Epigallocatechin Gallate for Management of Heavy Metal-Induced Oxidative Stress: Mechanisms of Action, Efficacy, and Concerns
Abstract
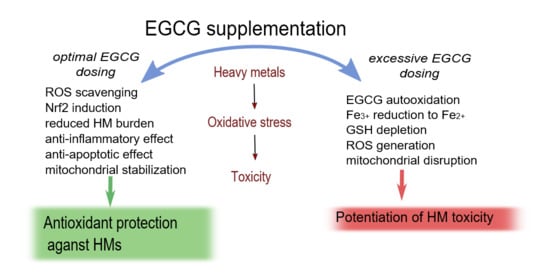
:1. Background
2. Heavy Metals As Toxicants: Health Risks and Sources for Exposure
2.1. Lead
2.2. Arsenic
2.3. Cadmium
2.4. Chromium (VI)
2.5. Nickel
2.6. Mercury
3. Absorption, Metabolism, and Bioavailability of Epigallocatechin Gallate
4. Mechanistic Considerations of the Protective Effects of EGCG against HM Toxicity
4.1. Direct Antioxidant Effects of EGCG via Scavenging Cytotoxic Reactive Species and Metal Chelation
4.2. Regulation of the Nrf2 Antioxidant Pathway
4.3. Regulation of Inflammatory Responses
4.4. Regulation of Mitochondrial Functions
5. Toxic Effects Triggered by EGCG during HM Exposure
6. Potential Obstacles in the Use of EGCG in HM Intoxication Treatment
7. Conclusions and Future Directions
- Estimation of optimal EGCG dose ranges which are both safe and effective in the treatment of HM toxicity;
- Investigation of the indirect mechanisms by which EGCG can modulate HM toxicity, including mitochondrial functions and Nrf2 activity, using different mammalian cells or tissues that are particularly prone to HM intoxication such as the lung, brain, liver, or kidney;
- Analysis and comparison of the efficacy of native EGCG and nanoEGCG in HM toxicity treatment;
- Verification of the possible synergistic effect between EGCG and chelation agents on HM toxicity;
- Investigation of the effects of microbial ring-fission metabolites of EGCG and their contribution to EGCG effects on HM toxicity.
Funding
Conflicts of Interest
References
- Gavrilescu, M. Behaviour of Persistent Pollutants and Risks Associated with Their Presence in The Environment–Integrated Studies. Environ. Eng. Manag. J. 2009, 8, 1517–1531. [Google Scholar] [CrossRef]
- Anyanwu, B.O.; Ezejiofor, A.N.; Igweze, Z.N.; Orisakwe, O.E. Heavy Metal Mixture Exposure and Effects in Developing Nations: An Update. Toxics 2018, 6, 65. [Google Scholar] [CrossRef] [Green Version]
- Tóth, G.; Hermann, T.; Szatmári, G.; Pásztor, L. Maps of Heavy Metals in the Soils of the European Union and Proposed Priority Areas for Detailed Assessment. Sci. Total Environ. 2016, 565, 1054–1062. [Google Scholar] [CrossRef]
- Angelovičová, L.; Fazekašová, D. Contamination of the Soil and Water Environment by Heavy Metals in the Former Mining Area of Rudňany (Slovakia). Soil Water Res. 2014, 9, 18–24. [Google Scholar] [CrossRef] [Green Version]
- Sodango, T.H.; Li, X.; Sha, J.; Bao, Z. Review of the Spatial Distribution, Source and Extent of Heavy Metal Pollution of Soil in China: Impacts and Mitigation Approaches. J. Health Pollut. 2018, 8, 53–70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fasinu, P.; Orisakwe, O.E. Heavy Metal Pollution in Sub-Saharan Africa and Possible Implications in Cancer Epidemiology. Asian Pac. J. Cancer Prev. 2013, 14, 3393–3402. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Serrazina, D.C.; De Andrade, V.L.; Cota, M.; Mateus, M.L.; Aschner, M.; Dos Santos, A.P.M. Biomarkers of Exposure and Effect in a Working Population Exposed to Lead, Manganese and Arsenic. J. Toxicol. Environ. Health A 2018, 81, 983–997. [Google Scholar] [CrossRef] [PubMed]
- Romero-Zarazua, M.F.; Sanchez-Salas, J.L.; Quiroz-Alfaro, M.A.; Bandala, E.R.; Méndez-Rojas, M.A. Occupational Exposure to Heavy Metals in a Metal-Mechanical Auto Part Manufacturing Plant in Puebla, Mexico. Int. J. Environ. Health Eng. 2015, 4, 8. [Google Scholar] [CrossRef]
- Yang, A.M.; Cheng, Z.Y.; Pu, H.Q.; Cheng, N.; Li, H.Y.; Liu, S.M.; Ding, J.; Li, J.S.; Hu, X.B.; Ren, X.W.; et al. Heavy Metal Assessment among Chinese Nonferrous Metal-Exposed Workers from the Jinchang Cohort Study. Biomed. Environ. Sci. 2017, 30, 530–534. [Google Scholar] [CrossRef] [PubMed]
- Hormozi, M.; Mirzaei, R.; Nakhaee, A.; Izadi, S.; Haghighi, J.D. The Biochemical Effects of Occupational Exposure to Lead and Cadmium on Markers of Oxidative Stress and Antioxidant Enzymes Activity in the Blood of Glazers in Tile Industry. Toxicol. Ind. Health 2018, 34, 459–467. [Google Scholar] [CrossRef]
- Sung, J.H.; Oh, I.; Kim, A.; Lee, J.; Sim, C.S.; Yoo, C.; Park, S.J.; Kim, G.-B.; Kim, Y. Environmental and Body Concentrations of Heavy Metals at Sites Near and Distant from Industrial Complexes in Ulsan, Korea. J. Korean Med. Sci. 2017, 33. [Google Scholar] [CrossRef] [Green Version]
- Li, P.; Feng, X.; Qiu, G. Methylmercury Exposure and Health Effects from Rice and Fish Consumption: A Review. Int. J. Environ. Res. Public Health 2010, 7, 2666–2691. [Google Scholar] [CrossRef]
- Substance Priority List | ATSDR. Available online: https://www.atsdr.cdc.gov/spl/index.html (accessed on 31 January 2021).
- IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Arsenic, Metals, Fibres and Dusts; International Agency for Research on Cancer: Bethesda, MD, USA, 2012. [Google Scholar]
- Amadi, C.N.; Offor, S.J.; Frazzoli, C.; Orisakwe, O.E. Natural Antidotes and Management of Metal Toxicity. Environ. Sci. Pollut. Res. 2019, 26, 18032–18052. [Google Scholar] [CrossRef] [PubMed]
- Briffa, J.; Sinagra, E.; Blundell, R. Heavy Metal Pollution in the Environment and Their Toxicological Effects on Humans. Heliyon 2020, 6, e04691. [Google Scholar] [CrossRef] [PubMed]
- Patel, M.M.; Hoepner, L.; Garfinkel, R.; Chillrud, S.; Reyes, A.; Quinn, J.W.; Perera, F.; Miller, R.L. Ambient Metals, Elemental Carbon, and Wheeze and Cough in New York City Children through 24 Months of Age. Am. J. Respir. Crit. Care Med. 2009, 180, 1107–1113. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Fagerberg, B.; Sallsten, G.; Borné, Y.; Hedblad, B.; Engström, G.; Barregard, L.; Andersson, E.M. Smoking-Induced Risk of Future Cardiovascular Disease Is Partly Mediated by Cadmium in Tobacco: Malmö Diet and Cancer Cohort Study. Environ. Health 2019, 18, 56. [Google Scholar] [CrossRef] [Green Version]
- Nogawa, K.; Suwazono, Y.; Nishijo, M.; Sakurai, M.; Ishizaki, M.; Morikawa, Y.; Watanabe, Y.; Kido, T.; Nakagawa, H. Relationship between Mortality and Rice Cadmium Concentration in Inhabitants of the Polluted Jinzu River Basin, Toyama, Japan: A 26 Year Follow-Up. J. Appl. Toxicol. 2018, 38, 855–861. [Google Scholar] [CrossRef] [PubMed]
- Chung, R.T.M. Detoxification Effects of Phytonutrients against Environmental Toxicants and Sharing of Clinical Experience on Practical Applications. Environ. Sci. Pollut. Res. 2017, 24, 8946–8956. [Google Scholar] [CrossRef]
- Oh, C.C.; Jin, A.-Z.; Yuan, J.-M.; Koh, W.-P. Fish Intake and Risk of Nonmelanoma Skin Cancer in a Chinese Population: The Singapore Chinese Health Study. Clin. Exp. Dermatol. 2020, 45, 461–463. [Google Scholar] [CrossRef]
- Xue, L.; Zhao, Z.; Zhang, Y.; Liao, J.; Wu, M.; Wang, M.; Sun, J.; Gong, H.; Guo, M.; Li, S.; et al. Dietary Exposure to Arsenic and Human Health Risks in Western Tibet. Sci. Total. Environ. 2020, 731, 138840. [Google Scholar] [CrossRef]
- Hosoki, M.; Nishigawa, K.; Miyamoto, Y.; Ohe, G.; Matsuka, Y. Allergic Contact Dermatitis Caused by Titanium Screws and Dental Implants. J. Prosthodont. Res. 2016, 60, 213–219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Engelhart, S.; Segal, R.J. Allergic Reaction to Vanadium Causes a Diffuse Eczematous Eruption and Titanium Alloy Orthopedic Implant Failure. Cutis 2017, 99, 245–249. [Google Scholar]
- Mehta, V.; Midha, V.; Mahajan, R.; Narang, V.; Wander, P.; Sood, R.; Sood, A. Lead Intoxication Due to Ayurvedic Medications as a Cause of Abdominal Pain in Adults. Clin. Toxicol. 2017, 55, 97–101. [Google Scholar] [CrossRef] [PubMed]
- Nędzarek, A.; Tórz, A.; Karakiewicz, B.; Clark, J.S.; Laszczyńska, M.; Kaleta, A.; Adler, G. Concentrations of Heavy Metals (Mn, Co, Ni, Cr, Ag, Pb) in Coffee. Acta Biochim. Pol. 2013, 60, 623–627. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borowska, S.; Brzóska, M.M. Metals in Cosmetics: Implications for Human Health. J. Appl. Toxicol. 2015, 35, 551–572. [Google Scholar] [CrossRef]
- Blanusa, M.; Varnai, V.M.; Piasek, M.; Kostial, K. Chelators as Antidotes of Metal Toxicity: Therapeutic and Experimental Aspects. Curr. Med. Chem. 2005, 12, 2771–2794. [Google Scholar] [CrossRef] [PubMed]
- Flora, S.J.; Pachauri, V. Chelation in Metal Intoxication. Int. J. Environ. Res. Public Health 2010, 7, 2745–2788. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mazumder, D.N.G. Chapter 4: Diagnosis and Treatment of Chronic Arsenic Poisoning. In United Nations Synthesis Report on Arsenic in Drinking Water; WHO: Geneva, Switzerland, 2000. [Google Scholar]
- Sears, M.E. Chelation: Harnessing and Enhancing Heavy Metal Detoxification—A Review. Sci. World J. 2013, 2013, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Li, D.; An, D.; Zhou, Y.; Liu, J.; Waalkes, M.P. Current Status and Prevention Strategy for Coal-Arsenic Poisoning in Guizhou, China. J. Health Popul. Nutr. 2006, 24, 273–276. [Google Scholar]
- Winiarska-Mieczan, A. Protective Effect of Tea against Lead and Cadmium-Induced Oxidative Stress-a Review. Biometals 2018, 31, 909–926. [Google Scholar] [CrossRef] [Green Version]
- Zwolak, I. Protective Effects of Dietary Antioxidants against Vanadium-Induced Toxicity: A Review. Oxidative Med. Cell. Longev. 2020, 2020, 1490316. [Google Scholar] [CrossRef] [PubMed]
- Omidkhoda, S.F.; Razavi, B.M.; Hosseinzadeh, H. Protective Effects of Ginkgo Biloba L. against Natural Toxins, Chemical Toxicities, and Radiation: A Comprehensive Review. Phytother. Res. 2019, 33, 2821–2840. [Google Scholar] [CrossRef] [PubMed]
- Maiti, S.; Acharyya, N.; Ghosh, T.K.; Ali, S.S.; Manna, E.; Nazmeen, A.; Sinha, N.K. Green Tea (Camellia Sinensis) Protects Against Arsenic Neurotoxicity via Antioxidative Mechanism and Activation of Superoxide Dismutase Activity. Cent. Nerv. Syst. Agents Med. Chem. 2017, 17, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Anacleto, P.; Barbosa, V.; Alves, R.N.; Maulvault, A.L.; Bronze, M.R.; Marques, A. Green Tea Infusion Reduces Mercury Bioaccessibility and Dietary Exposure from Raw and Cooked Fish. Food Chem. Toxicol. 2020, 145, 111717. [Google Scholar] [CrossRef]
- Abdelrazek, H.M.A.; Helmy, S.A.; Elsayed, D.H.; Ebaid, H.M.; Mohamed, R.M. Ameliorating Effects of Green Tea Extract on Cadmium Induced Reproductive Injury in Male Wistar Rats with Respect to Androgen Receptors and Caspase-3. Reprod. Biol. 2016, 16, 300–308. [Google Scholar] [CrossRef] [PubMed]
- Pervin, M.; Unno, K.; Takagaki, A.; Isemura, M.; Nakamura, Y. Function of Green Tea Catechins in the Brain: Epigallocatechin Gallate and Its Metabolites. Int. J. Mol. Sci. 2019, 20, 3630. [Google Scholar] [CrossRef] [Green Version]
- Legeay, S.; Rodier, M.; Fillon, L.; Faure, S.; Clere, N. Epigallocatechin Gallate: A Review of Its Beneficial Properties to Prevent Metabolic Syndrome. Nutrients 2015, 7, 5443–5468. [Google Scholar] [CrossRef] [Green Version]
- He, J.; Xu, L.; Yang, L.; Wang, X. Epigallocatechin Gallate Is the Most Effective Catechin Against Antioxidant Stress via Hydrogen Peroxide and Radical Scavenging Activity. Med. Sci. Monit. 2018, 24, 8198–8206. [Google Scholar] [CrossRef]
- Grzesik, M.; Naparło, K.; Bartosz, G.; Sadowska-Bartosz, I. Antioxidant Properties of Catechins: Comparison with Other Antioxidants. Food Chem. 2018, 241, 480–492. [Google Scholar] [CrossRef]
- Tian, B.; Sun, Z.; Xu, Z.; Hua, Y. Chemiluminescence Analysis of the Prooxidant and Antioxidant Effects of Epigallocatechin-3-Gallate. Asia Pac. J. Clin. Nutr 2007, 16 (Suppl. S1), 153–157. [Google Scholar]
- Chu, C.; Deng, J.; Man, Y.; Qu, Y. Green Tea Extracts Epigallocatechin-3-Gallate for Different Treatments. Biomed Res. Int. 2017, 2017, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Kanwar, J.; Taskeen, M.; Mohammad, I.; Huo, C.; Chan, T.H.; Dou, Q.P. Recent Advances on Tea Polyphenols. Front. Biosci. 2012, 4, 111–131. [Google Scholar] [CrossRef]
- Kanlaya, R.; Thongboonkerd, V. Protective Effects of Epigallocatechin-3-Gallate from Green Tea in Various Kidney Diseases. Adv. Nutr. 2019, 10, 112–121. [Google Scholar] [CrossRef]
- Singh, N.A.; Mandal, A.K.A.; Khan, Z.A. Potential Neuroprotective Properties of Epigallocatechin-3-Gallate (EGCG). Nutr. J. 2016, 15, 60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, F.; Wang, Y.; Li, D.; Chen, Y.; Qiao, X.; Fardous, R.; Lewandowski, A.; Liu, J.; Chan, T.-H.; Dou, Q.P. Perspectives on the Recent Developments with Green Tea Polyphenols in Drug Discovery. Expert Opin. Drug Discov. 2018, 13, 643–660. [Google Scholar] [CrossRef] [PubMed]
- Nanjo, F.; Mori, M.; Goto, K.; Hara, Y. Radical Scavenging Activity of Tea Catechins and Their Related Compounds. BioSci. Biotechnol. Biochem. 1999, 63, 1621–1623. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ryan, P.; Hynes, M.J. The Kinetics and Mechanisms of the Complex Formation and Antioxidant Behaviour of the Polyphenols EGCg and ECG with Iron(III). J. Inorg. Biochem. 2007, 101, 585–593. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Xu, L.; Liang, Q.; Sun, Q.; Chen, C.; Zhang, Y.; Ding, Y.; Zhou, P. Metal Chelator EGCG Attenuates Fe(III)-Induced Conformational Transition of α-Synuclein and Protects AS-PC12 Cells against Fe(III)-Induced Death. J. Neurochem. 2017, 143, 136–146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jia, G.; Aroor, A.R.; Martinez-Lemus, L.A.; Sowers, J.R. Mitochondrial Functional Impairment in Response to Environmental Toxins in the Cardiorenal Metabolic Syndrome. Arch. Toxicol. 2015, 89, 147–153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lead Poisoning and Health. Available online: https://www.who.int/news-room/fact-sheets/detail/lead-poisoning-and-health (accessed on 5 February 2021).
