The Role of Leptin in Fetal Growth during Pre-Eclampsia
Abstract
:1. Introduction
2. Pre-Eclampsia—A Severe Complication of Pregnancy
3. Fetal Growth Restriction in Pre-Eclampsia
4. An Introduction to Leptin and Its Receptors
5. Leptin and Fetal Growth in Normal Pregnancy
5.1. Maternal Leptin
5.2. Fetal Leptin
5.3. Placental Leptin
6. Leptin in Pre-Eclampsia
6.1. Maternal Leptin in PE
6.2. Fetal Leptin in PE
6.3. Placental Leptin in PE
7. Leptin and Fetal Growth in Pre-Eclampsia
7.1. Maternal Leptin and Fetal Growth in PE
7.2. Fetal Leptin and Fetal Growth in PE
7.3. Placental Leptin and Fetal Growth in PE
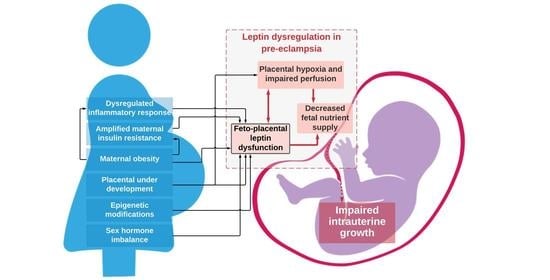
8. Mechanisms for Dysregulated Leptin in Pre-Eclampsia and Effects on Fetal Growth
- Leptin has auto- and paracrine roles in placental development and function where it regulates implantation and placentation and modulates functions in placental cells [4,51]. Abnormal trophoblast proliferation or invasion has been associated with abnormal placental leptin release in pregnancies complicated by FGR [89] and may also be involved in placental growth in PE.
- It has been suggested that placental production of leptin is augmented in PE because of placental hypoxia, which is a consequence of reduced placental perfusion (Figure 2) [75]. Hypoxia has been proven to be a positive regulator of placental leptin gene expression and production [75]. Placental hypoxia-inducible factor 1alpha (HIF1alpha) mRNA level and level of placental leptin mRNA expression are positively correlated [93], which implies that the rapid increase in maternal serum leptin levels seen in the third trimester of pre-eclamptic pregnancies [37] may be, at least in part, explained by placental hypoxia. Alternatively, abnormalities within HIF1alpha’s oxygen sensing properties, rather than hypoxia, may promote conditions for the development of PE [94]. Either way, it remains unclear what impact this has on fetal growth, as pregnancies complicated by FGR have not typically been characterized by high placental leptin mRNA expression and leptin protein levels [56], which suggests that pregnancies complicated by FGR do not suffer from placental hypoxia as in PE.
- 4.
- Exaggerated maternal hyperleptinemia in PE may be a compensatory response to impaired placental perfusion in order to boost nutrient delivery to the fetus [56] and is supported by findings of an increased placental leptin content and a positive correlation with the resistance index of the umbilical artery in both PE and FGR [81]. Furthermore, animal experiments with rats showed significantly increased maternal serum leptin levels in animals with reduced placental perfusion [95]. It has been hypothesized that PE occurs in women who cannot accommodate for or tolerate the exaggerated increase in leptin whilst FGR occurs in women who do not respond enough to compensatory hyperleptinemia [56].
- 5.
- Leptin is both associated with angiogenic molecules in PE [61] and is itself a angiogenic factor that can enhance vascular endothelial growth factor synthesis during pregnancy (Figure 3) [96]. Maternal serum leptin and endothelin-1 (a highly potent vasoconstrictor that is released in conditions of hypoxia) were found to be increased in women with PE and were correlated positively with the degree of FGR in women with PE [97]. The increase in leptin may be a response to under-perfusion of the placenta in an attempt to support neovascularization and improvement of nutrient delivery [2,4,61,81].
- 6.
