COVID-19: Direct and Indirect Mechanisms of Statins
Abstract
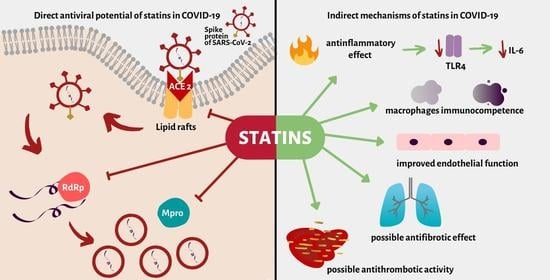
:1. Introduction
2. Lipids in SARS-CoV-2 Infection
3. Direct Effect of Statins on SARS-CoV-2 Endocytosis via ACE2 Protein
4. Statins Possibly Attenuate SARS-CoV-2 Replication Directly via Main Protease and RNA-Dependent RNA Polymerase
5. Statins May Reduce Inflammation in COVID-19: Interleukin-6 and Toll-Like Receptor 4
6. Statins Can Possibly Attenuate Macrophage Activation Syndrome
7. Statins Improve Endothelial Functions
8. Statins May Reduce the Risk of Thrombosis in COVID-19
9. Statins May Alleviate Post-COVID Pulmonary Fibrosis
10. The Safety of Statins
11. Summary
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| SARS-CoV-2 | Severe Acute Respiratory Syndrome Coronavirus 2 |
| COVID-19 | Coronavirus Disease 2019 |
| WHO | World Health Organisation |
| aHR | adjusted Hazard Ratio |
| CI | Confidence Interval |
| ARDS | Acute Respiratory Distress Syndrome |
| IRR | Incidence rate ratio |
| LDL | Low Density Lipoprotein |
| ACE2 | Angiotensin Converting Enzyme 2 |
| TLR | Toll-like receptor |
| CK | Creatine Kinase |
| ALT | Alanine Transaminase |
| ULN | Upper Limit Norm |
| MβCD | Methyl-beta-cyclodextrin |
| ApoE | Apolipoprotein E |
| PLA2 | Phospholipase A2 |
| SARS-PV | retroviruses pseudotyped with SARS-CoV-2 S proteins |
| cPLA2 | cytosolic phospholipase A2 |
| Py-2 | pyrrolidyne 2 |
| TC | total cholesterol |
| HDL | High Density Lipoprotein |
| RNA | ribonucleic acidSARS-CoV-1—Severe Acute Respiratory Syndrome Coronavirus 1 |
| RAA | Renin Angiotensin Aldosterone |
| AT1R | Angiotensine 1 receptor |
| RSV | Respiratory Syncytial Virus |
| H3-Ac | histone H3 acetylation |
| HCD | High Cholesterol Diet |
| PPAR-γ | peroxisome proliferator γ receptor |
| ARBs | Angiotensin Receptor Blockers |
| Mpro | main protease |
| RdRp | RNA-dependent RNA polymerase |
| HBV | Hepatitis B Virus |
| HCV | Hepatitis C virus |
| HIV | Human Immunodeficiency Virus |
| IL-6 | interleukin 6 |
| MAS | Macrophage activating syndrome |
| CRP | C reactive protein |
| MyD88 | Myeloid differentiation primary response 88 |
| TRIF | Toll/IL-1R domain-contactiong adaptor-inducing IFN-βTRIF—Toll/IL-1R domain-contactiong adaptor-inducing IFN-β |
| IFN-β | Interferon beta |
| NF- κB | nuclear factor kappa-light-chain-enhancer of activated B cells |
| CD8+ | cluster of differentiation 8 |
| sHLH | secondary hemophagocytic lymphohistiocytosis |
| TNF-α | Tumor Necrosis Factor alpha |
| LPS | Lipopolysaccharides |
| ROS | reactive oxygen spieces |
| miRNA-221 | MicroRNA-221 |
| eNOS | endothelial nitric oxide synthase |
| KLF-2 | Krüppel-like Factor 2 |
| CSE | cystathionine γ-lyase |
| NLRP3 | NOD-, LRR- and pyrin domain-containing protein 3 |
| PXR | pregnane X receptor |
| EPCs | Endothelial Progenitor Cells |
| PE | pulmonary embolismr |
| VTE | recurrence of venous thromboembolism |
| aSHR | adjusted Hazard Ratio |
| VKA | vitamin K antagonist |
| PAI-1 | plasminogen activator inhibitor –1 |
| CT | computed tomography |
| VEGF | Vascular endothelial growth factor |
| TGFβ | transforming growth factor β |
| IPF | Idiopathic Pulmonary Fibrosis |
| OR | Odds Ratio |
| AST | aspartate aminotransferase |
References
- World Health Organization. Timeline: WHO’s COVID-19 Response. Available online: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/interactive-timeline# (accessed on 5 February 2021).