- ATSDR. Lead (Pb) Toxicity: What is the Biological Fate of Lead in the Body? | ATSDR-Environmental Medicine & Environmental Health Education-CSEM. Available online: https://www.atsdr.cdc.gov/csem/csem.asp?csem=34&po=9 (accessed on 5 February 2021).
- Charkiewicz, A.E.; Backstrand, J.R. Lead Toxicity and Pollution in Poland. Int. J. Environ. Res. Public Health 2020, 17, 4385. [Google Scholar] [CrossRef] [PubMed]
- Neurogenetic Developmental Disorders | MIT CogNet. Available online: http://cognet.mit.edu/book/neurogenetic-developmental-disorders (accessed on 31 January 2021).
- Hauptman, M.; Bruccoleri, R.; Woolf, A.D. An Update on Childhood Lead Poisoning. Clin. Pediatr. Emerg. Med. 2017, 18, 181–192. [Google Scholar] [CrossRef] [PubMed]
- Preventing Disease through Healthy Environments: Exposure to Lead: A Major Public Health Concern. Available online: https://www.who.int/publications-detail-redirect/WHO-CED-PHE-EPE-19.4.7-eng (accessed on 5 February 2021).
- Tchounwou, P.B.; Yedjou, C.G.; Patlolla, A.K.; Sutton, D.J. Heavy Metals Toxicity and the Environment. EXS 2012, 101, 133–164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Natasha; Shahid, M.; Imran, M.; Khalid, S.; Murtaza, B.; Niazi, N.K.; Zhang, Y.; Hussain, I. Arsenic Environmental Contamination Status in South Asia. In Arsenic in Drinking Water and Food; Srivastava, S., Ed.; Springer: Singapore, 2020; pp. 13–39. [Google Scholar] [CrossRef]
- Jaishankar, M.; Tseten, T.; Anbalagan, N.; Mathew, B.B.; Beeregowda, K.N. Toxicity, Mechanism and Health Effects of Some Heavy Metals. Interdiscip. Toxicol. 2014, 7, 60–72. [Google Scholar] [CrossRef] [Green Version]
- Clemens, S.; Ma, J.F. Toxic Heavy Metal and Metalloid Accumulation in Crop Plants and Foods. Annu. Rev. Plant Biol. 2016, 67, 489–512. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Arsenic and Arsenic Compounds; International Agency for Research on Cancer: Bethesda, MD, USA, 2012. [Google Scholar]
- Anonymous. Cadmium in Food-Scientific Opinion of the Panel on Contaminants in the Food Chain. EFSA J. 2009, 7, 980. [Google Scholar] [CrossRef]
- Rahimzadeh, M.R.; Rahimzadeh, M.R.; Kazemi, S.; Moghadamnia, A. Cadmium Toxicity and Treatment: An Update. Casp. J. Intern. Med. 2017, 8, 135–145. [Google Scholar] [CrossRef]
- Zwolak, I. The Role of Selenium in Arsenic and Cadmium Toxicity: An Updated Review of Scientific Literature. Biol. Trace Elem. Res. 2020, 193, 44–63. [Google Scholar] [CrossRef] [Green Version]
- Wang, P.; Chen, H.; Kopittke, P.M.; Zhao, F.-J. Cadmium Contamination in Agricultural Soils of China and the Impact on Food Safety. Environ. Pollut. 2019, 249, 1038–1048. [Google Scholar] [CrossRef]
- IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Chromium (Vi) Compounds; International Agency for Research on Cancer: Bethesda, MD, USA, 2012. [Google Scholar]
- Linos, A.; Petralias, A.; Christophi, C.A.; Christoforidou, E.; Kouroutou, P.; Stoltidis, M.; Veloudaki, A.; Tzala, E.; Makris, K.C.; Karagas, M.R. Oral Ingestion of Hexavalent Chromium through Drinking Water and Cancer Mortality in an Industrial Area of Greece--an Ecological Study. Environ. Health 2011, 10, 50. [Google Scholar] [CrossRef] [Green Version]
- Velma, V.; Vutukuru, S.S.; Tchounwou, P.B. Ecotoxicology of Hexavalent Chromium in Freshwater Fish: A Critical Review. Rev. Environ. Health 2009, 24, 129–145. [Google Scholar] [CrossRef] [Green Version]
- Mensoor, M.; Said, A.M. Determination of Heavy Metals in Freshwater Fishes of the Tigris River in Baghdad. Fishes 2018, 3, 23. [Google Scholar] [CrossRef] [Green Version]
- ATSDR-Toxicological Profile: Nickel. Available online: https://www.atsdr.cdc.gov/toxprofiles/tp.asp?id=245&tid=44 (accessed on 6 February 2021).
- IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Nickel and Nickel Compounds; International Agency for Research on Cancer: Bethesda, MD, USA, 2012. [Google Scholar]
- Tramontana, M.; Bianchi, L.; Hansel, K.; Agostinelli, D.; Stingeni, L. Nickel Allergy: Epidemiology, Pathomechanism, Clinical Patterns, Treatment and Prevention Programs. Endocr. Metab. Immune Disord. Drug Targets 2020, 20, 992–1002. [Google Scholar] [CrossRef] [PubMed]
- Tammaro, A.; Magri, F.; Pigliacelli, F.; Gelormini, E.; Francesca, R.P.; Chello, C.; Persechino, S. Allergic Contact Dermatitis to a Cell Phone. Acta Dermatovenerol. Croat. 2018, 26, 339–340. [Google Scholar] [PubMed]
- Mcdonagh, A.J.G.; Wright, A.L.; Cork, M.J.; Gawkrodger, D.J. Nickel Sensitivity: The Influence of Ear Piercing and Atopy. Br. J. Dermatol. 1992, 126, 16–18. [Google Scholar] [CrossRef]
- Guntani, A.; Kawakubo, E.; Yoshiga, R.; Mii, S. Metallic Allergy Requiring Removal of Iliac Stent: Report of a Case. Surg. Case Rep. 2020, 6, 82. [Google Scholar] [CrossRef] [Green Version]
- Park, J.-D.; Zheng, W. Human Exposure and Health Effects of Inorganic and Elemental Mercury. J. Prev. Med. Public Health 2012, 45, 344–352. [Google Scholar] [CrossRef]
- US EPA. How People are Exposed to Mercury. Available online: https://www.epa.gov/mercury/how-people-are-exposed-mercury (accessed on 1 February 2021).
