed]
- Do, H.T.T.; Lee, C.H.; Cho, J. Chemokines and their Receptors: Multifaceted Roles in Cancer Progression and Potential Value as Cancer Prognostic Markers. Cancers 2020, 12, 287. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matloubian, M.; David, A.; Engel, S.; Ryan, J.E.; Cyster, J.G. A transmembrane CXC chemokine is a ligand for HIV-coreceptor Bonzo. Nat. Immunol. 2000, 1, 298–304. [Google Scholar] [CrossRef] [PubMed]
- Wilbanks, A.; Zondlo, S.C.; Murphy, K.; Mak, S.; Soler, D.; Langdon, P.; Andrew, D.P.; Wu, L.; Briskin, M. Expression cloning of the STRL33/BONZO/TYMSTRligand reveals elements of CC, CXC, and CX3C chemokines. J. Immunol. 2001, 166, 5145–5154. [Google Scholar] [CrossRef] [Green Version]
- Shimaoka, T.; Kume, N.; Minami, M.; Hayashida, K.; Kataoka, H.; Kita, T.; Yonehara, S. Molecular cloning of a novel scavenger receptor for oxidized low density lipoprotein, SR-PSOX, on macrophages. J. Biol. Chem. 2000, 275, 40663–40666. [Google Scholar] [CrossRef] [Green Version]
- Van der Voort, R.; Verweij, V.; de Witte, T.M.; Lasonder, E.; Adema, G.J.; Dolstra, H. An alternatively spliced CXCL16 isoform expressed by dendritic cells is a secreted chemoattractant for CXCR6+ cells. J. Leukoc. Biol. 2010, 87, 1029–1039. [Google Scholar] [CrossRef] [Green Version]
- Fong, A.M.; Erickson, H.P.; Zachariah, J.P.; Poon, S.; Schamberg, N.J.; Imai, T.; Patel, D.D. Ultrastructure and function of the fractalkine mucin domain in CX(3)C chemokine domain presentation. J. Biol. Chem. 2000, 275, 3781–3786. [Google Scholar] [CrossRef] [Green Version]
- Shimaoka, T.; Nakayama, T.; Kume, N.; Takahashi, S.; Yamaguchi, J.; Minami, M.; Hayashida, K.; Kita, T.; Ohsumi, J.; Yoshie, O.; et al. Cutting edge: SR-PSOX/CXC chemokine ligand 16 mediates bacterial phagocytosis by APCs through its chemokine domain. J. Immunol. 2003, 171, 1647–1651. [Google Scholar] [CrossRef] [Green Version]
- Shimaoka, T.; Nakayama, T.; Fukumoto, N.; Kume, N.; Takahashi, S.; Yamaguchi, J.; Minami, M.; Hayashida, K.; Kita, T.; Ohsumi, J.; et al. Cell surface-anchored SR-PSOX/CXC chemokine ligand 16 mediates firm adhesion of CXC chemokine receptor 6-expressing cells. J. Leukoc. Biol. 2004, 75, 267–274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abel, S.; Hundhausen, C.; Mentlein, R.; Schulte, A.; Berkhout, T.A.; Broadway, N.; Hartmann, D.; Sedlacek, R.; Dietrich, S.; Muetze, B.; et al. The transmembrane CXC-chemokine ligand 16 is induced by IFN-gamma and TNF-alpha and shed by the activity of the disintegrin-like metalloproteinase ADAM10. J. Immunol. 2004, 172, 6362–6372. [Google Scholar] [CrossRef] [Green Version]
- Gutwein, P.; Schramme, A.; Sinke, N.; Abdel-Bakky, M.S.; Voss, B.; Obermüller, N.; Doberstein, K.; Koziolek, M.; Fritzsche, F.; Johannsen, M.; et al. Tumoural CXCL16 expression is a novel prognostic marker of longer survival times in renal cell cancer patients. Eur. J. Cancer 2009, 45, 478–489. [Google Scholar] [CrossRef]
- Schulte, A.; Schulz, B.; Andrzejewski, M.G.; Hundhausen, C.; Mletzko, S.; Achilles, J.; Reiss, K.; Paliga, K.; Weber, C.; John, S.R.; et al. Sequential processing of the transmembrane chemokines CX3CL1 and CXCL16 by alpha- and gamma-secretases. Biochem. Biophys. Res. Commun. 2007, 358, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Gutwein, P.; Abdel-Bakky, M.S.; Schramme, A.; Doberstein, K.; Kämpfer-Kolb, N.; Amann, K.; Hauser, I.A.; Obermüller, N.; Bartel, C.; Abdel-Aziz, A.A.; et al. CXCL16 is expressed in podocytes and acts as a scavenger receptor for oxidized low-density lipoprotein. Am. J. Pathol. 2009, 174, 2061–2072. [Google Scholar] [CrossRef] [Green Version]
- Schramme, A.; Abdel-Bakky, M.S.; Kämpfer-Kolb, N.; Pfeilschifter, J.; Gutwein, P. The role of CXCL16 and its processing metalloproteinases ADAM10 and ADAM17 in the proliferation and migration of human mesangial cells. Biochem. Biophys. Res. Commun. 2008, 370, 311–316. [Google Scholar] [CrossRef]
- Tohyama, M.; Sayama, K.; Komatsuzawa, H.; Hanakawa, Y.; Shirakata, Y.; Dai, X.; Yang, L.; Tokumaru, S.; Nagai, H.; Hirakawa, S.; et al. CXCL16 is a novel mediator of the innate immunity of epidermal keratinocytes. Int. Immunol. 2007, 19, 1095–1102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tabata, S.; Kadowaki, N.; Kitawaki, T.; Shimaoka, T.; Yonehara, S.; Yoshie, O.; Uchiyama, T. Distribution and kinetics of SR-PSOX/CXCL16 and CXCR6 expression on human dendritic cell subsets and CD4+ T cells. J. Leukoc. Biol. 2005, 77, 777–786. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hofnagel, O.; Engel, T.; Severs, N.J.; Robenek, H.; Buers, I. SR-PSOX at sites predisposed to atherosclerotic lesion formation mediates monocyte-endothelial cell adhesion. Atherosclerosis 2011, 217, 371–378. [Google Scholar] [CrossRef] [PubMed]
- Hattermann, K.; Bartsch, K.; Gebhardt, H.H.; Mehdorn, H.M.; Synowitz, M.; Schmitt, A.D.; Mentlein, R.; Held-Feindt, J. “Inverse signaling” of the transmembrane chemokine CXCL16 contributes to proliferative and anti-apoptotic effects in cultured human meningioma cells. Cell Commun. Signal. 2016, 14, 26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adamski, V.; Mentlein, R.; Lucius, R.; Synowitz, M.; Held-Feindt, J.; Hattermann, K. The Chemokine Receptor CXCR6 Evokes Reverse Signaling via the Transmembrane Chemokine CXCL16. Int. J. Mol. Sci. 2017, 18, 1468. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hattermann, K.; Gebhardt, H.; Krossa, S.; Ludwig, A.; Lucius, R.; Held-Feindt, J.; Mentlein, R. Transmembrane chemokines act as receptors in a novel mechanism termed inverse signaling. Elife 2016, 5, e10820. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Ma, J.; Lin, J. Activation of the CXCL16/CXCR6 Axis by TNF-α Contributes to Ectopic Endometrial Stromal Cells Migration and Invasion. Reprod. Sci. 2019, 26, 420–427. [Google Scholar] [CrossRef]
- Koenen, A.; Babendreyer, A.; Schumacher, J.; Pasqualon, T.; Schwarz, N.; Seifert, A.; Deupi, X.; Ludwig, A.; Dreymueller, D. The DRF motif of CXCR6 as chemokine receptor adaptation to adhesion. PLoS ONE 2017, 12, e0173486. [Google Scholar] [CrossRef] [Green Version]
- Chandrasekar, B.; Bysani, S.; Mummidi, S. CXCL16 signals via Gi, phosphatidylinositol 3-kinase, Akt, I kappa B kinase, and nuclear factor-kappa B and induces cell-cell adhesion and aortic smooth muscle cell proliferation. J. Biol. Chem. 2004, 279, 3188–3196. [Google Scholar] [CrossRef] [Green Version]
- Hong, L.; Wang, S.; Li, W.; Wu, D.; Chen, W. Tumor-associated macrophages promote the metastasis of ovarian carcinoma cells by enhancing CXCL16/CXCR6 expression. Pathol. Res. Pract. 2018, 214, 1345–1351. [Google Scholar] [CrossRef] [PubMed]
- Liang, K.; Liu, Y.; Eer, D.; Liu, J.; Yang, F.; Hu, K. High CXC Chemokine Ligand 16 (CXCL16) Expression Promotes Proliferation and Metastasis of Lung Cancer via Regulating the NF-κB Pathway. Med. Sci. Monit. 2018, 24, 405–411. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.; Lu, Y.; Wang, J.; Koch, A.E.; Zhang, J.; Taichman, R.S. CXCR6 induces prostate cancer progression by the AKT/mammalian target of rapamycin signaling pathway. Cancer Res. 2008, 68, 10367–10376. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, Z.; Yu, R.; Zhu, Q.; Sun, L.; Jian, L.; Wang, X.; Zhao, J.; Li, C.; Liu, X. CXCL16/CXCR6 axis promotes bleomycin-induced fibrotic process in MRC-5 cells via the PI3K/AKT/FOXO3a pathway. Int. Immunopharmacol. 2020, 81, 106035. [Google Scholar] [CrossRef] [PubMed]