- Parohan, M.; Yaghoubi, S.; Seraji, A.; Javanbakht, M.H.; Sarraf, P.; Djalali, M. Risk factors for mortality in patients with Coronavirus disease 2019 (COVID-19) infection: A systematic review and meta-analysis of observational studies. Aging Male 2020, 8, 1–9. [Google Scholar] [CrossRef]
- Mach, F.; Baigent, C.; Catapano, A.L.; Koskinas, K.C.; Casula, M.; Badimon, L.; Chapman, M.J.; De Backer, G.G.; Delgado, V.; Ference, B.A.; et al. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: Lipid modification to reduce cardiovascular risk: The Task Force for the management of dyslipidaemias of the European Society of Cardiology (ESC) and European Atherosclerosis Society (EAS). Eur. Heart J. 2020, 41, 111–188. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.-J.; Qin, J.-J.; Cheng, X.; Shen, L.; Zhao, Y.-C.; Yuan, Y.; Lei, F.; Chen, M.-M.; Yang, H.; Bai, L.; et al. In-Hospital Use of Statins Is Associated with a Reduced Risk of Mortality among Individuals with COVID-19. Cell Metab. 2020, 32, 176–187.e4. [Google Scholar] [CrossRef]
- Kow, C.S.; Hasan, S.S. Meta-analysis of Effect of Statins in Patients with COVID-19. Am. J. Cardiol. 2020, 134, 153–155. [Google Scholar] [CrossRef]
- Niedzielski, M.; Broncel, M.; Gorzelak-Pabiś, P.; Woźniak, E. New possible pharmacological targets for statins and ezetimibe. Biomed. Pharmacother. 2020, 129, 110388. [Google Scholar] [CrossRef]
- Shin, Y.H.; Min, J.J.; Lee, J.-H.; Kim, E.-H.; Kim, G.E.; Kim, M.H.; Lee, J.J.; Ahn, H.J. The effect of fluvastatin on cardiac fibrosis and angiotensin-converting enzyme-2 expression in glucose-controlled diabetic rat hearts. Hear. Vessel. 2016, 32, 618–627. [Google Scholar] [CrossRef]
- Bourgonje, A.R.; Abdulle, A.E.; Timens, W.; Hillebrands, J.; Navis, G.J.; Gordijn, S.J.; Bolling, M.C.; Dijkstra, G.; Voors, A.A.; Osterhaus, A.D.M.E.; et al. Angiotensin-converting enzyme 2 (ACE2), SARS-CoV-2 and the pathophysiology of coronavirus disease 2019 ( COVID-19). J. Pathol. 2020, 251, 228–248. [Google Scholar] [CrossRef]
- Reiner, Ž.; Hatamipour, M.; Banach, M.; Pirro, M.; Al-Rasadi, K.; Jamialahmadi, T.; Radenkovic, D.; Montecucco, F.; Sahebkar, A. Statins and the COVID-19 main protease: In silico evidence on direct interaction. Arch. Med Sci. 2020, 16, 490–496. [Google Scholar] [CrossRef]
- Baby, K.; Maity, S.; Mehta, C.H.; Suresh, A.; Nayak, U.Y.; Nayak, Y. Targeting SARS-CoV-2 RNA-dependent RNA polymerase: An in silico drug repurposing for COVID-19. F1000Research 2020, 9, 1166. [Google Scholar] [CrossRef]
- Chansrichavala, P.; Chantharaksri, U.; Sritara, P.; Chaiyaroj, S.C. Atorvastatin attenuates TLR4-mediated NF-κB activation in a MyD88-dependent pathway. Asian Pacific J. Allergy Immunol. 2009, 27, 49–57. [Google Scholar]
- Santos, J.C.; Passos, G.A. The high infectivity of SARS-CoV-2 B.1.1.7 is associated with increased interaction force between Spike-ACE2 caused by the viral N501Y mutation. bioRxiv 2020. [Google Scholar] [CrossRef]
- Cariou, B.; Goronflot, T.; Rimbert, A.; Boullu, S.; Le May, C.; Moulin, P.; Pichelin, M.; Potier, L.; Smati, S.; Sultan, A.; et al. Routine use of statins and increased COVID-19 related mortality in inpatients with type 2 diabetes: Results from the CORONADO study. Diabetes Metab. 2021, 47, 101202. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Yuan, Z.; Pavel, M.A.; Hansen, S. Cholesterol and COVID19 lethality in elderly. bioRxiv 2020, 15, 1–14. [Google Scholar] [CrossRef]