- Caravati, E.M.; Erdman, A.R.; Christianson, G.; Nelson, L.S.; Woolf, A.D.; Booze, L.L.; Cobaugh, D.J.; Chyka, P.A.; Scharman, E.J.; Manoguerra, A.S.; et al. Elemental Mercury Exposure: An Evidence-Based Consensus Guideline for out-of-Hospital Management. Clin. Toxicol. 2008, 46, 1–21. [Google Scholar] [CrossRef] [Green Version]
- Dórea, J.G. Low-Dose Thimerosal (Ethyl-Mercury) Is Still Used in Infants’ Vaccines: Should We Be Concerned with This Form of Exposure? J. Trace Elem. Med. Biol. 2018, 49, 134–139. [Google Scholar] [CrossRef]
- Dórea, J.G.; Farina, M.; Rocha, J.B.T. Toxicity of Ethylmercury (and Thimerosal): A Comparison with Methylmercury. J. Appl. Toxicol. 2013, 33, 700–711. [Google Scholar] [CrossRef]
- Cai, Z.-Y.; Li, X.-M.; Liang, J.-P.; Xiang, L.-P.; Wang, K.-R.; Shi, Y.-L.; Yang, R.; Shi, M.; Ye, J.-H.; Lu, J.-L.; et al. Bioavailability of Tea Catechins and Its Improvement. Molecules 2018, 23, 2346. [Google Scholar] [CrossRef] [Green Version]
- Clifford, M.N.; van der Hooft, J.J.; Crozier, A. Human Studies on the Absorption, Distribution, Metabolism, and Excretion of Tea Polyphenols. Am. J. Clin. Nutr. 2013, 98 (Suppl. S6), 1619S–1630S. [Google Scholar] [CrossRef]
- Lee, M.-J.; Maliakal, P.; Chen, L.; Meng, X.; Bondoc, F.Y.; Prabhu, S.; Lambert, G.; Mohr, S.; Yang, C.S. Pharmacokinetics of Tea Catechins after Ingestion of Green Tea and (-)-Epigallocatechin-3-Gallate by Humans: Formation of Different Metabolites and Individual Variability. Cancer Epidemiol. Biomarkers Prev. 2002, 11 Pt 1, 1025–1032. [Google Scholar]
- Raneva, V.G.; Shimizu, Y.; Shimasaki, H. Antioxidant Activity in Plasma and Tissues Distribution of (-)-Epigallocatechin Gallate after Oral Administration to Rats. J. Oleo Sci. 2005, 54, 289–298. [Google Scholar] [CrossRef] [Green Version]
- Lu, H.; Meng, X.; Yang, C.S. Enzymology of Methylation of Tea Catechins and Inhibition of Catechol-O-Methyltransferase by (-)-Epigallocatechin Gallate. Drug Metab. Dispos. 2003, 31, 572–579. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, H.; Meng, X.; Li, C.; Sang, S.; Patten, C.; Sheng, S.; Hong, J.; Bai, N.; Winnik, B.; Ho, C.-T.; et al. Glucuronides of Tea Catechins: Enzymology of Biosynthesis and Biological Activities. Drug Metab. Dispos. 2003, 31, 452–461. [Google Scholar] [CrossRef] [Green Version]
- Kohri, T.; Matsumoto, N.; Yamakawa, M.; Suzuki, M.; Nanjo, F.; Hara, Y.; Oku, N. Metabolic Fate of (-)-[4-(3)H]Epigallocatechin Gallate in Rats after Oral Administration. J. Agric. Food Chem. 2001, 49, 4102–4112. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Sang, S.; Zhu, N.; Lu, H.; Sheng, S.; Lee, M.-J.; Ho, C.-T.; Yang, C.S. Identification and Characterization of Methylated and Ring-Fission Metabolites of Tea Catechins Formed in Humans, Mice, and Rats. Chem. Res. Toxicol. 2002, 15, 1042–1050. [Google Scholar] [CrossRef]
- Kohri, T.; Nanjo, F.; Suzuki, M.; Seto, R.; Matsumoto, N.; Yamakawa, M.; Hojo, H.; Hara, Y.; Desai, D.; Amin, S.; et al. Synthesis of (−)-[4-3H]Epigallocatechin Gallate and Its Metabolic Fate in Rats after Intravenous Administration. J. Agric. Food Chem. 2001, 49, 1042–1048. [Google Scholar] [CrossRef]
- Dai, W.; Ruan, C.; Zhang, Y.; Wang, J.; Han, J.; Shao, Z.; Sun, Y.; Liang, J. Bioavailability Enhancement of EGCG by Structural Modification and Nano-Delivery: A Review. J. Funct. Foods 2020, 65, 103732. [Google Scholar] [CrossRef]
- Zou, L.; Peng, S.; Liu, W.; Gan, L.; Liu, W.; Liang, R.; Liu, C.; Niu, J.; Cao, Y.; Liu, Z.; et al. Improved in Vitro Digestion Stability of (−)-Epigallocatechin Gallate through Nanoliposome Encapsulation. Food Res. Int. 2014, 64, 492–499. [Google Scholar] [CrossRef]
- Nakagawa, K.; Miyazawa, T. Chemiluminescence-High-Performance Liquid Chromatographic Determination of Tea Catechin, (-)-Epigallocatechin 3-Gallate, at Picomole Levels in Rat and Human Plasma. Anal. Biochem. 1997, 248, 41–49. [Google Scholar] [CrossRef]
- Naumovski, N.; Blades, B.L.; Roach, P.D. Food Inhibits the Oral Bioavailability of the Major Green Tea Antioxidant Epigallocatechin Gallate in Humans. Antioxidants 2015, 4, 373–393. [Google Scholar] [CrossRef]
- Nakagawa, K.; Miyazawa, T. Absorption and Distribution of Tea Catechin, (-)-Epigallocatechin-3-Gallate, in the Rat. J. Nutr. Sci. Vitaminol. 1997, 43, 679–684. [Google Scholar] [CrossRef] [Green Version]
- Calani, L.; Del Rio, D.; Callegari, M.L.; Morelli, L.; Brighenti, F. Updated Bioavailability and 48 h Excretion Profile of Flavan-3-Ols from Green Tea in Humans. Int. J. Food Sci. Nutr. 2012, 63, 513–521. [Google Scholar] [CrossRef]
- Mizoi, M.; Takabayashi, F.; Nakano, M.; An, Y.; Sagesaka, Y.; Kato, K.; Okada, S.; Yamanaka, K. The Role of Trivalent Dimethylated Arsenic in Dimethylarsinic Acid-Promoted Skin and Lung Tumorigenesis in Mice: Tumor-Promoting Action through the Induction of Oxidative Stress. Toxicol. Lett. 2005, 158, 87–94. [Google Scholar] [CrossRef]
- Yu, N.-H.; Pei, H.; Huang, Y.-P.; Li, Y.-F. (-)-Epigallocatechin-3-Gallate Inhibits Arsenic-Induced Inflammation and Apoptosis through Suppression of Oxidative Stress in Mice. Cell. Physiol. Biochem. 2017, 41, 1788–1800. [Google Scholar] [CrossRef] [Green Version]
- García-Rodríguez, M.D.C.; Montaño-Rodríguez, A.R.; Altamirano-Lozano, M.A. Modulation of Hexavalent Chromium-Induced Genotoxic Damage in Peripheral Blood of Mice by Epigallocatechin-3-Gallate (EGCG) and Its Relationship to the Apoptotic Activity. J. Toxicol. Environ. Health Part A 2016, 79, 28–38. [Google Scholar] [CrossRef]
- Singh, G.; Thaker, R.; Sharma, A.; Parmar, D. Therapeutic Effects of Biochanin A, Phloretin, and Epigallocatechin-3-Gallate in Reducing Oxidative Stress in Arsenic-Intoxicated Mice. Environ. Sci. Pollut. Res. Int. 2021, 1–20. [Google Scholar] [CrossRef]
- Kaushal, S.; Ahsan, A.U.; Sharma, V.L.; Chopra, M. Epigallocatechin Gallate Attenuates Arsenic Induced Genotoxicity via Regulation of Oxidative Stress in Balb/C Mice. Mol. Biol. Rep. 2019, 46, 5355–5369. [Google Scholar] [CrossRef]
- Guvvala, P.R.; Ravindra, J.P.; Rajani, C.V.; Sivaram, M.; Selvaraju, S. Protective Role of Epigallocatechin-3-Gallate on Arsenic Induced Testicular Toxicity in Swiss Albino Mice. Biomed. Pharm. 2017, 96, 685–694. [Google Scholar] [CrossRef]
- Han, X.-D.; Zhang, Y.-Y.; Wang, K.-L.; Huang, Y.-P.; Yang, Z.-B.; Liu, Z. The Involvement of Nrf2 in the Protective Effects of (-)-Epigallocatechin-3-Gallate (EGCG) on NaAsO2-Induced Hepatotoxicity. Oncotarget 2017, 8, 65302–65312. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, T.-L.; Liu, Z.; Qi, Z.-J.; Huang, Y.-P.; Gao, X.-Q.; Zhang, Y.-Y. (-)-Epigallocatechin-3-Gallate (EGCG) Attenuates Arsenic-Induced Cardiotoxicity in Rats. Food Chem. Toxicol. 2016, 93, 102–110. [Google Scholar] [CrossRef]