- Xiao, G.; Wang, X.; Wang, J.; Zu, L.; Cheng, G.; Hao, M.; Sun, X.; Xue, Y.; Lu, J.; Wang, J. CXCL16/CXCR6 chemokine signaling mediates breast cancer progression by pERK1/2-dependent mechanisms. Oncotarget 2015, 6, 14165–14178. [Google Scholar] [CrossRef] [Green Version]
- Gao, Q.; Zhao, Y.J.; Wang, X.Y.; Qiu, S.J.; Shi, Y.H.; Sun, J.; Yi, Y.; Shi, J.Y.; Shi, G.M.; Ding, Z.B.; et al. CXCR6 upregulation contributes to a proinflammatory tumor microenvironment that drives metastasis and poor patient outcomes in hepatocellular carcinoma. Cancer Res. 2012, 72, 3546–3556. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, J.M.; Weng, M.Z.; Song, F.B.; Chen, J.Y.; Zhang, J.Y.; Wu, J.Y.; Qin, J.; Jin, T.; Wang, X.L. Blockade of the CXCR6 signaling inhibits growth and invasion of hepatocellular carcinoma cells through inhibition of the VEGF expression. Int. J. Immunopathol. Pharmacol. 2014, 27, 553–561. [Google Scholar] [CrossRef] [PubMed]
- Wågsäter, D.; Olofsson, P.S.; Norgren, L.; Stenberg, B.; Sirsjö, A. The chemokine and scavenger receptor CXCL16/SR-PSOX is expressed in human vascular smooth muscle cells and is induced by interferon gamma. Biochem. Biophys. Res. Commun. 2004, 325, 1187–1193. [Google Scholar] [CrossRef]
- Matsumura, S.; Wang, B.; Kawashima, N.; Braunstein, S.; Badura, M.; Cameron, T.O.; Babb, J.S.; Schneider, R.J.; Formenti, S.C.; Dustin, M.L.; et al. Radiation-induced CXCL16 release by breast cancer cells attracts effector T cells. J. Immunol. 2008, 181, 3099–3107. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Z.; Yang, S.; Su, Y.; Wang, W.; Zhang, H.; Zhang, M.; Zhang, K.; Tian, Y.; Cao, Y.; Yin, L.; et al. Alteration of the inflammatory molecule network after irradiation of soft tissue. Adv. Exp. Med. Biol. 2013, 765, 335–341. [Google Scholar]
- Ye, L.Y.; Chen, W.; Bai, X.L.; Xu, X.Y.; Zhang, Q.; Xia, X.F.; Sun, X.; Li, G.G.; Hu, Q.D.; Fu, Q.H.; et al. Hypoxia-Induced Epithelial-to-Mesenchymal Transition in Hepatocellular Carcinoma Induces an Immunosuppressive Tumor Microenvironment to Promote Metastasis. Cancer Res. 2016, 76, 818–830. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, X.; Zhao, R.; Lin, S.; Bai, X.; Zhang, L.; Yuan, S.; Sun, L. CXCL16 induces angiogenesis in autocrine signaling pathway involving hypoxia-inducible factor 1α in human umbilical vein endothelial cells. Oncol. Rep. 2016, 35, 1557–1565. [Google Scholar] [CrossRef]
- Liao, F.; Alkhatib, G.; Peden, K.W.; Sharma, G.; Berger, E.A.; Farber, J.M. STRL33, A novel chemokine receptor-like protein, functions as a fusion cofactor for both macrophage-tropic and T cell line-tropic HIV-1. J. Exp. Med. 1997, 185, 2015–2023. [Google Scholar] [CrossRef]
- Sharron, M.; Pöhlmann, S.; Price, K.; Lolis, E.; Tsang, M.; Kirchhoff, F.; Doms, R.W.; Lee, B. Expression and coreceptor activity of STRL33/Bonzo on primary peripheral blood lymphocytes. Blood 2000, 96, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Unutmaz, D.; Xiang, W.; Sunshine, M.J.; Campbell, J.; Butcher, E.; Littman, D.R. The primate lentiviral receptor Bonzo/STRL33 is coordinately regulated with CCR5 and its expression pattern is conserved between human and mouse. J. Immunol. 2000, 165, 3284–3292. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, C.H.; Kunkel, E.J.; Boisvert, J.; Johnston, B.; Campbell, J.J.; Genovese, M.C.; Greenberg, H.B.; Butcher, E.C. Bonzo/CXCR6 expression defines type 1-polarized T-cell subsets with extralymphoid tissue homing potential. J. Clin. Investig. 2001, 107, 595–601. [Google Scholar] [CrossRef] [Green Version]
- Xuan, W.; Qu, Q.; Zheng, B.; Xiong, S.; Fan, G.H. The chemotaxis of M1 and M2 macrophages is regulated by different chemokines. J. Leukoc. Biol. 2015, 97, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Hattermann, K.; Ludwig, A.; Gieselmann, V.; Held-Feindt, J.; Mentlein, R. The chemokine CXCL16 induces migration and invasion of glial precursor cells via its receptor CXCR6. Mol. Cell. Neurosci. 2008, 39, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.P.; Foley, J.F.; Zhang, H.H.; Hurt, D.E.; Richards, J.L.; Smith, C.S.; Liao, F.; Farber, J.M. Selectivity in the Use of Gi/o Proteins Is Determined by the DRF Motif in CXCR6 and Is Cell-Type Specific. Mol. Pharmacol. 2015, 88, 894–910. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borst, O.; Münzer, P.; Gatidis, S.; Schmidt, E.M.; Schönberger, T.; Schmid, E.; Towhid, S.T.; Stellos, K.; Seizer, P.; May, A.E.; et al. The inflammatory chemokine CXC motif ligand 16 triggers platelet activation and adhesion via CXC motif receptor 6-dependent phosphatidylinositide 3-kinase/Akt signaling. Circ. Res. 2012, 111, 1297–1307. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Isozaki, T.; Arbab, A.S.; Haas, C.S.; Amin, M.A.; Arendt, M.D.; Koch, A.E.; Ruth, J.H. Evidence that CXCL16 is a potent mediator of angiogenesis and is involved in endothelial progenitor cell chemotaxis: Studies in mice with K/BxN serum-induced arthritis. Arthritis Rheumatol. 2013, 65, 1736–1746. [Google Scholar] [CrossRef] [Green Version]
- Galkina, E.; Harry, B.L.; Ludwig, A.; Liehn, E.A.; Sanders, J.M.; Bruce, A.; Weber, C.; Ley, K. CXCR6 promotes atherosclerosis by supporting T-cell homing, interferon-gamma production, and macrophage accumulation in the aortic wall. Circulation 2007, 116, 1801–1811. [Google Scholar] [CrossRef] [Green Version]
- Minami, M.; Kume, N.; Shimaoka, T.; Kataoka, H.; Hayashida, K.; Yonehara, S.; Kita, T. Expression of scavenger receptor for phosphatidylserine and oxidized lipoprotein (SR-PSOX) in human atheroma. Ann. N. Y. Acad. Sci. 2001, 947, 373–376. [Google Scholar] [CrossRef] [PubMed]
- Aslanian, A.M.; Charo, I.F. Targeted disruption of the scavenger receptor and chemokine CXCL16 accelerates atherosclerosis. Circulation 2006, 114, 583–590. [Google Scholar] [CrossRef] [Green Version]
- Ma, K.L.; Wu, Y.; Zhang, Y.; Wang, G.H.; Hu, Z.B.; Ruan, X.Z. Activation of the CXCL16/CXCR6 pathway promotes lipid deposition in fatty livers of apolipoprotein E knockout mice and HepG2 cells. Am. J. Transl. Res. 2018, 10, 1802–1816. [Google Scholar] [PubMed]
- Jiang, L.; Yang, M.; Li, X.; Wang, Y.; Zhou, G.; Zhao, J. CXC Motif Ligand 16 Promotes Nonalcoholic Fatty Liver Disease Progression via Hepatocyte-Stellate Cell Crosstalk. J. Clin. Endocrinol. Metab. 2018, 103, 3974–3985. [Google Scholar] [CrossRef]
- Liepelt, A.; Wehr, A.; Kohlhepp, M.; Mossanen, J.C.; Kreggenwinkel, K.; Denecke, B.; Costa, I.G.; Luedde, T.; Trautwein, C.; Tacke, F. CXCR6 protects from inflammation and fibrosis in NEMOLPC-KO mice. Biochim. Biophys. Acta Mol. Basis Dis. 2019, 1865, 391–402. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.B.; Gong, Y.P.; Cheng, J.; Chu, Y.W.; Xiong, S.D. CXCL16 participates in pathogenesis of immunological liver injury by regulating T lymphocyte infiltration in liver tissue. World J. Gastroenterol. 2005, 11, 4979–4985. [Google Scholar] [CrossRef]