- Lorizate, M.; Kräusslich, H.-G. Role of Lipids in Virus Replication. Cold Spring Harb. Perspect. Biol. 2011, 3, a004820. [Google Scholar] [CrossRef] [Green Version]
- Sanchez, E.L.; Lagunoff, M. Viral activation of cellular metabolism. Virology 2015, 479-480, 609–618. [Google Scholar] [CrossRef] [Green Version]
- Li, G.-M.; Li, Y.-G.; Yamate, M.; Li, S.-M.; Ikuta, K. Lipid rafts play an important role in the early stage of severe acute respiratory syndrome-coronavirus life cycle. Microbes Infect. 2007, 9, 96–102. [Google Scholar] [CrossRef]
- Barberis, E.; Timo, S.; Amede, E.; Vanella, V.V.; Puricelli, C.; Cappellano, G.; Raineri, D.; Cittone, M.G.; Rizzi, E.; Pedrinelli, A.R.; et al. Large-Scale Plasma Analysis Revealed New Mechanisms and Molecules Associated with the Host Response to SARS-CoV-2. Int. J. Mol. Sci. 2020, 21, 8623. [Google Scholar] [CrossRef]
- Hammock, B.D.; Wang, W.; Gilligan, M.M.; Panigrahy, D. Eicosanoids: The Overlooked Storm in Coronavirus Disease 2019 (COVID-19)? Am. J. Pathol. 2020, 190, 1782–1788. [Google Scholar] [CrossRef]
- Müller, C.; Hardt, M.; Schwudke, D.; Neuman, B.W.; Pleschka, S.; Ziebuhr, J. Inhibition of cytosolic phospholipase A2α impairs an early step of coronavirus replication in cell culture. J. Virol. 2017, 92, JVI.01463-17. [Google Scholar] [CrossRef] [Green Version]
- Hu, X.; Chen, D.; Wu, L.; He, G.; Ye, W. Low Serum Cholesterol Level Among Patients with COVID-19 Infection in Wenzhou, China. SSRN Electron. J. 2020. [Google Scholar] [CrossRef]
- Wei, X.; Zeng, W.; Su, J.; Wan, H.; Yu, X.; Cao, X.; Tan, W.; Wang, H. Hypolipidemia is associated with the severity of COVID-19. J. Clin. Lipidol. 2020, 14, 297–304. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Zhang, Q.; Zhao, X.; Dong, H.; Wu, C.; Wu, F.; Yu, B.; Lv, J.; Zhang, S.; Wu, G.; et al. Low high-density lipoprotein level is correlated with the severity of COVID-19 patients: An observational study. Lipids Heal. Dis. 2020, 19, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Li, L.; Zhang, Q.; Clarke, R.; Chen, J.; Guo, Y.; Bian, Z.; Pan, X.; Peto, R.; Tao, R.; et al. Use of drug treatment for secondary prevention of cardiovascular disease in urban and rural communities of China: China Kadoorie Biobank Study of 0.5million people. Int. J. Cardiol. 2014, 172, 88–95. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Daniels, L.B.; Sitapati, A.M.; Zhang, J.; Zou, J.; Bui, Q.M.; Ren, J.; Longhurst, C.A.; Criqui, M.H.; Messer, K. Relation of Statin Use Prior to Admission to Severity and Recovery Among COVID-19 Inpatients. Am. J. Cardiol. 2020, 136, 149–155. [Google Scholar] [CrossRef]
- Abu-Farha, M.; Thanaraj, T.A.; Qaddoumi, M.G.; Hashem, A.; Abubaker, J.; Al-Mulla, F. The Role of Lipid Metabolism in COVID-19 Virus Infection and as a Drug Target. Int. J. Mol. Sci. 2020, 21, 3544. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, F.; Santos, R.D. Statins and COVID-19: To Suspend or Not to Suspend? That is the Question! Estatinas e COVID-19: Suspender ou não Suspender? Eis a Questão! Arq. Bras. Cardiol. 2021, 116, 147–152. [Google Scholar] [CrossRef]
- Kuba, K.; Imai, Y.; Rao, S.; Gao, H.; Guo, F.; Guan, B.; Huan, Y.; Yang, P.; Zhang, Y.; Deng, W.; et al. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus–induced lung injury. Nat. Med. 2005, 11, 875–879. [Google Scholar] [CrossRef] [PubMed]