- Chen, J.; Du, L.; Li, J.; Song, H. Epigallocatechin-3-Gallate Attenuates Cadmium-Induced Chronic Renal Injury and Fibrosis. Food Chem. Toxicol. 2016, 96, 70–78. [Google Scholar] [CrossRef]
- Yin, S.-T.; Tang, M.-L.; Su, L.; Chen, L.; Hu, P.; Wang, H.-L.; Wang, M.; Ruan, D.-Y. Effects of Epigallocatechin-3-Gallate on Lead-Induced Oxidative Damage. Toxicology 2008, 249, 45–54. [Google Scholar] [CrossRef]
- Hassan, E.; Kahilo, K.; Kamal, T.; Hassan, M.; Elgawish, M.S. The Protective Effect of Epigallocatechin-3-Gallate on Testicular Oxidative Stress in Lead-Induced Toxicity Mediated by Cyp19 Gene/Estradiol Level. Toxicology 2019, 422, 76–83. [Google Scholar] [CrossRef]
- Nakazato, T.; Ito, K.; Ikeda, Y.; Kizaki, M. Green Tea Component, Catechin, Induces Apoptosis of Human Malignant B Cells via Production of Reactive Oxygen Species. Clin. Cancer Res. 2005, 11, 6040–6049. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.-Y.; Choi, J.-Y.; Lee, H.-J.; Byun, C.J.; Park, J.-H.; Park, J.H.; Cho, H.-S.; Cho, S.-J.; Jo, S.A.; Jo, I. The Green Tea Component (-)-Epigallocatechin-3-Gallate Sensitizes Primary Endothelial Cells to Arsenite-Induced Apoptosis by Decreasing c-Jun N-Terminal Kinase-Mediated Catalase Activity. PLoS ONE 2015, 10, e0138590. [Google Scholar] [CrossRef] [Green Version]
- Sarkar, N.; Sinha, D. Epigallocatechin-3-Gallate Partially Restored Redox Homeostasis in Arsenite-Stressed Keratinocytes. J. Appl. Toxicol. 2018, 38, 1071–1080. [Google Scholar] [CrossRef]
- Yu, H.-N.; Shen, S.-R.; Yin, J.-J. Effects of Interactions of EGCG and Cd(2+) on the Growth of PC-3 Cells and Their Mechanisms. Food Chem. Toxicol. 2007, 45, 244–249. [Google Scholar] [CrossRef]
- Abib, R.T.; Peres, K.C.; Barbosa, A.M.; Peres, T.V.; Bernardes, A.; Zimmermann, L.M.; Quincozes-Santos, A.; Fiedler, H.D.; Leal, R.B.; Farina, M.; et al. Epigallocatechin-3-Gallate Protects Rat Brain Mitochondria against Cadmium-Induced Damage. Food Chem. Toxicol. 2011, 49, 2618–2623. [Google Scholar] [CrossRef]
- An, Z.; Qi, Y.; Huang, D.; Gu, X.; Tian, Y.; Li, P.; Li, H.; Zhang, Y. EGCG Inhibits Cd(2+)-Induced Apoptosis through Scavenging ROS Rather than Chelating Cd(2+) in HL-7702 Cells. Toxicol. Mech. Methods 2014, 24, 259–267. [Google Scholar] [CrossRef]
- Bondad, S.E.C.; Kurasaki, M. Analysis of Cadmium, Epigallocatechin Gallate, and Vitamin C Co-Exposure on PC12 Cellular Mechanisms. Biol. Trace Elem. Res. 2020, 198, 627–635. [Google Scholar] [CrossRef]
- Wu, F.; Sun, H.; Kluz, T.; Clancy, H.A.; Kiok, K.; Costa, M. Epigallocatechin-3-Gallate (EGCG) Protects against Chromate-Induced Toxicity in Vitro. Toxicol. Appl. Pharmacol. 2012, 258, 166–175. [Google Scholar] [CrossRef] [Green Version]
- Chen, M.; Wang, F.; Cao, J.-J.; Han, X.; Lu, W.-W.; Ji, X.; Chen, W.-H.; Lu, W.-Q.; Liu, A.-L. (-)-Epigallocatechin-3-Gallate Attenuates the Toxicity of Methylmercury in Caenorhabditis Elegans by Activating SKN-1. Chem. Biol. Interact. 2019, 307, 125–135. [Google Scholar] [CrossRef]
- Gu, Y.; Wang, Y.; Zhou, Q.; Bowman, L.; Mao, G.; Zou, B.; Xu, J.; Liu, Y.; Liu, K.; Zhao, J.; et al. Inhibition of Nickel Nanoparticles-Induced Toxicity by Epigallocatechin-3-Gallate in JB6 Cells May Be through Down-Regulation of the MAPK Signaling Pathways. PLoS ONE 2016, 11, e0150954. [Google Scholar] [CrossRef]
- Chen, L.; Yang, X.; Jiao, H.; Zhao, B. Tea Catechins Protect against Lead-Induced Cytotoxicity, Lipid Peroxidation, and Membrane Fluidity in HepG2 Cells. Toxicol. Sci. 2002, 69, 149–156. [Google Scholar] [CrossRef] [Green Version]
- Ayyalasomayajula, N.; Ajumeera, R.; Chellu, C.S.; Challa, S. Mitigative Effects of Epigallocatechin Gallate in Terms of Diminishing Apoptosis and Oxidative Stress Generated by the Combination of Lead and Amyloid Peptides in Human Neuronal Cells. J. Biochem. Mol. Toxicol. 2019, 33, e22393. [Google Scholar] [CrossRef]
- Weinreb, O.; Amit, T.; Mandel, S.; Youdim, M.B.H. Neuroprotective Molecular Mechanisms of (−)-Epigallocatechin-3-Gallate: A Reflective Outcome of Its Antioxidant, Iron Chelating and Neuritogenic Properties. Genes Nutr. 2009, 4, 283–296. [Google Scholar] [CrossRef] [Green Version]
- Papuc, C.; Goran, G.V.; Predescu, C.N.; Nicorescu, V.; Stefan, G. Plant Polyphenols as Antioxidant and Antibacterial Agents for Shelf-Life Extension of Meat and Meat Products: Classification, Structures, Sources, and Action Mechanisms. Compr. Rev. Food Sci. Food Saf. 2017, 16, 1243–1268. [Google Scholar] [CrossRef] [Green Version]
- Pannala, A.S.; Rice-Evans, C.A.; Halliwell, B.; Singh, S. Inhibition of Peroxynitrite-Mediated Tyrosine Nitration by Catechin Polyphenols. Biochem. Biophys. Res. Commun. 1997, 232, 164–168. [Google Scholar] [CrossRef]
- Valcic, S.; Burr, J.A.; Timmermann, B.N.; Liebler, D.C. Antioxidant Chemistry of Green Tea Catechins. New Oxidation Products of (-)-Epigallocatechin Gallate and (-)-Epigallocatechin from Their Reactions with Peroxyl Radicals. Chem. Res. Toxicol. 2000, 13, 801–810. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Zhao, B.; Li, M.; Shen, S.; Xin, W. Studies on Protective Mechanisms of Four Components of Green Tea Polyphenols against Lipid Peroxidation in Synaptosomes. Biochim. Biophys. Acta 1996, 1304, 210–222. [Google Scholar] [CrossRef]
- Teng, Y.; Zhao, J.; Ding, L.; Ding, Y.; Zhou, P. Complex of EGCG with Cu(II) Suppresses Amyloid Aggregation and Cu(II)-Induced Cytotoxicity of α-Synuclein. Molecules 2019, 24, 2940. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, D.; Kim, D.; Shin, C.-H.; Zhao, Y.; Park, J.-S.; Ryu, M. Evaluation of Epigallocatechin Gallate (EGCG) to Remove Pb(II) Using Spectroscopic and Quantum Chemical Calculation Method. Environ. Earth Sci. 2019, 78, 138. [Google Scholar] [CrossRef]
- Wang, Y.; Tang, Y.; Li, Z.; Hua, Q.; Wang, L.; Song, X.; Zou, B.; Ding, M.; Zhao, J.; Tang, C. JoInt Toxicity of a Multi-Heavy Metal Mixture and Chemoprevention in Sprague Dawley Rats. Int. J. Environ. Res. Public Health 2020, 17, 1451. [Google Scholar] [CrossRef] [Green Version]
- Pall, M.L.; Levine, S. Nrf2, a Master Regulator of Detoxification and Also Antioxidant, Anti-Inflammatory and Other Cytoprotective Mechanisms, Is Raised by Health Promoting Factors. Sheng Li Xue Bao 2015, 67, 1–18. [Google Scholar]