- Wehr, A.; Baeck, C.; Heymann, F.; Niemietz, P.M.; Hammerich, L.; Martin, C.; Zimmermann, H.W.; Pack, O.; Gassler, N.; Hittatiya, K.; et al. Chemokine receptor CXCR6-dependent hepatic NK T Cell accumulation promotes inflammation and liver fibrosis. J. Immunol. 2013, 190, 5226–5236. [Google Scholar] [CrossRef] [Green Version]
- Wehr, A.; Baeck, C.; Ulmer, F.; Gassler, N.; Hittatiya, K.; Luedde, T.; Neumann, U.P.; Trautwein, C.; Tacke, F. Pharmacological inhibition of the chemokine CXCL16 diminishes liver macrophage infiltration and steatohepatitis in chronic hepatic injury. PLoS ONE 2014, 9, e112327. [Google Scholar] [CrossRef]
- Xu, H.; Xu, W.; Chu, Y.; Gong, Y.; Jiang, Z.; Xiong, S. Involvement of up-regulated CXC chemokine ligand 16/scavenger receptor that binds phosphatidylserine and oxidized lipoprotein in endotoxin-induced lethal liver injury via regulation of T-cell recruitment and adhesion. Infect. Immun. 2005, 73, 4007–4016. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, X.; Shimaoka, T.; Kojo, S.; Harada, M.; Watarai, H.; Wakao, H.; Ohkohchi, N.; Yonehara, S.; Taniguchi, M.; Seino, K. Cutting edge: Critical role of CXCL16/CXCR6 in NKT cell trafficking in allograft tolerance. J. Immunol. 2005, 175, 2051–2055. [Google Scholar] [CrossRef] [Green Version]
- Sato, T.; Thorlacius, H.; Johnston, B.; Staton, T.L.; Xiang, W.; Littman, D.R.; Butcher, E.C. Role for CXCR6 in recruitment of activated CD8+ lymphocytes to inflamed liver. J. Immunol. 2005, 174, 277–283. [Google Scholar] [CrossRef] [Green Version]
- Chen, G.; Lin, S.C.; Chen, J.; He, L.; Dong, F.; Xu, J.; Han, S.; Du, J.; Entman, M.L.; Wang, Y. CXCL16 recruits bone marrow-derived fibroblast precursors in renal fibrosis. J. Am. Soc. Nephrol. 2011, 22, 1876–1886. [Google Scholar] [CrossRef] [Green Version]
- Xia, Y.; Yan, J.; Jin, X.; Entman, M.L.; Wang, Y. The chemokine receptor CXCR6 contributes to recruitment of bone marrow-derived fibroblast precursors in renal fibrosis. Kidney Int. 2014, 86, 327–337. [Google Scholar] [CrossRef] [Green Version]
- Satoh-Takayama, N.; Serafini, N.; Verrier, T.; Rekiki, A.; Renauld, J.C.; Frankel, G.; Di Santo, J.P. The chemokine receptor CXCR6 controls the functional topography of interleukin-22 producing intestinal innate lymphoid cells. Immunity 2014, 41, 776–788. [Google Scholar] [CrossRef] [Green Version]
- Uza, N.; Nakase, H.; Yamamoto, S.; Yoshino, T.; Takeda, Y.; Ueno, S.; Inoue, S.; Mikami, S.; Matsuura, M.; Shimaoka, T.; et al. SR-PSOX/CXCL16 plays a critical role in the progression of colonic inflammation. Gut 2011, 60, 1494–1505. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mandai, Y.; Takahashi, D.; Hase, K.; Obata, Y.; Furusawa, Y.; Ebisawa, M.; Nakagawa, T.; Sato, T.; Katsuno, T.; Saito, Y.; et al. Distinct Roles for CXCR6+ and CXCR6− CD4+ T Cells in the Pathogenesis of Chronic Colitis. PLoS ONE 2013, 8, e65488. [Google Scholar] [CrossRef]
- Deng, H.K.; Unutmaz, D.; KewalRamani, V.N.; Littman, D.R. Expression cloning of new receptors used by simian and human immunodeficiency viruses. Nature 1997, 388, 296–300. [Google Scholar] [CrossRef]
- Zhang, Y.J.; Zhang, L.; Ketas, T.; Korber, B.T.; Moore, J.P. HIV type 1 molecular clones able to use the Bonzo/STRL-33 coreceptor for virus entry. AIDS Res. Hum. Retrovir. 2001, 17, 217–227. [Google Scholar] [CrossRef]
- Blaak, H.; Boers, P.H.; Gruters, R.A.; Schuitemaker, H.; van der Ende, M.E.; Osterhaus, A.D. CCR5, GPR15, and CXCR6 are major coreceptors of human immunodeficiency virus type 2 variants isolated from individuals with and without plasma viremia. J. Virol. 2005, 79, 1686–1700. [Google Scholar] [CrossRef] [Green Version]
- Limou, S.; Coulonges, C.; Herbeck, J.T.; van Manen, D.; An, P.; Le Clerc, S.; Delaneau, O.; Diop, G.; Taing, L.; Montes, M.; et al. Multiple-cohort genetic association study reveals CXCR6 as a new chemokine receptor involved in long-term nonprogression to AIDS. J. Infect. Dis. 2010, 202, 908–915. [Google Scholar] [CrossRef] [PubMed]
- Picton, A.C.P.; Paximadis, M.; Chaisson, R.E.; Martinson, N.A.; Tiemessen, C.T. CXCR6 gene characterization in two ethnically distinct South African populations and association with viraemic disease control in HIV-1-infected black South African individuals. Clin. Immunol. 2017, 180, 69–79. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Ambikan, A.T.; Sperk, M.; van Domselaar, R.; Nowak, P.; Noyan, K.; Russom, A.; Sönnerborg, A.; Neogi, U. Transcriptomics and Targeted Proteomics Analysis to Gain Insights Into the Immune-control Mechanisms of HIV-1 Infected Elite Controllers. EBioMedicine 2018, 27, 40–50. [Google Scholar] [CrossRef] [Green Version]
- Duggal, P.; An, P.; Beaty, T.H.; Strathdee, S.A.; Farzadegan, H.; Markham, R.B.; Johnson, L.; O’Brien, S.J.; Vlahov, D.; Winkler, C.A. Genetic influence of CXCR6 chemokine receptor alleles on PCP-mediated AIDS progression among African Americans. Genes Immun. 2003, 4, 245–250. [Google Scholar] [CrossRef] [Green Version]
- Passam, A.M.; Sourvinos, G.; Krambovitis, E.; Miyakis, S.; Stavrianeas, N.; Zagoreos, I.; Spandidos, D.A. Polymorphisms of Cx(3)CR1 and CXCR6 receptors in relation to HAART therapy of HIV type 1 patients. AIDS Res. Hum. Retrovir. 2007, 23, 1026–1032. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Landrø, L.; Damås, J.K.; Halvorsen, B.; Fevang, B.; Ueland, T.; Otterdal, K.; Heggelund, L.; Frøland, S.S.; Aukrust, P. CXCL16 in HIV infection—A link between inflammation and viral replication. Eur. J. Clin. Investig. 2009, 39, 1017–1024. [Google Scholar] [CrossRef] [PubMed]
- Hattermann, K.; Held-Feindt, J.; Ludwig, A.; Mentlein, R. The CXCL16-CXCR6 chemokine axis in glial tumors. J. Neuroimmunol. 2013, 260, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Liu, Y.; Zhou, W.; Si, L.; Ren, L. CXCL16 and CXCR6 are coexpressed in human lung cancer in vivo and mediate the invasion of lung cancer cell lines in vitro. PLoS ONE 2014, 9, e99056. [Google Scholar] [CrossRef]
- Hald, S.M.; Kiselev, Y.; Al-Saad, S.; Richardsen, E.; Johannessen, C.; Eilertsen, M.; Kilvaer, T.K.; Al-Shibli, K.; Andersen, S.; Busund, L.T.; et al. Prognostic impact of CXCL16 and CXCR6 in non-small cell lung cancer: Combined high CXCL16 expression in tumor stroma and cancer cells yields improved survival. BMC Cancer 2015, 15, 441. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mir, H.; Singh, R.; Kloecker, G.H.; Lillard, J.W., Jr.; Singh, S. CXCR6 expression in non-small cell lung carcinoma supports metastatic process via modulating metalloproteinases. Oncotarget 2015, 6, 9985–9998. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, F.; Zhang, Y.; Tang, H.; Zhou, X.; Wu, Z.; Tang, D.; Zhao, T. CXC chemokine ligand 16, inversely correlated with CD99 expression in Hodgkin Reed-Sternberg cells, is widely expressed in diverse types of lymphomas. Oncol. Rep. 2013, 30, 783–792. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Zou, C.; Zhang, Z.; Qian, C.N.; Yang, X.; Shi, J.; Xia, Y.; Zhang, J.; Lu, Y. MEK inhibitor diminishes nasopharyngeal carcinoma (NPC) cell growth and NPC-induced osteoclastogenesis via modulating CCL2 and CXCL16 expressions. Tumour Biol. 2015, 36, 8811–8818. [Google Scholar] [CrossRef]