- Yeung, M.L.; Teng, J.L.L.; Jia, L.; Zhang, C.; Huang, C.; Cai, J.-P.; Zhou, R.; Chan, K.-H.; Zhao, H.; Zhu, L.; et al. Soluble ACE2-mediated cell entry of SARS-CoV-2 via interaction with proteins related to the renin-angiotensin system. Cell 2021, 184, 2212–2228. [Google Scholar] [CrossRef]
- Silva, A.C.S.E.; Silveira, K.D.; Ferreira, A.J.; Teixeira, M.M. ACE2, angiotensin-(1-7) and Mas receptor axis in inflammation and fibrosis. Br. J. Pharmacol. 2013, 169, 477–492. [Google Scholar] [CrossRef] [Green Version]
- Gu, H.; Xie, Z.; Li, T.; Zhang, S.; Lai, C.; Zhu, P.; Wang, K.; Han, L.; Duan, Y.; Zhao, Z.; et al. Angiotensin-converting enzyme 2 inhibits lung injury induced by respiratory syncytial virus. Sci. Rep. 2016, 6, 19840. [Google Scholar] [CrossRef]
- Zoufaly, A.; Poglitsch, M.; Aberle, J.H.; Hoepler, W.; Seitz, T.; Traugott, M.; Grieb, A.; Pawelka, E.; Laferl, H.; Wenisch, C.; et al. Human recombinant soluble ACE2 in severe COVID-19. Lancet Respir. Med. 2020, 8, 1154–1158. [Google Scholar] [CrossRef]
- Monteil, V.; Kwon, H.; Prado, P.; Hagelkrüys, A.; Wimmer, R.A.; Stahl, M.; Leopoldi, A.; Garreta, E.; Del Pozo, C.H.; Prosper, F.; et al. Inhibition of SARS-CoV-2 Infections in Engineered Human Tissues Using Clinical-Grade Soluble Human ACE2. Cell 2020, 181, 905–913. [Google Scholar] [CrossRef]
- Tikoo, K.; Patel, G.; Kumar, S.; Karpe, P.A.; Sanghavi, M.; Malek, V.; Srinivasan, K. Tissue specific up regulation of ACE2 in rabbit model of atherosclerosis by atorvastatin: Role of epigenetic histone modifications. Biochem. Pharmacol. 2015, 93, 343–351. [Google Scholar] [CrossRef]
- Fukuda, K.; Matsumura, T.; Senokuchi, T.; Ishii, N.; Kinoshita, H.; Yamada, S.; Murakami, S.; Nakao, S.; Motoshima, H.; Kondo, T.; et al. Statins meditate anti-atherosclerotic action in smooth muscle cells by peroxisome proliferator-activated receptor-γ activation. Biochem. Biophys. Res. Commun. 2015, 457, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Fedson, D.S. Treating the host response to emerging virus diseases: Lessons learned from sepsis, pneumonia, influenza and Ebola. Ann. Transl. Med. 2016, 4, 421. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, L.; Lin, D.; Sun, X.; Curth, U.; Drosten, C.; Sauerhering, L.; Becker, S.; Rox, K.; Hilgenfeld, R. Crystal structure of SARS-CoV-2 main protease provides a basis for design of improved α-ketoamide inhibitors. Science 2020, 368, 409–412. [Google Scholar] [CrossRef] [Green Version]
- Rut, W.; Groborz, K.; Zhang, L.; Sun, X.; Zmudzinski, M.; Hilgenfeld, R.; Drag, M. Substrate specificity profiling of SARS-CoV-2 Mpro protease provides basis for anti-COVID-19 drug design. BioRxiv 2020. [Google Scholar] [CrossRef]
- Singh, P.; Sharma, A.; Nandi, S.P. Identification of Potent Inhibitors of COVID-19 Main Protease Enzyme by Molecular Docking Study. ChemRxiv 2020. [Google Scholar] [CrossRef]
- Kandeel, M.; Al-Nazawi, M. Virtual screening and repurposing of FDA approved drugs against COVID-19 main protease. Life Sci. 2020, 251, 117627. [Google Scholar] [CrossRef]
- Jin, Z.; Du, X.; Xu, Y.; Deng, Y.; Liu, M.; Zhao, Y.; Zhang, B.; Li, X.; Zhang, L.; Peng, C.; et al. Structure of Mpro from SARS-CoV-2 and discovery of its inhibitors. Nature 2020, 582, 289–293. [Google Scholar] [CrossRef] [Green Version]
- World Health Organization. Clinical Management of COVID-19. Available online: https://www.who.int/publications/i/item/clinical-management-of-covid-19 (accessed on 27 May 2020).