- Increased insulin resistance from the second trimester and onwards ensures adequate nutrient supply to the growing fetus and is regulated by a range of maternal, placental, and fetal hormones [33]. However, in pregnancies complicated by PE, insulin resistance is amplified above the normal range [98]. Insulin is a positive regulator of leptin production [99], and it has been speculated that maternal hyperinsulinemia upregulates placental leptin gene expression and production in pre-eclamptic women (Figure 2) [56,100]. Furthermore, the development of insulin resistance can originate from adipose tissue in pre-eclamptic women with overweight or obesity [101]. Fetal growth may be affected by perturbed maternal energy balance and reduced glucose availability resulting from increased leptin and insulin resistance [87]. Metabolic disturbances involving leptin and insulin may also trigger a cascade of changes within compensatory mechanisms in immune, inflammatory, and endothelial pathways that ultimately end in placental insufficiency and FGR [102].
- 7.
- Overweight and obesity are known risk factors for PE [55,103]. Several studies have proposed that the normal relationship between serum leptin concentrations and adiposity is disrupted in PE and is potentiated by increasing BMI [62,69,72,104]. As in lean women, PE in maternal obesity is associated with FGR rather than fetal overgrowth, which otherwise is common in normotensive pregnancies [105]. There are several hypotheses as to how leptin might be involved in the increased risk of PE and FGR in overweight and obese women. In pregnant women with BMI values on the extreme ends of the scale, normal compensatory mechanisms may be inadequate in controlling metabolic homeostasis [72]. Increased insulin resistance originating from adipose tissue can be a contributing factor [101]. Placental insulin resistance may reduce amino acid transfer across the placenta [104]. Finally, obesity is itself an inflammatory condition where increased production of leptin, proinflammatory cytokines, and complement proteins from adipose tissue can aggravate endothelial dysfunction and cause reductions in placental perfusion [106].
- 8.
- An exaggerated maternal systemic inflammatory response to pregnancy with activation of both the innate and the adaptive immune system plays a central role in the pathogenesis of PE [107]. The increased shedding of syncytiotrophoblast microparticles from the placenta, persistent hypoxic conditions, and/or alterations in oxygen-sensing mechanisms in the placenta may promote activation of maternal leukocytes and endothelial cell dysfunction [108]. Class 1 cytokines, which induce inflammation, are upregulated and class 2 cytokines, which regulate inflammation, are down-regulated in PE [107]. Leptin has also been shown to play a role in immunity [109]. Inflammatory stimuli or immune dysfunction could alter maternal leptin expression in PE (Figure 2) [110]. Leptin is itself a class 1 cytokine with proinflammatory properties. There is evidence that inflammatory mediators increase serum leptin concentrations [111] and that leptin contributes to the increased circulating levels of pro-inflammatory mediators in PE [2,112].
- 9.
- Epigenetic modifications with differential placental and fetal gene expression for leptin and its receptors, as well as factors involved in cytokine and endothelin signaling pathways, protein modification, and regulation of JAK-STAT have been reported for both PE and FGR [6,84]. Furthermore, transmembrane leptin receptors in syncytiotrophoblast and trophoblast cells are accessible to maternal leptin (of both placental and maternal adipose origin) and may signal differently in pregnancies with increased leptin production [51]. Further, the increased expression of a soluble leptin receptor in cytotrophoblast cells of pre-eclamptic placentas may modify leptin-binding capabilities and may modulate free leptin levels [51]. Further examination of genetic variations in leptin and its receptors is warranted to understand the possible effect on fetal growth.
- 10.