- Guo, H.; Wang, F.; Diao, Y.; Zhang, Z.; Chen, Q.; Qian, C.N.; Keller, E.T.; Zhang, J.; Lu, Y. Knockdown of Notch1 inhibits nasopharyngeal carcinoma cell growth and metastasis via downregulation of CCL2, CXCL16, and uPA. Mol. Carcinog. 2019, 58, 1886–1896. [Google Scholar] [CrossRef] [PubMed]
- Cerami, E.; Gao, J.; Dogrusoz, U.; Gross, B.E.; Sumer, S.O.; Aksoy, B.A.; Jacobsen, A.; Byrne, C.J.; Heuer, M.L.; Larsson, E.; et al. The cBio cancer genomics portal: An open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2012, 2, 401–404. [Google Scholar] [CrossRef] [Green Version]
- Gao, J.; Aksoy, B.A.; Dogrusoz, U.; Dresdner, G.; Gross, B.; Sumer, S.O.; Sun, Y.; Jacobsen, A.; Sinha, R.; Larsson, E.; et al. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci. Signal. 2013, 6, pl1. [Google Scholar] [CrossRef] [Green Version]
- Darash-Yahana, M.; Gillespie, J.W.; Hewitt, S.M.; Chen, Y.Y.; Maeda, S.; Stein, I.; Singh, S.P.; Bedolla, R.B.; Peled, A.; Troyer, D.A.; et al. The chemokine CXCL16 and its receptor, CXCR6, as markers and promoters of inflammation-associated cancers. PLoS ONE 2009, 4, e6695. [Google Scholar] [CrossRef]
- Xiao, Q.; Zhu, X.; Yang, S.; Wang, J.; Yin, R.; Song, J.; Ma, A.; Pan, X. LPS induces CXCL16 expression in HUVECs through the miR-146a-mediated TLR4 pathway. Int. Immunopharmacol. 2019, 69, 143–149. [Google Scholar] [CrossRef]
- Ouaguia, L.; Moralès, O.; Aoudjehane, L.; Wychowski, C.; Kumar, A.; Dubuisson, J.; Calmus, Y.; Conti, F.; Delhem, N. Hepatitis C Virus Improves Human Tregs Suppressive Function and Promotes Their Recruitment to the Liver. Cells 2019, 8, 1296. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, Y.; Gao, X.M.; Yang, J.; Xu, D.; Zhang, Y.; Lu, M.; Zhang, Z.; Sheng, Y.Y.; Li, J.H.; Yu, X.X.; et al. C-C chemokine receptor type 1 mediates osteopontin-promoted metastasis in hepatocellular carcinoma. Cancer Sci. 2018, 109, 710–723. [Google Scholar] [CrossRef] [PubMed]
- Chalabi-Dchar, M.; Cassant-Sourdy, S.; Duluc, C.; Fanjul, M.; Lulka, H.; Samain, R.; Roche, C.; Breibach, F.; Delisle, M.B.; Poupot, M.; et al. Loss of Somatostatin Receptor Subtype 2 Promotes Growth of KRAS-Induced Pancreatic Tumors in Mice by Activating PI3K Signaling and Overexpression of CXCL16. Gastroenterology 2015, 148, 1452–1465. [Google Scholar] [CrossRef] [PubMed]
- Ajona, D.; Zandueta, C.; Corrales, L.; Moreno, H.; Pajares, M.J.; Ortiz-Espinosa, S.; Martínez-Terroba, E.; Perurena, N.; de Miguel, F.J.; Jantus-Lewintre, E.; et al. Blockade of the Complement C5a/C5aR1 Axis Impairs Lung Cancer Bone Metastasis by CXCL16-mediated Effects. Am. J. Respir. Crit. Care Med. 2018, 197, 1164–1176. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Huang, W.; Sheng, M.; Liu, T. MiR-451 inhibits cell growth and invasion by targeting CXCL16 and is associated with prognosis of osteosarcoma patients. Tumour Biol. 2015, 36, 2041–2048. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Liu, W.; Wang, C.; Ai, Z. miR-873-5p Inhibits Cell Migration and Invasion of Papillary Thyroid Cancer via Regulation of CXCL16. OncoTargets Ther. 2020, 13, 1037–1046. [Google Scholar] [CrossRef] [Green Version]
- Sun, J.J.; Chen, G.Y.; Xie, Z.T. MicroRNA-361-5p Inhibits Cancer Cell Growth by Targeting CXCR6 in Hepatocellular Carcinoma. Cell. Physiol. Biochem. 2016, 38, 777–785. [Google Scholar] [CrossRef] [PubMed]
- Morris, M.R.; Gentle, D.; Abdulrahman, M.; Clarke, N.; Brown, M.; Kishida, T.; Yao, M.; Teh, B.T.; Latif, F.; Maher, E.R. Functional epigenomics approach to identify methylated candidate tumour suppressor genes in renal cell carcinoma. Br. J. Cancer 2008, 98, 496–501. [Google Scholar] [CrossRef] [Green Version]
- Fang, Y.; Henderson, F.C., Jr.; Yi, Q.; Lei, Q.; Li, Y.; Chen, N. Chemokine CXCL16 expression suppresses migration and invasiveness and induces apoptosis in breast cancer cells. Mediat. Inflamm. 2014, 2014, 478641. [Google Scholar] [CrossRef]
- Takiguchi, G.; Nishita, M.; Kurita, K.; Kakeji, Y.; Minami, Y. Wnt5a-Ror2 signaling in mesenchymal stem cells promotes proliferation of gastric cancer cells by activating CXCL16-CXCR6 axis. Cancer Sci. 2016, 107, 290–297. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ikeda, T.; Nishita, M.; Hoshi, K.; Honda, T.; Kakeji, Y.; Minami, Y. Mesenchymal stem cell-derived CXCL16 promotes progression of gastric cancer cells by STAT3-mediated expression of Ror1. Cancer Sci. 2020, 111, 1254–1265. [Google Scholar] [CrossRef] [Green Version]
- Han, E.C.; Lee, J.; Ryu, S.W.; Choi, C. Tumor-conditioned Gr-1+CD11b+ myeloid cells induce angiogenesis through the synergistic action of CCL2 and CXCL16 in vitro. Biochem. Biophys. Res. Commun. 2014, 443, 1218–1225. [Google Scholar] [CrossRef]
- Chung, B.; Esmaeili, A.A.; Gopalakrishna-Pillai, S.; Murad, J.P.; Andersen, E.S.; Kumar Reddy, N.; Srinivasan, G.; Armstrong, B.; Chu, C.; Kim, Y.; et al. Human brain metastatic stroma attracts breast cancer cells via chemokines CXCL16 and CXCL12. NPJ Breast Cancer 2017, 3, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsushita, K.; Toiyama, Y.; Tanaka, K.; Saigusa, S.; Hiro, J.; Uchida, K.; Inoue, Y.; Kusunoki, M. Soluble CXCL16 in preoperative serum is a novel prognostic marker and predicts recurrence of liver metastases in colorectal cancer patients. Ann. Surg. Oncol. 2012, 19 (Suppl. S3), S518–S527. [Google Scholar] [CrossRef] [PubMed]
- Lepore, F.; D’Alessandro, G.; Antonangeli, F.; Santoro, A.; Esposito, V.; Limatola, C.; Trettel, F. CXCL16/CXCR6 Axis Drives Microglia/Macrophages Phenotype in Physiological Conditions and Plays a Crucial Role in Glioma. Front. Immunol. 2018, 9, 2750. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taghizadeh, R.; Noh, M.; Huh, Y.H.; Ciusani, E.; Sigalotti, L.; Maio, M.; Arosio, B.; Nicotra, M.R.; Natali, P.; Sherley, J.L.; et al. CXCR6, a newly defined biomarker of tissue-specific stem cell asymmetric self-renewal, identifies more aggressive human melanoma cancer stem cells. PLoS ONE 2010, 5, e15183. [Google Scholar] [CrossRef]
- Held-Feindt, J.; Rehmke, B.; Mentlein, R.; Hattermann, K.; Knerlich, F.; Hugo, H.H.; Ludwig, A.; Mehdorn, H.M. Overexpression of CXCL16 and its receptor CXCR6/Bonzo promotes growth of human schwannomas. Glia 2008, 56, 764–774. [Google Scholar] [CrossRef]
- Jin, J.J.; Dai, F.X.; Long, Z.W.; Cai, H.; Liu, X.W.; Zhou, Y.; Hong, Q.; Dong, Q.Z.; Wang, Y.N.; Huang, H. CXCR6 predicts poor prognosis in gastric cancer and promotes tumor metastasis through epithelial-mesenchymal transition. Oncol. Rep. 2017, 37, 3279–3286. [Google Scholar] [CrossRef] [Green Version]
- Ma, Y.; Xu, X.; Luo, M. CXCR6 promotes tumor cell proliferation and metastasis in osteosarcoma through the Akt pathway. Cell. Immunol. 2017, 311, 80–85. [Google Scholar] [CrossRef]
- Sellerio, A.L.; Ciusani, E.; Ben-Moshe, N.B.; Coco, S.; Piccinini, A.; Myers, C.R.; Sethna, J.P.; Giampietro, C.; Zapperi, S.; La Porta, C.A.M. Overshoot during phenotypic switching of cancer cell populations. Sci. Rep. 2015, 5, 15464. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, H.; Qiu, B.; Chen, S.; Xun, Y.; Pan, Y.; Chen, M.; Li, W.X.; Liao, W.; El-Ashram, S.; Yang, A.; et al. Soluble CXCL16 promotes TNF-α-induced apoptosis in DLBCL via the AMAD10-NF-κB regulatory feedback loop. Cell Biol. Int. 2019, 43, 863–874. [Google Scholar] [CrossRef] [PubMed]