- Gubernatorova, E.; Gorshkova, E.; Polinova, A.; Drutskaya, M. IL-6: Relevance for immunopathology of SARS-CoV-2. Cytokine Growth Factor Rev. 2020, 53, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Coomes, E.A.; Haghbayan, H. Interleukin-6 in Covid-19: A systematic review and meta-analysis. Rev. Med. Virol. 2020, 30, 1–9. [Google Scholar] [CrossRef]
- Velazquez-Salinas, L.; Verdugo-Rodriguez, A.; Rodriguez, L.L.; Borca, M.V. The Role of Interleukin 6 during Viral Infections. Front. Microbiol. 2019, 10, 1057. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Toniati, P.; Piva, S.; Cattalini, M.; Garrafa, E.; Regola, F.; Castelli, F.; Franceschini, F.; Airò, P.; Bazzani, C.; Beindorf, E.-A.; et al. Tocilizumab for the treatment of severe COVID-19 pneumonia with hyperinflammatory syndrome and acute respiratory failure: A single center study of 100 patients in Brescia, Italy. Autoimmun. Rev. 2020, 19, 102568. [Google Scholar] [CrossRef]
- McGonagle, D.; Sharif, K.; O’Regan, A.; Bridgewood, C. The Role of Cytokines including Interleukin-6 in COVID-19 induced Pneumonia and Macrophage Activation Syndrome-Like Disease. Autoimmun. Rev. 2020, 19, 102537. [Google Scholar] [CrossRef]
- Henderson, L.A.; Cron, R.Q. Macrophage Activation Syndrome and Secondary Hemophagocytic Lymphohistiocytosis in Childhood Inflammatory Disorders: Diagnosis and Management. Pediatr. Drugs 2020, 22, 29–44. [Google Scholar] [CrossRef]
- Oesterle, A.; Laufs, U.; Liao, J.K. Pleiotropic Effects of Statins on the Cardiovascular System. Circ. Res. 2017, 120, 229–243. [Google Scholar] [CrossRef] [Green Version]
- Bonsu, K.O.; Reidpath, D.D.; Kadirvelu, A. Effects of Statin Treatment on Inflammation and Cardiac function in Heart Failure: An Adjusted Indirect Comparison Meta-analysis of Randomised Trials. Cardiovasc. Ther. 2015, 33, 338–346. [Google Scholar] [CrossRef] [Green Version]
- Xu, M.-J.; Liu, B.-J.; Wang, C.-L.; Wang, G.-H.; Tian, Y.; Wang, S.-H.; Li, J.; Li, P.-Y.; Zhang, R.-H.; Wei, D.; et al. Epigallocatechin-3-gallate inhibits TLR4 signaling through the 67-kDa laminin receptor and effectively alleviates acute lung injury induced by H9N2 swine influenza virus. Int. Immunopharmacol. 2017, 52, 24–33. [Google Scholar] [CrossRef]
- Choudhury, A.; Mukherjee, S. In silico studies on the comparative characterization of the interactions of SARS-CoV-2 spike glycoprotein with ACE-2 receptor homologs and human TLRs. J. Med Virol. 2020, 92, 2105–2113. [Google Scholar] [CrossRef]
- Wang, J.; Jiang, M.; Chen, X.; Montaner, L.J. Cytokine storm and leukocyte changes in mild versus severe SARS-CoV-2 infection: Review of 3939 COVID-19 patients in China and emerging pathogenesis and therapy concepts. J. Leukoc. Biol. 2020, 108, 17–41. [Google Scholar] [CrossRef]
- Prieto-Pérez, L.; Fortes, J.; Soto, C.; Vidal-González, Á.; Alonso-Riaño, M.; Lafarga, M.; Cortti, M.J.; Lazaro-Garcia, A.; Pérez-Tanoira, R.; Trascasa, Á.; et al. Histiocytic hyperplasia with hemophagocytosis and acute alveolar damage in COVID-19 infection. Mod. Pathol. 2020, 33, 2139–2146. [Google Scholar] [CrossRef] [PubMed]
- Soy, M.; Atagündüz, P.; Atagündüz, I.; Sucak, G.T. Hemophagocytic lymphohistiocytosis: A review inspired by the COVID-19 pandemic. Rheumatol. Int. 2021, 41, 7–18. [Google Scholar] [CrossRef] [PubMed]
- Härdtner, C.; Kornemann, J.; Krebs, K.; Ehlert, C.A.; Jander, A.; Zou, J.; Starz, C.; Rauterberg, S.; Sharipova, D.; Dufner, B.; et al. Inhibition of macrophage proliferation dominates plaque regression in response to cholesterol lowering. Basic Res. Cardiol. 2020, 115, 1–19. [Google Scholar] [CrossRef]
- Zhang, T.; Shao, B.; Liu, G.-A. Rosuvastatin promotes the differentiation of peripheral blood monocytes into M2 macrophages in patients with atherosclerosis by activating PPAR-γ. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 4464–4471. [Google Scholar] [PubMed]