- Toyama, T.; Sumi, D.; Shinkai, Y.; Yasutake, A.; Taguchi, K.; Tong, K.I.; Yamamoto, M.; Kumagai, Y. Cytoprotective Role of Nrf2/Keap1 System in Methylmercury Toxicity. Biochem. Biophys. Res. Commun. 2007, 363, 645–650. [Google Scholar] [CrossRef]
- Toyama, T.; Shinkai, Y.; Yasutake, A.; Uchida, K.; Yamamoto, M.; Kumagai, Y. Isothiocyanates Reduce Mercury Accumulation via an Nrf2-Dependent Mechanism during Exposure of Mice to Methylmercury. Environ. Health Perspect. 2011, 119, 1117–1122. [Google Scholar] [CrossRef]
- Stefanson, A.L.; Bakovic, M. Dietary Regulation of Keap1/Nrf2/ARE Pathway: Focus on Plant-Derived Compounds and Trace Minerals. Nutrients 2014, 6, 3777–3801. [Google Scholar] [CrossRef] [Green Version]
- Gogoi, K.; Manna, P.; Dey, T.; Kalita, J.; Unni, B.G.; Ozah, D.; Baruah, P.K. Circulatory Heavy Metals (Cadmium, Lead, Mercury, and Chromium) Inversely Correlate with Plasma GST Activity and GSH Level in COPD Patients and Impair NOX4/Nrf2/GCLC/GST Signaling Pathway in Cultured Monocytes. Toxicol. Vitr. 2019, 54, 269–279. [Google Scholar] [CrossRef]
- Matzinger, M.; Fischhuber, K.; Heiss, E.H. Activation of Nrf2 Signaling by Natural Products-Can It Alleviate Diabetes? Biotechnol. Adv. 2018, 36, 1738–1767. [Google Scholar] [CrossRef]
- Na, H.-K.; Kim, E.-H.; Jung, J.-H.; Lee, H.-H.; Hyun, J.-W.; Surh, Y.-J. (-)-Epigallocatechin Gallate Induces Nrf2-Mediated Antioxidant Enzyme Expression via Activation of PI3K and ERK in Human Mammary Epithelial Cells. Arch. Biochem. Biophys. 2008, 476, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Zwolak, I. Vanadium Carcinogenic, Immunotoxic and Neurotoxic Effects: A Review of in Vitro Studies. Toxicol. Mech. Methods 2014, 24, 1–12. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.-C. NF-ΚB Signaling in Inflammation. Signal Transduct. Target. Ther. 2017, 2, 17023. [Google Scholar] [CrossRef] [Green Version]
- Ye, N.; Ding, Y.; Wild, C.; Shen, Q.; Zhou, J. Small Molecule Inhibitors Targeting Activator Protein 1 (AP-1). J. Med. Chem. 2014, 57, 6930–6948. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, M.R.; Nabavi, S.F.; Daglia, M.; Rastrelli, L.; Nabavi, S.M. Epigallocatechin Gallate and Mitochondria-A Story of Life and Death. Pharmacol. Res. 2016, 104, 70–85. [Google Scholar] [CrossRef]
- Schroeder, E.K.; Kelsey, N.A.; Doyle, J.; Breed, E.; Bouchard, R.J.; Loucks, F.A.; Harbison, R.A.; Linseman, D.A. Green Tea Epigallocatechin 3-Gallate Accumulates in Mitochondria and Displays a Selective Antiapoptotic Effect against Inducers of Mitochondrial Oxidative Stress in Neurons. Antioxid. Redox Signal. 2009, 11, 469–480. [Google Scholar] [CrossRef]
- Pan, H.; Chen, J.; Shen, K.; Wang, X.; Wang, P.; Fu, G.; Meng, H.; Wang, Y.; Jin, B. Mitochondrial Modulation by Epigallocatechin 3-Gallate Ameliorates Cisplatin Induced Renal Injury through Decreasing Oxidative/Nitrative Stress, Inflammation and NF-KB in Mice. PLoS ONE 2015, 10, e0124775. [Google Scholar] [CrossRef] [Green Version]
- Sang, S.; Yang, I.; Buckley, B.; Ho, C.-T.; Yang, C.S. Autoxidative Quinone Formation in Vitro and Metabolite Formation in Vivo from Tea Polyphenol (-)-Epigallocatechin-3-Gallate: Studied by Real-Time Mass Spectrometry Combined with Tandem Mass Ion Mapping. Free. Radic. Biol. Med. 2007, 43, 362–371. [Google Scholar] [CrossRef] [Green Version]
- Sergi, C.M. Epigallocatechin-3-Gallate Toxicity in Children: A Potential and Current Toxicological Event in the Differential Diagnosis with Virus-Triggered Fulminant Hepatic Failure. Front. Pharmacol. 2020, 10. [Google Scholar] [CrossRef]
- Kajiya, K.; Kumazawa, S.; Nakayama, T. Steric Effects on Interaction of Tea Catechins with Lipid Bilayers. BioSci. Biotechnol. Biochem. 2001, 65, 2638–2643. [Google Scholar] [CrossRef]
- Kucera, O.; Mezera, V.; Moravcova, A.; Endlicher, R.; Lotkova, H.; Drahota, Z.; Cervinkova, Z. In Vitro Toxicity of Epigallocatechin Gallate in Rat Liver Mitochondria and Hepatocytes. Oxidative Med. Cell. Longev. 2015, 2015, 476180. [Google Scholar] [CrossRef] [Green Version]
- Elbling, L.; Weiss, R.-M.; Teufelhofer, O.; Uhl, M.; Knasmueller, S.; Schulte-Hermann, R.; Berger, W.; Micksche, M. Green Tea Extract and (-)-Epigallocatechin-3-Gallate, the Major Tea Catechin, Exert Oxidant but Lack Antioxidant Activities. Faseb J. 2005, 19, 807–809. [Google Scholar] [CrossRef]
- Grzesik, M.; Bartosz, G.; Stefaniuk, I.; Pichla, M.; Namieśnik, J.; Sadowska-Bartosz, I. Dietary Antioxidants as a Source of Hydrogen Peroxide. Food Chem. 2019, 278, 692–699. [Google Scholar] [CrossRef]
- Long, L.; Halliwell, B. Artefacts in Cell Culture: Pyruvate as a Scavenger of Hydrogen Peroxide Generated by Ascorbate or Epigallocatechin Gallate in Cell Culture Media. Biochem. Biophys. Res. Commun. 2009, 388, 700–704. [Google Scholar] [CrossRef]
- Scholl, C.; Lepper, A.; Lehr, T.; Hanke, N.; Schneider, K.L.; Brockmöller, J.; Seufferlein, T.; Stingl, J.C. Population Nutrikinetics of Green Tea Extract. PLoS ONE 2018, 13, e0193074. [Google Scholar] [CrossRef]
- Haratifar, S.; Meckling, K.; Corredig, M. Bioefficacy of Tea Catechins Encapsulated in Casein Micelles Tested on a Normal Mouse Cell Line (4D/WT) and Its Cancerous Counterpart (D/v-Src) before and after in Vitro Digestion. Food Funct. 2014, 5, 1160–1166. [Google Scholar] [CrossRef] [PubMed]
- Dube, A.; Nicolazzo, J.A.; Larson, I. Chitosan Nanoparticles Enhance the Plasma Exposure of (-)-Epigallocatechin Gallate in Mice through an Enhancement in Intestinal Stability. Eur. J. Pharm. Sci. 2011, 44, 422–426. [Google Scholar] [CrossRef]
- Peters, C.M.; Green, R.J.; Janle, E.M.; Ferruzzi, M.G. Formulation with Ascorbic Acid and Sucrose Modulates Catechin Bioavailability from Green Tea. Food Res. Int. 2010, 43, 95–102. [Google Scholar] [CrossRef] [Green Version]
- Mazzanti, G.; Menniti-Ippolito, F.; Moro, P.A.; Cassetti, F.; Raschetti, R.; Santuccio, C.; Mastrangelo, S. Hepatotoxicity from Green Tea: A Review of the Literature and Two Unpublished Cases. Eur. J. Clin. Pharm. 2009, 65, 331–341. [Google Scholar] [CrossRef]
- Wang, D.; Taylor, E.W.; Wang, Y.; Wan, X.; Zhang, J. Encapsulated Nanoepigallocatechin-3-Gallate and Elemental Selenium Nanoparticles as Paradigms for Nanochemoprevention. Int. J. Nanomed. 2012, 7, 1711–1721. [Google Scholar] [CrossRef] [Green Version]
- National Toxicology Program (NTP). NTP Technical Report on the Toxicology Studies of Green Tea Extract in F344/NTac Rats and B6C3F1/N Mice and Toxicology and Carcinogenesis Studies of Green Tea Extract in Wistar Han [Crl:Wi(Han)] Rats and B6C3F1/N Mice (Gavage Studies); NCBI: Bethesda, MD, USA, 2016; p. 585. [Google Scholar] [CrossRef]