- Wente, M.N.; Gaida, M.M.; Mayer, C.; Michalski, C.W.; Haag, N.; Giese, T.; Felix, K.; Bergmann, F.; Giese, N.A.; Friess, H. Expression and potential function of the CXC chemokine CXCL16 in pancreatic ductal adenocarcinoma. Int. J. Oncol. 2008, 33, 297–308. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xing, Y.N.; Zhang, J.Y.; Xu, H.M. The roles of serum CXCL16 in circulating Tregs and gastrointestinal stromal tumor cells. OncoTargets Ther. 2016, 9, 3939–3949. [Google Scholar]
- Richardsen, E.; Ness, N.; Melbø-Jørgensen, C.; Johannesen, C.; Grindstad, T.; Nordbakken, C.; Al-Saad, S.; Andersen, S.; Dønnem, T.; Nordby, Y.; et al. The prognostic significance of CXCL16 and its receptor C-X-C chemokine receptor 6 in prostate cancer. Am. J. Pathol. 2015, 185, 2722–2730. [Google Scholar] [CrossRef]
- Kee, J.Y.; Ito, A.; Hojo, S.; Hashimoto, I.; Igarashi, Y.; Tsuneyama, K.; Tsukada, K.; Irimura, T.; Shibahara, N.; Takasaki, I.; et al. CXCL16 suppresses liver metastasis of colorectal cancer by promoting TNF-α-induced apoptosis by tumor-associated macrophages. BMC Cancer 2014, 14, 949. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, S.; Sun, L.; Hu, J.; Wan, S.; Zhao, R.; Yuan, S.; Zhang, L. Chemokine C-X-C motif receptor 6 contributes to cell migration during hypoxia. Cancer Lett. 2009, 279, 108–117. [Google Scholar] [CrossRef]
- Lee, H.S.; Hong, J.E.; Kim, E.J.; Kim, S.H. Escin suppresses migration and invasion involving the alteration of CXCL16/CXCR6 axis in human gastric adenocarcinoma AGS cells. Nutr. Cancer 2014, 66, 938–945. [Google Scholar] [CrossRef]
- Ou, D.L.; Chen, C.L.; Lin, S.B.; Hsu, C.H.; Lin, L.I. Chemokine receptor expression profiles in nasopharyngeal carcinoma and their association with metastasis and radiotherapy. J. Pathol. 2006, 210, 363–373. [Google Scholar] [CrossRef] [PubMed]
- Mir, H.; Kaur, G.; Kapur, N.; Bae, S.; Lillard, J.W., Jr.; Singh, S. Higher CXCL16 exodomain is associated with aggressive ovarian cancer and promotes the disease by CXCR6 activation and MMP modulation. Sci. Rep. 2019, 9, 2527. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.W.; Kim, Y.A.; Sun, H.J.; Kim, Y.A.; Oh, B.C.; Yi, K.H.; Park, D.J.; Park, Y.J. CXCL16 signaling mediated macrophage effects on tumor invasion of papillary thyroid carcinoma. Endocr. Relat. Cancer 2016, 23, 113–124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, W.; Zhen, X.; Xiong, B.; Wang, B.; Zhang, W.; Zhou, W. CXCR6 is expressed in human prostate cancer in vivo and is involved in the in vitro invasion of PC3 and LNCap cells. Cancer Sci. 2008, 99, 1362–1369. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Wang, J.; Xu, Y.; Koch, A.E.; Cai, Z.; Chen, X.; Galson, D.L.; Taichman, R.S.; Zhang, J. CXCL16 functions as a novel chemotactic factor for prostate cancer cells in vitro. Mol. Cancer Res. 2008, 6, 546–554. [Google Scholar] [CrossRef] [Green Version]
- Singh, R.; Kapur, N.; Mir, H.; Singh, N.; Lillard, J.W., Jr.; Singh, S. CXCR6-CXCL16 axis promotes prostate cancer by mediating cytoskeleton rearrangement via Ezrin activation and αvβ3 integrin clustering. Oncotarget 2016, 7, 7343–7353. [Google Scholar] [CrossRef]
- Li, Y.; Fu, L.X.; Zhu, W.L.; Shi, H.; Chen, L.J.; Ye, B. Blockade of CXCR6 reduces invasive potential of gastric cancer cells through inhibition of AKT signaling. Int. J. Immunopathol. Pharmacol. 2015, 28, 194–200. [Google Scholar] [CrossRef] [PubMed]
- Airoldi, I.; Cocco, C.; Morandi, F.; Prigione, I.; Pistoia, V. CXCR5 may be involved in the attraction of human metastatic neuroblastoma cells to the bone marrow. Cancer Immunol. Immunother. 2008, 57, 541–548. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.H.; Dong, Y.Y.; Wang, W.M.; Xie, X.Y.; Wang, Z.M.; Chen, R.X.; Chen, J.; Gao, D.M.; Cui, J.F.; Ren, Z.G. Vascular endothelial cells facilitated HCC invasion and metastasis through the Akt and NF-κB pathways induced by paracrine cytokines. J. Exp. Clin. Cancer Res. 2013, 32, 51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hua, H.; Li, M.; Luo, T.; Yin, Y.; Jiang, Y. Matrix metalloproteinases in tumorigenesis: An evolving paradigm. Cell. Mol. Life Sci. 2011, 68, 3853–3868. [Google Scholar] [CrossRef]
- Cui, N.; Hu, M.; Khalil, R.A. Biochemical and Biological Attributes of Matrix Metalloproteinases. Prog. Mol. Biol. Transl. Sci. 2017, 147, 1–73. [Google Scholar] [PubMed] [Green Version]
- Belotti, D.; Paganoni, P.; Manenti, L.; Garofalo, A.; Marchini, S.; Taraboletti, G.; Giavazzi, R. Matrix metalloproteinases (MMP9 and MMP2) induce the release of vascular endothelial growth factor (VEGF) by ovarian carcinoma cells: Implications for ascites formation. Cancer Res. 2003, 63, 5224–5229. [Google Scholar] [PubMed]
- Hawinkels, L.J.; Zuidwijk, K.; Verspaget, H.W.; de Jonge-Muller, E.S.; van Duijn, W.; Ferreira, V.; Fontijn, R.D.; David, G.; Hommes, D.W.; Lamers, C.B.; et al. VEGF release by MMP-9 mediated heparan sulphate cleavage induces colorectal cancer angiogenesis. Eur. J. Cancer 2008, 44, 1904–1913. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Zhang, J.; Cui, Z.M.; Zhao, J.; Zheng, Y. Expression of the CXCL12/CXCR4 and CXCL16/CXCR6 axes in cervical intraepithelial neoplasia and cervical cancer. Chin. J. Cancer 2013, 32, 289–296. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Na, K.Y.; Kim, H.S.; Jung, W.W.; Sung, J.Y.; Kalil, R.K.; Kim, Y.W.; Park, Y.K. CXCL16 and CXCR6 in Ewing sarcoma family tumor. Hum. Pathol. 2014, 45, 753–760. [Google Scholar] [CrossRef] [PubMed]
- Seidl, H.; Richtig, E.; Tilz, H.; Stefan, M.; Schmidbauer, U.; Asslaber, M.; Zatloukal, K.; Herlyn, M.; Schaider, H. Profiles of chemokine receptors in melanocytic lesions: De novo expression of CXCR6 in melanoma. Hum. Pathol. 2007, 38, 768–780. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Cui, Z.M.; Zhang, J.; Huang, Y. Chemokine axes CXCL12/CXCR4 and CXCL16/CXCR6 correlate with lymph node metastasis in epithelial ovarian carcinoma. Chin. J. Cancer 2011, 30, 336–343. [Google Scholar] [CrossRef]
- Nakayama, T.; Hieshima, K.; Izawa, D.; Tatsumi, Y.; Kanamaru, A.; Yoshie, O. Cutting edge: Profile of chemokine receptor expression on human plasma cells accounts for their efficient recruitment to target tissues. J. Immunol. 2003, 170, 1136–1140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.; Liu, C.; Chang, X.; Qi, Y.; Zhu, Z.; Yang, X. Fibrosis of mesothelial cell-induced peritoneal implantation of ovarian cancer cells. Cancer Manag. Res. 2018, 10, 6641–6647. [Google Scholar] [CrossRef] [Green Version]
- Zhuge, X.; Murayama, T.; Arai, H.; Yamauchi, R.; Tanaka, M.; Shimaoka, T.; Yonehara, S.; Kume, N.; Yokode, M.; Kita, T. CXCL16 is a novel angiogenic factor for human umbilical vein endothelial cells. Biochem. Biophys. Res. Commun. 2005, 331, 1295–1300. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heidemann, J.; Ogawa, H.; Dwinell, M.B.; Rafiee, P.; Maaser, C.; Gockel, H.R.; Otterson, M.F.; Ota, D.M.; Lugering, N.; Domschke, W.; et al. Angiogenic effects of interleukin 8 (CXCL8) in human intestinal microvascular endothelial cells are mediated by CXCR2. J. Biol. Chem. 2003, 278, 8508–8515. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, G.; Tao, L.; Shen, S.; Chen, L. Hypoxia induced CCL28 promotes angiogenesis in lung adenocarcinoma by targeting CCR3 on endothelial cells. Sci. Rep. 2016, 6, 27152. [Google Scholar] [CrossRef] [Green Version]