- Fu, H.; Alabdullah, M.; Großmann, J.; Spieler, F.; Abdosh, R.; Lutz, V.; Kalies, K.; Knöpp, K.; Rieckmann, M.; Koch, S.; et al. The differential statin effect on cytokine production of monocytes or macrophages is mediated by differential geranylgeranylation-dependent Rac1 activation. Cell Death Dis. 2019, 10, 880. [Google Scholar] [CrossRef] [Green Version]
- Becker, R.C. COVID-19-associated vasculitis and vasculopathy. J. Thromb. Thrombolysis 2020, 50, 499–511. [Google Scholar] [CrossRef] [PubMed]
- Becker, R.C. COVID-19 update: Covid-19-associated coagulopathy. J. Thromb. Thrombolysis 2020, 50, 54–67. [Google Scholar] [CrossRef]
- Ackermann, M.; Verleden, S.E.; Kuehnel, M.; Haverich, A.; Welte, T.; Laenger, F.; Vanstapel, A.; Werlein, C.; Stark, H.; Tzankov, A.; et al. Pulmonary Vascular Endothelialitis, Thrombosis, and Angiogenesis in Covid-19. N. Engl. J. Med. 2020, 383, 120–128. [Google Scholar] [CrossRef]
- Magro, C.; Mulvey, J.J.; Berlin, D.; Nuovo, G.; Salvatore, S.; Harp, J.; Baxter-Stoltzfus, A.; Laurence, J. Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID-19 infection: A report of five cases. Transl. Res. 2020, 220, 1–13. [Google Scholar] [CrossRef]
- Sega, F.V.D.; Aquila, G.; Fortini, F.; Vaccarezza, M.; Secchiero, P.; Rizzo, P.; Campo, G. Context-dependent function of ROS in the vascular endothelium: The role of the Notch pathway and shear stress. BioFactors 2017, 43, 475–485. [Google Scholar] [CrossRef] [PubMed]
- Förstermann, U.; Xia, N.; Li, H. Roles of Vascular Oxidative Stress and Nitric Oxide in the Pathogenesis of Atherosclerosis. Circ. Res. 2017, 120, 713–735. [Google Scholar] [CrossRef] [PubMed]
- Cerda, A.; Fajardo, C.M.; Basso, R.G.; Hirata, M.H.; Hirata, R.D.C. Role of microRNAs 221/222 on Statin Induced Nitric Oxide Release in Human Endothelial Cells. Arq. Bras. Cardiol. 2014, 104, 195–200. [Google Scholar] [CrossRef]
- Förstermann, U.; Li, H. Therapeutic effect of enhancing endothelial nitric oxide synthase (eNOS) expression and preventing eNOS uncoupling. Br. J. Pharmacol. 2011, 164, 213–223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, D.; Kassab, G.S. Role of shear stress and stretch in vascular mechanobiology. J. R. Soc. Interface 2011, 8, 1379–1385. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bibli, S.-I.; Hu, J.; Leisegang, M.S.; Wittig, J.; Zukunft, S.; Kapasakalidi, A.; Fisstlhaller, B.; Tsilimigras, D.; Zografos, G.; Filis, K.; et al. Shear stress regulates cystathionine γ lyase expression to preserve endothelial redox balance and reduce membrane lipid peroxidation. Redox Biol. 2020, 28, 101379. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Xie, X.; Lei, T.; Zhang, K.; Lai, B.; Zhang, Z.; Guan, Y.; Mao, G.; Xiao, L.; Wang, N. Statins Attenuate Activation of the NLRP3 Inflammasome by Oxidized LDL or TNFα in Vascular Endothelial Cells through a PXR-Dependent Mechanism. Mol. Pharmacol. 2017, 92, 256–264. [Google Scholar] [CrossRef] [Green Version]
- Kou, F.; Zhu, C.; Wan, H.; Xue, F.; Wang, J.; Xiang, L.; Li, J. Endothelial progenitor cells as the target for cardiovascular disease prediction, personalized prevention, and treatments: Progressing beyond the state-of-the-art. EPMA J. 2020, 11, 629–643. [Google Scholar] [CrossRef]
- Oikonomou, E.; Siasos, G.; Zaromitidou, M.; Hatzis, G.; Mourouzis, K.; Chrysohoou, C.; Zisimos, K.; Mazaris, S.; Tourikis, P.; Athanasiou, D.; et al. Atorvastatin treatment improves endothelial function through endothelial progenitor cells mobilization in ischemic heart failure patients. Atherosclerosis 2015, 238, 159–164. [Google Scholar] [CrossRef]
- Al-Samkari, H.; Leaf, R.S.K.; Dzik, W.H.; Carlson, J.C.T.; Fogerty, A.E.; Waheed, A.; Goodarzi, K.; Bendapudi, P.K.; Bornikova, L.; Gupta, S.; et al. COVID-19 and coagulation: Bleeding and thrombotic manifestations of SARS-CoV-2 infection. Blood 2020, 136, 489–500. [Google Scholar] [CrossRef]