- Emoto, Y.; Yoshizawa, K.; Kinoshita, Y.; Yuki, M.; Yuri, T.; Yoshikawa, Y.; Sayama, K.; Tsubura, A. Green Tea Extract-Induced Acute Hepatotoxicity in Rats. J. Toxicol. Pathol. 2014, 27, 163–174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pae, M.; Ren, Z.; Meydani, M.; Shang, F.; Smith, D.; Meydani, S.N.; Wu, D. Dietary Supplementation with High Dose of Epigallocatechin-3-Gallate Promotes Inflammatory Response in Mice. J. Nutr. Biochem. 2012, 23, 526–531. [Google Scholar] [CrossRef] [PubMed]
- Rasheed, N.O.A.; Ahmed, L.A.; Abdallah, D.M.; El-Sayeh, B.M. Nephro-Toxic Effects of Intraperitoneally Injected EGCG in Diabetic Mice: Involvement of Oxidative Stress, Inflammation and Apoptosis. Sci. Rep. 2017, 7, 40617. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, D.; Wei, Y.; Wang, T.; Wan, X.; Yang, C.S.; Reiter, R.J.; Zhang, J. Melatonin Attenuates (-)-Epigallocatehin-3-Gallate-Triggered Hepatotoxicity without Compromising Its Downregulation of Hepatic Gluconeogenic and Lipogenic Genes in Mice. J. Pineal Res. 2015, 59, 497–507. [Google Scholar] [CrossRef] [PubMed]
- Church, R.J.; Gatti, D.M.; Urban, T.J.; Long, N.; Yang, X.; Shi, Q.; Eaddy, J.S.; Mosedale, M.; Ballard, S.; Churchill, G.A.; et al. Sensitivity to Hepatotoxicity Due to Epigallocatechin Gallate Is Affected by Genetic Background in Diversity Outbred Mice. Food Chem. Toxicol. 2015, 76, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Lambert, J.D.; Kennett, M.J.; Sang, S.; Reuhl, K.R.; Ju, J.; Yang, C.S. Hepatotoxicity of High Oral Dose (-)-Epigallocatechin-3-Gallate in Mice. Food Chem. Toxicol. 2010, 48, 409–416. [Google Scholar] [CrossRef] [Green Version]


| HMs | Exposure Source | Adverse Effects on Human Health | Ref. |
|---|---|---|---|
| Ni, V | Fine particulate matter in the urban environment | Possible contribution to respiratory symptoms in children (in New York City) | [17] |
| Cd | Cigarette smoke | Increased risk of cardiovascular diseases (in Sweden) | [18] |
| Cd | Rice | Increased risk of kidney diseases (in Japan) | [19] |
| Cd, Al | Shellfish | Allergic eczema | [20] |
| As | Fish | Possible contribution to nonmelanoma skin cancer (in Singapore) | [21] |
| As | Highland barley | Increased probability of cancer risk (in western Tibet) | [22] |
| Ti | Titanium-based dental and orthopedic implants | Allergic eczema | [23] |
| V | Titanium-based orthopedic implants | Systemic dermatitis | [24] |
| Pb | Ayurvedic medicines | Abdominal pain | [25] |
| Pb | Coffee | Possible contribution to disease burden in heavy coffee drinkers | [26] |
| Al | Cosmetics (antiperspirants) | Possible contribution to breast cancer | [27] |
| EGCG Dose | HM Dose | Duration of Treatment | Model | Relevant EGCG Interferences | Suggested Mechanism/s of Action | Ref. |
|---|---|---|---|---|---|---|
| 0.05% in powder chow | DMA(V): 400 ppm, drinking water following lung tumor initiator 4NQO injection | DMA(V) and EGCG cotreatment for 25 weeks | Mice | ↓ incidence of lung tumors ↓ 8-oxodG level in lungs | ROS scavenging | [98] |
| 10 mg/kg i.g. | NaAsO2 10 mg/kg, i.g. | NaAsO2 and EGCG cotreatment for 30 days | Mice | ↓ NO level and IL-1β, IL-6, and TNFα release in serum; ↓ CD8 (cytotoxic) T cell and ↑ CD4 (helper) cell frequency; ↑ CD3-positive T cell and CD19-positive B cell frequency; ↓ As content in the thymus and spleen; In the thymus: ↓ ROS, ↓ caspase-3 activity, ↑ MMP, ↓ apoptotic and necrotic cell number | ROS scavenging/anti-inflammatory effects/metal chelation | [99] |
| 10 mg/kg, i.g. | CrO3 20 mg/kg i.p. | Single EGCG treatment followed by CrO3 injection | Mice | Peripheral blood: ↓ micronucleated polychromatic erythrocytes, ↓ cell viability, ↑ apoptotic and necrotic cell number | Proapoptotic effects | [100] |
| 40 mg/kg i.g. | NaAsO2 20 mg/kg i.g. | NaAsO2 and EGCG cotreatment for 14 days | Mice | ↑ sperm motility; ↓ As content in the liver and kidney; ↓ LPO level in the kidney and lung; ↓ PCC level in the lung and brain; ↑ GSH level in the liver, kidney, testis, and brain; ↑ SOD activity in the testis and brain; ↑ GST activity in the liver, kidney, lung, testis, and brain; ↑ BChE in the brain; ↑ Nrf2 expression in kidney; ↓ histopathological changes in the brain; no effect on DNA damage in blood cells | ROS scavenging/metal chelation/increased Nrf2 signaling | [101] |
| 25 and 50 mg/kg orally | NaAsO2 1.5 mg/kg i.p. | (1) EGCG pretreatment for 15 days followed by 10 days of As treatment (2) As treatment for 10 days followed by EGCG post-treatment for 15 days | Mice |
| ROS scavenging/metal chelation | [102] |
| 20 mg/kg i.p. | As 200 ppm (drinking water) | As and EGCG cotreatment for 40 days | Mice |
| ROS scavenging/stabilization of mitochondria | [103] |
| 50 mg/kg i.g. | NaAsO2 5 mg/kg i.g. | NaAsO2 and EGCG co-treatment for 30 days | Rats | In the liver: ↓ AST, ALT, ALP, and LDH activity, ↑ SOD and CAT activity, ↑ GSH level, ↓ MDA and ROS level, ↓ As content, ↓ histopathological changes | ROS scavenging/metal chelation | [104] |
| 50 mg/kg i.g. | NaAsO2 5 mg/kg i.g. | NaAsO2 and EGCG co-treatment for 30 days | Rats | Heart tissue: improved morphology and ultrastructure, ↓ As content, ↓ apoptotic cell number, ↑ integrity of plasma membrane, ↑ SOD, CAT and GPx activity, ↓ MDA level, ↓ intracellular Ca2+ concentration | ROS scavenging/maintenance of intracellular Ca2+ levels | [105] |
| 100 and 200 mg/kg i.g. | CdCl2 250 mg/L in (drinking water) | CdCl2 and EGCG cotreatment for 16 weeks | Rats | ↓ blood urea nitrogen and serum creatinine; in the kidneys: improved morphology, ↓ collagen deposition and fibrosis, ↓ TGF-β1 and p-Smad3 level, ↑ GSH level, ↑ SOD and GPx activity, ↓ MDA and NO level, ↓ miR-21 and miR-192 level and ↑ miR-29a/b/c level | ROS scavenging/anti-inflammatory effects/modulation of microRNA levels | [106] |
| 10, 25, and 50 mg/kg i.p. | Pb acetate 1090 ppm (drinking water) | Pb acetate treatment from PND1-20 (via mother’s milk) and PND21–23 (via drinking water) EGCG cotreatment from PND14–23 | Rats (pups) | ↑ Pb in blood; in the hippocampus: ↑ long-term potentiation amplitude in the CA1 area, ↑ GSH level and SOD activity, ↓ MDA level | ROS scavenging/metal chelation | [107] |
| 80 mg/kg i.p. | Pb acetate 50 mg/L (drinking water) | Pb acetate and EGCG cotreatment for 49 days | Rats | ↑ sperm motility, ↑ relative weight of testis and seminal vesicles, ↑ serum testosterone and 17β-estradiol level, in the testis: ↑ cyp19 (aromatase P450) gene expression, ↑ SOD, CAT, and GPx activity, ↓ MDA levels, ↑ testicular architecture and semen picture | ROS scavenging/increased cyp19 gene expression | [108] |