- Ludwig, A.; Schulte, A.; Schnack, C.; Hundhausen, C.; Reiss, K.; Brodway, N.; Held-Feindt, J.; Mentlein, R. Enhanced expression and shedding of the transmembrane chemokine CXCL16 by reactive astrocytes and glioma cells. J. Neurochem. 2005, 93, 1293–1303. [Google Scholar] [CrossRef] [PubMed]
- Allaoui, R.; Bergenfelz, C.; Mohlin, S.; Hagerling, C.; Salari, K.; Werb, Z.; Anderson, R.L.; Ethier, S.P.; Jirström, K.; Påhlman, S.; et al. Cancer-associated fibroblast-secreted CXCL16 attracts monocytes to promote stroma activation in triple-negative breast cancers. Nat. Commun. 2016, 7, 13050. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Hattermann, K.; Mentlein, R.; Mehdorn, H.M.; Held-Feindt, J. The transmembrane chemokines CXCL16 and CX3CL1 and their receptors are expressed in human meningiomas. Oncol. Rep. 2013, 29, 563–570. [Google Scholar] [CrossRef] [PubMed]
- Jung, Y.; Kim, J.K.; Shiozawa, Y.; Wang, J.; Mishra, A.; Joseph, J.; Berry, J.E.; McGee, S.; Lee, E.; Sun, H.; et al. Recruitment of mesenchymal stem cells into prostate tumours promotes metastasis. Nat. Commun. 2013, 4, 1795. [Google Scholar] [CrossRef] [Green Version]
- Behnan, J.; Isakson, P.; Joel, M.; Cilio, C.; Langmoen, I.A.; Vik-Mo, E.O.; Badn, W. Recruited brain tumor-derived mesenchymal stem cells contribute to brain tumor progression. Stem Cells 2014, 32, 1110–1123. [Google Scholar] [CrossRef]
- Chaturvedi, P.; Gilkes, D.M.; Takano, N.; Semenza, G.L. Hypoxia-inducible factor-dependent signaling between triple-negative breast cancer cells and mesenchymal stem cells promotes macrophage recruitment. Proc. Natl. Acad. Sci. USA 2014, 111, E2120–E2129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hattermann, K.; Sebens, S.; Helm, O.; Schmitt, A.D.; Mentlein, R.; Mehdorn, H.M.; Held-Feindt, J. Chemokine expression profile of freshly isolated human glioblastoma-associated macrophages/microglia. Oncol. Rep. 2014, 32, 270–276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karakasheva, T.A.; Waldron, T.J.; Eruslanov, E.; Kim, S.B.; Lee, J.S.; O’Brien, S.; Hicks, P.D.; Basu, D.; Singhal, S.; Malavasi, F.; et al. CD38-Expressing Myeloid-Derived Suppressor Cells Promote Tumor Growth in a Murine Model of Esophageal Cancer. Cancer Res. 2015, 75, 4074–4085. [Google Scholar] [CrossRef] [Green Version]
- Wang, X. Subsets of myeloid-derived suppressor cells in hepatocellular carcinoma express chemokines and chemokine receptors differentially. Int. Immunopharmacol. 2015, 26, 314–321. [Google Scholar]
- Krawczyk, K.M.; Nilsson, H.; Allaoui, R.; Lindgren, D.; Arvidsson, M.; Leandersson, K.; Johansson, M.E. Papillary renal cell carcinoma-derived chemerin, IL-8, and CXCL16 promote monocyte recruitment and differentiation into foam-cell macrophages. Lab. Investig. 2017, 97, 1296–1305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.Q.; Zhou, W.J.; Hou, X.X.; Fu, Q.; Li, D.J. Trophoblast-derived CXCL16 induces M2 macrophage polarization that in turn inactivates NK cells at the maternal-fetal interface. Cell. Mol. Immunol. 2018, 15, 1038–1046. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.J.; Sun, H.J.; Song, Y.S.; Yoo, S.K.; Kim, Y.A.; Seo, J.S.; Park, Y.J.; Cho, S.W. CXCL16 positively correlated with M2-macrophage infiltration, enhanced angiogenesis, and poor prognosis in thyroid cancer. Sci. Rep. 2019, 9, 13288. [Google Scholar] [CrossRef] [Green Version]
- Cai, H.; Zhu, X.D.; Ao, J.Y.; Ye, B.G.; Zhang, Y.Y.; Chai, Z.T.; Wang, C.H.; Shi, W.K.; Cao, M.Q.; Li, X.L.; et al. Colony-stimulating factor-1-induced AIF1 expression in tumor-associated macrophages enhances the progression of hepatocellular carcinoma. Oncoimmunology 2017, 6, e1333213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gaida, M.M.; Günther, F.; Wagner, C.; Friess, H.; Giese, N.A.; Schmidt, J.; Hänsch, G.M.; Wente, M.N. Expression of the CXCR6 on polymorphonuclear neutrophils in pancreatic carcinoma and in acute, localized bacterial infections. Clin. Exp. Immunol. 2008, 154, 216–223. [Google Scholar] [CrossRef] [PubMed]
- Parsonage, G.; Machado, L.R.; Hui, J.W.; McLarnon, A.; Schmaler, T.; Balasothy, M.; To, K.F.; Vlantis, A.C.; van Hasselt, C.A.; Lo, K.W.; et al. CXCR6 and CCR5 localize T lymphocyte subsets in nasopharyngeal carcinoma. Am. J. Pathol. 2012, 180, 1215–1222. [Google Scholar] [CrossRef]
- Oldham, K.A.; Parsonage, G.; Bhatt, R.I.; Wallace, D.M.; Deshmukh, N.; Chaudhri, S.; Adams, D.H.; Lee, S.P. T lymphocyte recruitment into renal cell carcinoma tissue: A role for chemokine receptors CXCR3, CXCR6, CCR5, and CCR6. Eur. Urol. 2012, 61, 385–394. [Google Scholar] [CrossRef] [PubMed]
- Gieniec, K.A.; Butler, L.M.; Worthley, D.L.; Woods, S.L. Cancer-associated fibroblasts-heroes or villains? Br. J. Cancer 2019, 121, 293–302. [Google Scholar] [CrossRef]
- Chanmee, T.; Ontong, P.; Konno, K.; Itano, N. Tumor-associated macrophages as major players in the tumor microenvironment. Cancers 2014, 6, 1670–1690. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hambardzumyan, D.; Gutmann, D.H.; Kettenmann, H. The role of microglia and macrophages in glioma maintenance and progression. Nat. Neurosci. 2016, 19, 20–27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hojo, S.; Koizumi, K.; Tsuneyama, K.; Arita, Y.; Cui, Z.; Shinohara, K.; Minami, T.; Hashimoto, I.; Nakayama, T.; Sakurai, H.; et al. High-level expression of chemokine CXCL16 by tumor cells correlates with a good prognosis and increased tumor-infiltrating lymphocytes in colorectal cancer. Cancer Res. 2007, 67, 4725–4731. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wågsäter, D.; Hugander, A.; Dimberg, J. Expression of CXCL16 in human rectal cancer. Int. J. Mol. Med. 2004, 14, 65–69. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Patel, S.; Tcyganov, E.; Gabrilovich, D.I. The Nature of Myeloid-Derived Suppressor Cells in the Tumor Microenvironment. Trends Immunol. 2016, 37, 208–220. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shaul, M.E.; Fridlender, Z.G. Tumour-associated neutrophils in patients with cancer. Nat. Rev. Clin. Oncol. 2019, 16, 601–620. [Google Scholar] [CrossRef] [PubMed]
- Ohue, Y.; Nishikawa, H. Regulatory T (Treg) cells in cancer: Can Treg cells be a new therapeutic target? Cancer Sci. 2019, 110, 2080–2089. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Kang, T.H.; Yoo, W.; Choi, H.; Jo, S.; Kong, K.; Lee, S.R.; Kim, S.U.; Kim, J.S.; Cho, D.; et al. An Antibody Designed to Improve Adoptive NK-Cell Therapy Inhibits Pancreatic Cancer Progression in a Murine Model. Cancer Immunol. Res. 2019, 7, 219–229. [Google Scholar] [CrossRef] [PubMed]
- Gillard-Bocquet, M.; Caer, C.; Cagnard, N.; Crozet, L.; Perez, M.; Fridman, W.H.; Sautès-Fridman, C.; Cremer, I. Lung tumor microenvironment induces specific gene expression signature in intratumoral NK cells. Front. Immunol. 2013, 4, 19. [Google Scholar] [CrossRef] [Green Version]