- Bompard, F.; Monnier, H.; Saab, I.; Tordjman, M.; Abdoul, H.; Fournier, L.; Sanchez, O.; Lorut, C.; Chassagnon, G.; Revel, M.-P. Pulmonary embolism in patients with COVID-19 pneumonia. Eur. Respir. J. 2020, 56, 2001365. [Google Scholar] [CrossRef]
- Sakr, Y.; Giovini, M.; Leone, M.; Pizzilli, G.; Kortgen, A.; Bauer, M.; Tonetti, T.; Duclos, G.; Zieleskiewicz, L.; Buschbeck, S.; et al. Pulmonary embolism in patients with coronavirus disease-2019 (COVID-19) pneumonia: A narrative review. Ann. Intensiv. Care 2020, 10, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Vadukul, P.; Sharma, D.S.; Vincent, P. Massive pulmonary embolism following recovery from COVID-19 infection: Inflammation, thrombosis and the role of extended thromboprophylaxis. BMJ Case Rep. 2020, 13, e238168. [Google Scholar] [CrossRef] [PubMed]
- Brummel-Ziedins, K.; Mann, K.; Undas, A. Anticoagulant effects of statins and their clinical implications. Thromb. Haemost. 2014, 111, 392–400. [Google Scholar] [CrossRef]
- Kronenberg, R.M.; Beglinger, S.; Stalder, O.; Méan, M.; Limacher, A.; Beer, J.H.; Aujesky, D.; Rodondi, N.; Feller, M. Statin therapy and recurrent venous thromboembolism in the elderly: A prospective cohort study. Sci. Rep. 2019, 9, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Biere-Rafi, S.; Hutten, B.A.; Squizzato, A.; Ageno, W.; Souverein, P.C.; De Boer, A.; Gerdes, V.E.; Büller, H.R.; Kamphuisen, P.W. Statin treatment and the risk of recurrent pulmonary embolism. Eur. Hear. J. 2013, 34, 1800–1806. [Google Scholar] [CrossRef] [Green Version]
- Sahebkar, A.; Catena, C.; Ray, K.; Vallejo-Vaz, A.; Reiner, Ž.; Sechi, L.; Colussi, G.; Vallejo-Vaz, A.J. Impact of statin therapy on plasma levels of plasminogen activator inhibitor-1. A systematic review and meta-analysis of randomised controlled trials. Thromb. Haemost. 2016, 116, 162–171. [Google Scholar] [CrossRef]
- Vasarmidi, E.; Tsitoura, E.; Spandidos, D.A.; Tzanakis, N.; Antoniou, K.M. Pulmonary fibrosis in the aftermath of the Covid-19 era (Review). Exp. Ther. Med. 2020, 20, 2557–2560. [Google Scholar] [CrossRef]
- Burnham, E.L.; Janssen, W.J.; Riches, D.W.H.; Moss, M.; Downey, G.P. The fibroproliferative response in acute respiratory distress syndrome: Mechanisms and clinical significance. Eur. Respir. J. 2014, 43, 276–285. [Google Scholar] [CrossRef] [Green Version]
- Burnham, E.L.; Hyzy, R.C.; Paine, R.; Coley, C.; Kelly, A.M.; Quint, L.E.; Lynch, D.; Janssen, W.J.; Moss, M.; Standiford, T.J. Chest CT Features are Associated With Poorer Quality of Life in Acute Lung Injury Survivors. Crit. Care Med. 2013, 41, 445–456. [Google Scholar] [CrossRef] [Green Version]
- Aydın, K.; Gülçelik, N.E.; Tuncel, M.; Balcı, C.; Akın, Ş.; Çınar, N.; Fırat, F.; Çağlar, M.; Usman, A.; Gürlek, A.; et al. Thyroid volumes and serum VEGF levels in dyslipidemic patients: Effects of statin treatment. Turk. J. Med Sci. 2019, 49, 738–745. [Google Scholar] [CrossRef] [PubMed]
- Calfee, C.S.; Delucchi, K.L.; Sinha, P.; Matthay, M.A.; Hackett, J.; Shankar-Hari, M.; McDowell, C.; Laffey, J.G.; O’Kane, C.M.; McAuley, D.F.; et al. Acute respiratory distress syndrome subphenotypes and differential response to simvastatin: Secondary analysis of a randomised controlled trial. Lancet Respir. Med. 2018, 6, 691–698. [Google Scholar] [CrossRef] [Green Version]
- Saito, A.; Horie, M.; Nagase, T. TGF-β Signaling in Lung Health and Disease. Int. J. Mol. Sci. 2018, 19, 2460. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, T.; Chen, M.; Sun, T. Simvastatin Attenuates TGF-β1-Induced Epithelial-Mesenchymal Transition in Human Alveolar Epithelial Cells. Cell. Physiol. Biochem. 2013, 31, 863–874. [Google Scholar] [CrossRef] [PubMed]