| EGCG Concentration | HM Dose | Duration of Treatment | Cell Type | Effects of EGCG on the Toxicity of HMs | Suggested Mechanism/s of EGCG Action | Ref. |
|---|---|---|---|---|---|---|
| 10 μM | As2O3 2 μM | As2O3 and EGCG coincubation for 3 or 24 h | Myeloma cells (RPMI 8226, IM9), Burkitt’s lymphoma cells (HS-sultan) | ↓ cell viability; ↑ apoptotic cells; ↑ intracellular ROS; ↓ GSH level; ↓ Bcl-2, Mcl-1, and procaspase-3 protein level | Increased ROS production/decreased GSH levels/proapoptotic effects | [109] |
| 20 μM | NaAsO2 20 μM | NaAsO2 and EGCG coincubation for 3–24 h | Primary bovine aortic endothelial cells (BAEC) | ↓ cell viability; ↑ number of apoptotic cells; ↑ caspase 3, 8, and 9 activity; ↑ bax translocation into mitochondria; ↑ ROS and MDA level; ↓ CAT activity; ↑ level of phosphorylated JNK (p-JNK) | JNK activation/increased ROS production/proapoptotic effects | [110] |
| 50 μM | NaAsO2 50 μM | NaAsO2 and EGCG coincubation for 24 h | Normal human keratinocytes HaCaT cells | ↑ ROS and MDA level; ↑ 8-OHdG content; ↑ DNA damage (comet assay); ↓ nuclear and ↑ cytosolic expression of Nrf2; ↑ nuclear expression of Keap1; ↑ protein expression of HO-1 and γ-GCSC; ↓ SOD, NQO1 and GST activity | Increased ROS production/modulation of Nrf2 signaling pathway | [111] |
| 30 and 150 μM | CdCl2 30 and 50 μM | CdCl2 and EGCG coincubation for 24 h | Human prostate cancer cell line PC-3 | ↓ cell viability; no complex of EGCG with Cd was formed at pH 7.0 | Modulation of Ca2+ and Zn2+ absorption by cells | [112] |
| 100 μM | CdCl2 200 μM | CdCl2 and EGCG coincubation for 2 h | Mitochondrial-enriched fractions from rat brain | ↑ mitochondrial viability; ↓ mitochondrial LPO; no effects on nonprotein thiol levels; formation of Cd:EGCG complex in a 1:1 ratio at pH 8.3 | ROS scavenging/stabilization of mitochondria/metal chelation | [113] |
| 20 μM | CdCl2 60 μM | CdCl2 incubation for 21 h followed by coincubation with EGCG for 3 h | Normal human liver cells HL-7702 | ↑ cell viability; ↓ apoptosis rate; ↓ ROS and MDA levels; ↑ MMP; ↓ caspase 3 activity; EGCG and Cd did not form complexes with each other at neutral pH (pH 7.2) | ROS scavenging/stabilization of mitochondria | [114] |
| 1.5 μM | CdCl2 5 μM | 1 h EGCG pretreatment followed by 48 h CdCl2 exposure | Rat pheochromocytoma cell line PC12 | ↓ cell viability; ↓ cell membrane integrity | Increased ROS production/cell membrane disruption | [115] |
| 5, 10 and 25 μM 25–200 μM | K2CrO4 10 μM K2CrO4 50 μM | K2CrO4 and EGCG coincubation for 24 h K2CrO4 and EGCG coincubation for 24 h | Human normal bronchial epithelial BEAS-2B cells Epstein-Barr virus-transformed human Burkitt’s lymphoma EBV-BL | ↑ cell viability; ↓ apoptotic cells; ↓ ROS; ↓ mRNA expression of cell death-related genes (GADD45A, PPP1R15A, EGR1); ↑ mRNA expression of genes involved in cell defense (SMUG1, XRCC4 and ERCC4) ↓ DNA-protein cross-links | ROS scavenging/modulation of gene expression | [116] |
| 10 mg/mL | CH3HgCl (MeHg) 2.5, 5 and 10 μM | 48 h EGCG pretreatment followed by 24 h MeHg exposure | Caenorhabditis elegans |
| Increased Nrf2 signaling pathway | [117] |
| 10 μM | Ni NPs 2.5–10 μg/cm2 | Ni NPs and EGCG coincubation for 24 h | Mouse epidermal cells JB6 | ↑ cell viability and morphology; ↑ G0/G1 phase arrest and ↓ G2/M phase arrest; ↓ apoptotic cells; ↓ intracellular ROS generation; ↓ AP-1 and NF-B activation; ↓ protein expression of p-ERK1/2, p-JNK, and p-p38 | ROS scavenging/anti-inflammatory effects/modulation of the MAPK signaling pathway | [118] |
| 5, 10, 15 μM | Pb2+ 100 μM | Pb2+ and EGCG coincubation for 24 h | Human hepatocellular carcinoma cell line HepG2 | ↑ cell viability; ↓ LPO; ↑ cell membrane fluidity | ROS scavenging/metal chelation/stabilization of cell membranes | [119] |
| 50 μM | Pb acetate 5 μM | Pb acetate and EGCG coincubation for 24 h | SH-SY5Y human neuroblastoma cells | ↓ apoptosis rate; ↓ ROS levels; ↓ caspase 3 activity; ↓ bax/bcl2 ratio | ROS scavenging/antiapoptotic effects | [120] |
| 50 μM | Pb acetate 20 μM | Pb acetate and EGCG coincubation for 24 h | Primary hippocampal neurons | ↑ cell viability; ↓ ROS levels; ↑ MMP | ROS scavenging/stabilization of mitochondria | [107] |
| EGCG Dose | Route of Administration | Duration | Animals | Toxic Effects | References |
|---|---|---|---|---|---|
| EGCG 100 mg/kg | i.p. | 4 d | Swiss albino mice  (diabetic) (diabetic) | Death (60% animals); ↑ serum cystatin C and NGAL (markers of kidney damage) In the kidney: ↑ NADPH oxidase, ↓ TAC, GSH, Nrf2, HO-1, and HSP 90, ↑ NF-κB and TNF-α, ↑ histopathological changes | [158] |
| EGCG 55 mg/kg | i.p. | 5 d | Kunming mice  | ↓ body weight; in the serum: ↑ ALT, AST (markers of liver damage), ↑ 4-HNE, IL-2, IL-6 and IL-10 | [159] |
| EGCG 50 mg/kg | i.p. | 3 d | DO mice  | Mild liver injury (0.55–9.94% liver necrosis) in 49% animals. Severe liver injury (10–86.8% liver necrosis) in 16% animals | [160] |
| GT extract 62.5, 125, 250, 500, and 1000 mg/kg containing 30.3–484 mg/kg of EGCG (48.4%) | i.g. | 14 weeks (5 days per week) | B6C3F1/N mice   |
| [155] |
| EGCG 1500 mg/kg 750 mg/kg | i.g. | Single dose 2–7 d | CF-1 mice  |
| [161] |
| GT extract 200 mg/kg containing 108 mg/kg of EGCG (54%) | i.p. | Single dose | SPF rats   |
| [156] |
| GT extract 62.5, 125, 250, 500, and 1000 mg/kg containing 30.3–484 mg/kg of EGCG (48.4%) | i.g. | 14 weeks (5 days per week) | F344/NTac rats   |
| [155] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zwolak, I. Epigallocatechin Gallate for Management of Heavy Metal-Induced Oxidative Stress: Mechanisms of Action, Efficacy, and Concerns. Int. J. Mol. Sci. 2021, 22, 4027. https://doi.org/10.3390/ijms22084027
Zwolak I. Epigallocatechin Gallate for Management of Heavy Metal-Induced Oxidative Stress: Mechanisms of Action, Efficacy, and Concerns. International Journal of Molecular Sciences. 2021; 22(8):4027. https://doi.org/10.3390/ijms22084027
Chicago/Turabian StyleZwolak, Iwona. 2021. "Epigallocatechin Gallate for Management of Heavy Metal-Induced Oxidative Stress: Mechanisms of Action, Efficacy, and Concerns" International Journal of Molecular Sciences 22, no. 8: 4027. https://doi.org/10.3390/ijms22084027
APA StyleZwolak, I. (2021). Epigallocatechin Gallate for Management of Heavy Metal-Induced Oxidative Stress: Mechanisms of Action, Efficacy, and Concerns. International Journal of Molecular Sciences, 22(8), 4027. https://doi.org/10.3390/ijms22084027