- Yoon, M.S.; Pham, C.T.; Phan, M.T.; Shin, D.J.; Jang, Y.Y.; Park, M.H.; Kim, S.K.; Kim, S.; Cho, D. Irradiation of breast cancer cells enhances CXCL16 ligand expression and induces the migration of natural killer cells expressing the CXCR6 receptor. Cytotherapy 2016, 18, 1532–1542. [Google Scholar] [CrossRef]
- Elemam, N.M.; Al-Jaderi, Z.; Hachim, M.Y.; Maghazachi, A.A. HCT-116 colorectal cancer cells secrete chemokines which induce chemoattraction and intracellular calcium mobilization in NK92 cells. Cancer Immunol. Immunother. 2019, 68, 883–895. [Google Scholar] [CrossRef]
- Terabe, M.; Berzofsky, J.A. Tissue-Specific Roles of NKT Cells in Tumor Immunity. Front. Immunol. 2018, 9, 1838. [Google Scholar] [CrossRef]
- Grujic, M.; Paivandy, A.; Gustafson, A.M.; Thomsen, A.R.; Öhrvik, H.; Pejler, G. The combined action of mast cell chymase, tryptase and carboxypeptidase A3 protects against melanoma colonization of the lung. Oncotarget 2017, 8, 25066–25079. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shimaoka, T.; Seino, K.; Kume, N.; Minami, M.; Nishime, C.; Suematsu, M.; Kita, T.; Taniguchi, M.; Matsushima, K.; Yonehara, S. Critical role for CXC chemokine ligand 16 (SR-PSOX) in Th1 response mediated by NKT cells. J. Immunol. 2007, 179, 8172–8179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mossanen, J.C.; Kohlhepp, M.; Wehr, A.; Krenkel, O.; Liepelt, A.; Roeth, A.A.; Möckel, D.; Heymann, F.; Lammers, T.; Gassler, N.; et al. CXCR6 Inhibits Hepatocarcinogenesis by Promoting Natural Killer T− and CD4+ T-Cell-Dependent Control of Senescence. Gastroenterology 2019, 156, 1877–1889.e4. [Google Scholar] [CrossRef] [PubMed]
- Cullen, R.; Germanov, E.; Shimaoka, T.; Johnston, B. Enhanced tumor metastasis in response to blockade of the chemokine receptor CXCR6 is overcome by NKT cell activation. J. Immunol. 2009, 183, 5807–5815. [Google Scholar] [CrossRef] [Green Version]
- Ma, C.; Han, M.; Heinrich, B.; Fu, Q.; Zhang, Q.; Sandhu, M.; Agdashian, D.; Terabe, M.; Berzofsky, J.A.; Fako, V.; et al. Gut microbiome-mediated bile acid metabolism regulates liver cancer via NKT cells. Science 2018, 360, eaan5931. [Google Scholar] [CrossRef] [Green Version]
- Kee, J.Y.; Ito, A.; Hojo, S.; Hashimoto, I.; Igarashi, Y.; Tsukada, K.; Irimura, T.; Shibahara, N.; Nakayama, T.; Yoshie, O.; et al. Chemokine CXCL16 suppresses liver metastasis of colorectal cancer via augmentation of tumor-infiltrating natural killer T cells in a murine model. Oncol. Rep. 2013, 29, 975–982. [Google Scholar] [CrossRef] [Green Version]
- Xiao, Y.S.; Gao, Q.; Xu, X.N.; Li, Y.W.; Ju, M.J.; Cai, M.Y.; Dai, C.X.; Hu, J.; Qiu, S.J.; Zhou, J.; et al. Combination of intratumoral invariant natural killer T cells and interferon-gamma is associated with prognosis of hepatocellular carcinoma after curative resection. PLoS ONE 2013, 8, e70345. [Google Scholar] [CrossRef]
- Veinotte, L.; Gebremeskel, S.; Johnston, B. CXCL16-positive dendritic cells enhance invariant natural killer T cell-dependent IFNγ production and tumor control. Oncoimmunology 2016, 5, e1160979. [Google Scholar] [CrossRef] [Green Version]
- AbdelMageed, M.; Ali, H.; Olsson, L.; Lindmark, G.; Hammarström, M.L.; Hammarström, S.; Sitohy, B. The Chemokine CXCL16 Is a New Biomarker for Lymph Node Analysis of Colon Cancer Outcome. Int. J. Mol. Sci. 2019, 20, 5793. [Google Scholar] [CrossRef] [Green Version]
- Chen, Z.; Dai, W.; Yang, L.; Yang, H.; Ding, L.; He, Y.; Song, X.; Cui, J. Elevated expression of CXCL16 correlates with poor prognosis in patients with colorectal cancer. Cancer Manag. Res. 2019, 11, 4691–4697. [Google Scholar] [CrossRef] [Green Version]
- Xing, Y.N.; Xu, X.Y.; Nie, X.C.; Yang, X.; Yu, M.; Xu, H.M.; Liu, Y.P.; Takano, Y.; Zheng, H.C. Role and clinicopathologic significance of CXC chemokine ligand 16 and chemokine (C-X-C motif) receptor 6 expression in gastric carcinomas. Hum. Pathol. 2012, 43, 2299–2307. [Google Scholar] [CrossRef]
- Sharma, I.; Singh, A.; Sharma, K.C.; Saxena, S. Gene Expression Profiling of Chemokines and Their Receptors in Low and High Grade Astrocytoma. Asian Pac. J. Cancer Prev. 2017, 18, 1307–1313. [Google Scholar]
- Ha, H.K.; Lee, W.; Park, H.J.; Lee, S.D.; Lee, J.Z.; Chung, M.K. Clinical significance of CXCL16/CXCR6 expression in patients with prostate cancer. Mol. Med. Rep. 2011, 4, 419–424. [Google Scholar]
- Del Prete, A.; Allavena, P.; Santoro, G.; Fumarulo, R.; Corsi, M.M.; Mantovani, A. Molecular pathways in cancer-related inflammation. Biochem. Med. 2011, 21, 264–275. [Google Scholar] [CrossRef]
- Lee, J.T.; Lee, S.D.; Lee, J.Z.; Chung, M.K.; Ha, H.K. Expression analysis and clinical significance of CXCL16/CXCR6 in patients with bladder cancer. Oncol. Lett. 2013, 5, 229–235. [Google Scholar] [CrossRef] [PubMed]
- Lang, K.; Bonberg, N.; Robens, S.; Behrens, T.; Hovanec, J.; Deix, T.; Braun, K.; Roghmann, F.; Noldus, J.; Harth, V.; et al. Soluble chemokine (C-X-C motif) ligand 16 (CXCL16) in urine as a novel biomarker candidate to identify high grade and muscle invasive urothelial carcinomas. Oncotarget 2017, 8, 104946–104959. [Google Scholar] [CrossRef]
- Ke, C.; Ren, Y.; Lv, L.; Hu, W.; Zhou, W. Association between CXCL16/CXCR6 expression and the clinicopathological features of patients with non-small cell lung cancer. Oncol. Lett. 2017, 13, 4661–4668. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gooden, M.J.; Wiersma, V.R.; Boerma, A.; Leffers, N.; Boezen, H.M.; ten Hoor, K.A.; Hollema, H.; Walenkamp, A.M.; Daemen, T.; Nijman, H.W.; et al. Elevated serum CXCL16 is an independent predictor of poor survival in ovarian cancer and may reflect pro-metastatic ADAM protease activity. Br. J. Cancer 2014, 110, 1535–1544. [Google Scholar] [CrossRef] [PubMed]
- Shibata, Y.; Kobayashi, N.; Sato, T.; Nakashima, K.; Kaneko, T. The clinical significance of CXCL16 in the treatment of advanced non-small cell lung cancer. Thorac. Cancer 2020, 11, 1258–1264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uhlén, M.; Fagerberg, L.; Hallström, B.M.; Lindskog, C.; Oksvold, P.; Mardinoglu, A.; Sivertsson, Å.; Kampf, C.; Sjöstedt, E.; Asplund, A.; et al. Proteomics. Tissue-based map of the human proteome. Science 2015, 347, 1260419. [Google Scholar] [CrossRef]
- Uhlen, M.; Zhang, C.; Lee, S.; Sjöstedt, E.; Fagerberg, L.; Bidkhori, G.; Benfeitas, R.; Arif, M.; Liu, Z.; Edfors, F.; et al. A pathology atlas of the human cancer transcriptome. Science 2017, 357, eaan2507. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, Q.Y.; Yang, C.K.; Rong, L.J.; Li, J.C.; Lei, L.M. Investigation of the association between C-X-C motif chemokine receptor subunits and tumor infiltration levels and prognosis in patients with early-stage pancreatic ductal adenocarcinoma. Oncol. Lett. 2020, 20, 16. [Google Scholar] [CrossRef]
- Wågsäter, D.; Dimberg, J. Expression of chemokine receptor CXCR6 in human colorectal adenocarcinomas. Anticancer Res. 2004, 24, 3711–3714. [Google Scholar]
- Chang, Y.; Zhou, L.; Xu, L.; Fu, Q.; Yang, Y.; Lin, Z.; Xu, J. High expression of CXC chemokine receptor 6 associates with poor prognosis in patients with clear cell renal cell carcinoma. Urol. Oncol. 2017, 35, e17–e675. [Google Scholar] [CrossRef]