- Saewong, S.; Thammasitboon, K.; Wattanaroonwong, N. Simvastatin induces apoptosis and disruption of the actin cytoskeleton in human dental pulp cells and periodontal ligament fibroblasts. Arch. Oral Biol. 2013, 58, 964–974. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.W.; Rhee, C.K.; Kim, T.J.; Kim, Y.H.; Lee, S.H.; Yoon, H.K.; Kim, S.C.; Lee, S.Y.; Kwon, S.S.; Kim, K.H.; et al. Effect of pravastatin on bleomycin-induced acute lung injury and pulmonary fibrosis. Clin. Exp. Pharmacol. Physiol. 2010, 37, 1055–1063. [Google Scholar] [CrossRef] [PubMed]
- Kreuter, M.; Bonella, F.; Maher, T.M.; Costabel, U.; Spagnolo, P.; Weycker, D.; Kirchgaessler, K.-U.; Kolb, M. Effect of statins on disease-related outcomes in patients with idiopathic pulmonary fibrosis. Thorax 2016, 72, 148–153. [Google Scholar] [CrossRef] [Green Version]
- Turner, R.M.; Pirmohamed, M. Statin-Related Myotoxicity: A Comprehensive Review of Pharmacokinetic, Pharmacogenomic and Muscle Components. J. Clin. Med. 2019, 9, 22. [Google Scholar] [CrossRef] [Green Version]
- Wang, D.; Hu, B.; Hu, C.; Zhu, F.; Liu, X.; Zhang, J.; Wang, B.; Xiang, H.; Cheng, Z.; Xiong, Y.; et al. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus–Infected Pneumonia in Wuhan, China. JAMA 2020, 323, 1061–1069. [Google Scholar] [CrossRef]
- De Rosa, A.; Verrengia, E.P.; Merlo, I.; Rea, F.; Siciliano, G.; Corrao, G.; Prelle, A.C. Muscle manifestations and CK levels in COVID infection: Results of a large cohort of patients inside a Pandemic COVID-19 Area. Available online: https://doi.org/10.21203/rs.3.rs-98470/v1 (accessed on 17 April 2021).
- Anklesaria, Z.; Frankman, J.; Gordin, J.; Zhan, J.; Liu, A.K. Fatal Rhabdomyolysis in a COVID-19 Patient on Rosuvastatin. Cureus 2020, 12, e11186. [Google Scholar] [CrossRef]
- Iqbal, Z.; Ho, J.H.; Adam, S.; France, M.; Syed, A.; Neely, D.; Rees, A.; Khatib, R.; Cegla, J.; Byrne, C.; et al. Heart UK’s Medical Scientific and Research Committee (2020). Managing hyperlipidaemia in patients with COVID-19 and during its pandemic: An expert panel position statement from HEART UK. Atherosclerosis 2020, 313, 126–136. [Google Scholar] [CrossRef]
- Sattar, N.; Preiss, D.; Murray, H.M.; Welsh, P.; Buckley, B.M.; de Craen, A.J.; Seshasai, S.R.K.; McMurray, J.J.; Freeman, D.J.; Jukema, J.W.; et al. Statins and risk of incident diabetes: A collaborative meta-analysis of randomised statin trials. Lancet 2010, 375, 735–742. [Google Scholar] [CrossRef]
- Lim, S.; Bae, J.H.; Kwon, H.-S.; Nauck, M.A. COVID-19 and diabetes mellitus: From pathophysiology to clinical management. Nat. Rev. Endocrinol. 2021, 17, 11–30. [Google Scholar] [CrossRef] [PubMed]
- Saeed, O.; Castagna, F.; Agalliu, I.; Xue, X.; Patel, S.R.; Rochlani, Y.; Kataria, R.; Vukelic, S.; Sims, D.B.; Alvarez, C.; et al. Statin Use and In-Hospital Mortality in Patients With Diabetes Mellitus and COVID-19. J. Am. Hear. Assoc. 2020, 9, e018475. [Google Scholar] [CrossRef]
- Moon, A.M.; Barritt, A.S. Elevated Liver Enzymes in Patients with COVID-19: Look, but Not Too Hard. Dig. Dis. Sci. 2020, 1–3. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pawlos, A.; Niedzielski, M.; Gorzelak-Pabiś, P.; Broncel, M.; Woźniak, E. COVID-19: Direct and Indirect Mechanisms of Statins. Int. J. Mol. Sci. 2021, 22, 4177. https://doi.org/10.3390/ijms22084177
Pawlos A, Niedzielski M, Gorzelak-Pabiś P, Broncel M, Woźniak E. COVID-19: Direct and Indirect Mechanisms of Statins. International Journal of Molecular Sciences. 2021; 22(8):4177. https://doi.org/10.3390/ijms22084177
Chicago/Turabian StylePawlos, Agnieszka, Mateusz Niedzielski, Paulina Gorzelak-Pabiś, Marlena Broncel, and Ewelina Woźniak. 2021. "COVID-19: Direct and Indirect Mechanisms of Statins" International Journal of Molecular Sciences 22, no. 8: 4177. https://doi.org/10.3390/ijms22084177
APA StylePawlos, A., Niedzielski, M., Gorzelak-Pabiś, P., Broncel, M., & Woźniak, E. (2021). COVID-19: Direct and Indirect Mechanisms of Statins. International Journal of Molecular Sciences, 22(8), 4177. https://doi.org/10.3390/ijms22084177