- Matsumura, S.; Demaria, S. Up-regulation of the pro-inflammatory chemokine CXCL16 is a common response of tumor cells to ionizing radiation. Radiat. Res. 2010, 173, 418–425. [Google Scholar] [CrossRef] [Green Version]
- Peddibhotla, S.; Hershberger, P.M.; Jason Kirby, R.; Sugarman, E.; Maloney, P.R.; Hampton Sessions, E.; Divlianska, D.; Morfa, C.J.; Terry, D.; Pinkerton, A.B.; et al. Discovery of small molecule antagonists of chemokine receptor CXCR6 that arrest tumor growth in SK-HEP-1 mouse xenografts as a model of hepatocellular carcinoma. Bioorg. Med. Chem. Lett. 2020, 30, 126899. [Google Scholar] [CrossRef]
- Kapur, N.; Mir, H.; Sonpavde, G.P.; Jain, S.; Bae, S.; Lillard, J.W., Jr.; Singh, S. Prostate cancer cells hyper-activate CXCR6 signaling by cleaving CXCL16 to overcome effect of docetaxel. Cancer Lett. 2019, 454, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.; Zhang, Z.; He, L.; Wang, Y. CXCL16 regulates cisplatin-induced acute kidney injury. Oncotarget 2016, 7, 31652–31662. [Google Scholar] [CrossRef] [Green Version]
- Adamski, V.; Hattermann, K.; Kubelt, C.; Cohrs, G.; Lucius, R.; Synowitz, M.; Sebens, S.; Held-Feindt, J. Entry and exit of chemotherapeutically-promoted cellular dormancy in glioblastoma cells is differentially affected by the chemokines CXCL12, CXCL16, and CX3CL1. Oncogene 2020, 39, 4421–4435. [Google Scholar] [CrossRef] [PubMed]
- Arora, A.; Somasundaram, K. Glioblastoma vs. temozolomide: Can the red queen race be won? Cancer Biol. Ther. 2019, 20, 1083–1090. [Google Scholar] [CrossRef] [PubMed]
- Roviello, G.; Bachelot, T.; Hudis, C.A.; Curigliano, G.; Reynolds, A.R.; Petrioli, R.; Generali, D. The role of bevacizumab in solid tumours: A literature based meta-analysis of randomised trials. Eur. J. Cancer 2017, 75, 245–258. [Google Scholar] [CrossRef] [PubMed]




| Ligand | Receptor | Activated Signaling Pathways | Physiological Significance | References |
|---|---|---|---|---|
| sCXCL16 | CXCR6 | G protein-coupled receptor, PKB, ERK MAPK, calcium mobilization | Proliferation, migration, fibrosis, VEGF and CXCL8/IL-8 expression | [7,12,24,25,26,27,28,29,30,31,32,33] |
| sCXCL16 | mCXCL16 | insensitive to pertussis toxin, ERK MAPK, PKB | Proliferation, apoptosis resistance | [21,23] |
| CXCR6 | mCXCL16 | insensitive to pertussis toxin, ERK MAPK | Migration but not proliferation | [22,23] |
| mCXCL16 | CXCR6 | Cell adhesion | [12,20] |
| Cell Type | Impact on Recruitment to the Cancer Niche | CXCL16 Expression | Cellular Effect | References |
|---|---|---|---|---|
| Astrocytes | X | [134] | ||
| Cancer-associated fibroblasts (CAF) | X | [76,135] | ||
| Endothelial cells (EC) | X | [74,76,120,134,136] | ||
| Mesenchymal stem cells (MSC) | X | X | Conversion into CAF | [94,95,137,138,139] |
| Microglia | X | Cause anti-inflammatory phenotype | [74,99,136,140] | |
| Myeloid-derived suppressor cells (MDSC) | X | Survival of MDSC | [96,141,142] | |
| Tumor-associated macrophages (TAM) | X | X | Polarization into M2 macrophage subset | [74,76,114,135,136,143,144,145,146] |
| Tumor-associated neutrophils (TAN) | X | [32,147] | ||
| Regulatory T cells (Treg) | X | Increase Treg growth at <0.3 ng/mL | [85,107,148,149] |
| Type of Cancer | Number of Patients | Overall Survival with Elevated Amounts of CXCL16 | Location | References |
|---|---|---|---|---|
| Bladder cancer | 155 | -- | tumor | [177] |
| Cervical cancer | 60 | ↓ | tumor, p = 0.089 | [125] |
| Colon cancer | 121 | ↓ | regional lymph nodes | [171] |
| Colorectal cancer | 58 | ↑ | tumor | [153] |
| Colorectal cancer | 314 | ↓ | serum | [98] |
| Colorectal cancer | 142 | ↓ | tumor | [172] |
| Ewing sarcoma family tumor | 61 | ↓ | tumor | [126] |
| Gastric carcinoma | 359 | ↑ | tumor | [173] |
| Gastrointestinal stromal tumor | 43 | ↓ | tumor | [107] |
| Gastrointestinal stromal tumor | 43 | ↓ | serum | [107] |
| Lung cancer (non-small cell lung cancer) | 58 | -- | tumor | [179] |
| Lung cancer (non-small cell lung cancer) | 58 | -- | serum | [179] |
| Lung cancer (non-small cell lung cancer) | 301 | ↑ | tumor | [78] |
| Lung cancer (non-small cell lung cancer) | 40 | -- | serum | [181] |
| Lung cancer | 56 | ↓ | tumor | [28] |
| Ovarian carcinoma | 56 | ↓ | tumor | [128] |
| Ovarian cancer | 273 | -- | tumor | [180] |
| Ovarian cancer | 118 | ↓ | serum | [180] |
| Prostate cancer | 470 | ↓ | tumor | [108] |
| Renal cell carcinoma | 104 | ↑ | tumor | [14] |
| Thyroid cancer (papillary thyroid cancer) | 492 | -- | tumor, from TCGA dataset | [145] |
| Type of Cancer | Overall Survival for Increased CXCL16 Expression in the Tumor | Overall Survival for Increased Expression of CXCR6 in the Tumor |
|---|---|---|
| Glioma | ↓p = 0.094 | ↓p = 0.078 |
| Thyroid cancer | ↑ | ↑ |
| Lung cancer | -- | ↑ |
| Colorectal cancer | -- | ↑ |
| Head and neck cancer | -- | ↑ |
| Stomach cancer | ↓ | ↑ |
| Liver cancer | ↓ | ↑ |
| Pancreatic cancer | ↑ | -- |
| Renal cancer | ↑ | ↓ |
| Urothelial cancer | -- | ↑ |
| Prostate cancer | -- | -- |
| Testis cancer | ↓ | ↓ |
| Breast cancer | ↑ | ↑ |
| Cervical cancer | ↑p = 0.052 | ↑ |
| Endometrial cancer | ↓ | ↑ |
| Ovarian cancer | -- | ↑ |
| Melanoma | -- | ↑ |
| Type of Cancer | Number of Patients | Overall Survival for An Increased Amount of CXCR6 | Comments | References |
|---|---|---|---|---|
| Bladder cancer | 155 | -- | [177] | |
| Cervical cancer | 60 | ↓ | [125] | |
| Ewing sarcoma family tumor | 61 | ↓ | [126] | |
| Gastric cancer | 352 | ↓ | [102] | |
| Gastrointestinal stromal tumor | 43 | ↓ | [107] | |
| Hepatocellular carcinoma | 240 | ↓ | p = 0.064 | [32] |
| Lung cancer (non-small cell lung cancer) | 58 | -- | [179] | |
| Ovarian carcinoma | 56 | -- | [128] | |
| Ovarian cancer | 268 | -- | [180] | |
| Pancreatic ductal adenocarcinoma | 112 | ↑ | Early stage of pancreatic ductal adenocarcinoma cases, from TCGA dataset | [184] |
| Prostate cancer | 476 | ↓ | [108] | |
| Renal cell carcinoma (clear cell renal cell carcinoma) | 239 | ↓ | [186] | |
| Renal cell carcinoma | 104 | -- | [14] | |
| Thyroid cancer (papillary thyroid cancer) | 136 | -- | [114] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Korbecki, J.; Bajdak-Rusinek, K.; Kupnicka, P.; Kapczuk, P.; Simińska, D.; Chlubek, D.; Baranowska-Bosiacka, I. The Role of CXCL16 in the Pathogenesis of Cancer and Other Diseases. Int. J. Mol. Sci. 2021, 22, 3490. https://doi.org/10.3390/ijms22073490
Korbecki J, Bajdak-Rusinek K, Kupnicka P, Kapczuk P, Simińska D, Chlubek D, Baranowska-Bosiacka I. The Role of CXCL16 in the Pathogenesis of Cancer and Other Diseases. International Journal of Molecular Sciences. 2021; 22(7):3490. https://doi.org/10.3390/ijms22073490
Chicago/Turabian StyleKorbecki, Jan, Karolina Bajdak-Rusinek, Patrycja Kupnicka, Patrycja Kapczuk, Donata Simińska, Dariusz Chlubek, and Irena Baranowska-Bosiacka. 2021. "The Role of CXCL16 in the Pathogenesis of Cancer and Other Diseases" International Journal of Molecular Sciences 22, no. 7: 3490. https://doi.org/10.3390/ijms22073490
APA StyleKorbecki, J., Bajdak-Rusinek, K., Kupnicka, P., Kapczuk, P., Simińska, D., Chlubek, D., & Baranowska-Bosiacka, I. (2021). The Role of CXCL16 in the Pathogenesis of Cancer and Other Diseases. International Journal of Molecular Sciences, 22(7), 3490. https://doi.org/10.3390/ijms22073490