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Masuzaki, H.; Ogawa, Y.; Sagawa, N.; Hosoda, K.; Matsumoto, T.; Mise, H.; Nishimura, H.; Yoshimasa, Y.; Tanaka, I.; Mori, T.; et al. Nonadipose tissue production of leptin: Leptin as a novel placenta-derived hormone in humans. Nat. Med. 1997, 3, 1029–1033. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Pérez, A.; Toro, A.; Vilariño-García, T.; Maymó, J.; Guadix, P.; Dueñas, J.L.; Fernández-Sánchez, M.; Varone, C.; Sánchez-Margalet, V. Leptin action in normal and pathological pregnancies. J. Cell. Mol. Med. 2017, 22, 716–727. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, E.; Duval, F.; Vialard, F.; Dieudonné, M.-N. The roles of leptin and adiponectin at the fetal-maternal interface in humans. Horm. Mol. Biol. Clin. Investig. 2015, 24, 47–63. [Google Scholar] [CrossRef] [PubMed]
- Mouzon, S.H.-D.; Lepercq, J.; Catalano, P. The known and unknown of leptin in pregnancy. Am. J. Obstet. Gynecol. 2006, 194, 1537–1545. [Google Scholar] [CrossRef]
- D’Ippolito, S.; Tersigni, C.; Scambia, G.; Di Simone, N. Adipokines, an adipose tissue and placental product with biological functions during pregnancy. BioFactors 2012, 38, 14–23. [Google Scholar] [CrossRef]
- Schanton, M.; Maymó, J.L.; Pérez-Pérez, A.; Sánchez-Margalet, V.; Varone, C.L. Involvement of leptin in the molecular physiology of the placenta. Reproduction 2018, 155, R1–R12. [Google Scholar] [CrossRef] [Green Version]
- Burton, G.J.; Redman, C.W.; Roberts, J.M.; Moffett, A. Pre-eclampsia: Pathophysiology and clinical implications. BMJ 2019, 366, l2381. [Google Scholar] [CrossRef] [Green Version]
- American College of Obstetricians and Gynecologists. Gestational Hypertension and Preeclampsia: ACOG Practice Bulletin Summary, Number 222. Obstet. Gynecol. 2020, 135, 1492–1495. [Google Scholar] [CrossRef]
- Hauspurg, A.; Countouris, M.E.; Catov, J.M. Hypertensive Disorders of Pregnancy and Future Maternal Health: How Can the Evidence Guide Postpartum Management? Curr. Hypertens. Rep. 2019, 21, 96. [Google Scholar] [CrossRef]
- Karatza, A.A.; Dimitriou, G. Preeclampsia Emerging as a Novel Risk Factor for Cardiovascular Disease in the Offspring. Curr. Pediatr. Rev. 2020, 16, 194–199. [Google Scholar] [CrossRef]
- Alonso-Ventura, V.; Li, Y.; Pasupuleti, V.; Roman, Y.M.; Hernandez, A.V.; Pérez-López, F.R. Effects of preeclampsia and eclampsia on maternal metabolic and biochemical outcomes in later life: A systematic review and meta-analysis. Metabolism 2020, 102, 154012. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brown, M.A.; Magee, L.A.; Kenny, L.C.; Karumanchi, S.A.; McCarthy, F.P.; Saito, S.; Hall, D.R.; Warren, C.E.; Adoyi, G.; Ishaku, S. Hypertensive Disorders of Pregnancy: ISSHP Classification, Diagnosis, and Management Recommendations for International Practice. Hypertension 2018, 72, 24–43. [Google Scholar] [CrossRef] [Green Version]
- Bartsch, E.; Medcalf, K.E.; Park, A.L.; Ray, J.G. High Risk of Pre-eclampsia Identification Group Clinical Risk Factors for Pre-Eclampsia Determined in Early Pregnancy: Systematic Review and Meta-Analysis of Large Cohort Studies. BMJ 2016, 353, i1753. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mol, B.W.J.; Roberts, C.T.; Thangaratinam, S.; Magee, L.A.; de Groot, C.J.M.; Hofmeyr, G.J. Pre-eclampsia. Lancet 2016, 387, 999–1011. [Google Scholar] [CrossRef]
- Roberts, J.; Hubel, C. The Two Stage Model of Preeclampsia: Variations on the Theme. Placenta 2009, 30, 32–37. [Google Scholar] [CrossRef] [Green Version]
- Roberts, J.M.; Escudero, C. The placenta in preeclampsia. Pregnancy Hypertens. 2012, 2, 72–83. [Google Scholar] [CrossRef] [Green Version]
- Burton, G.J.; Jauniaux, E. Pathophysiology of placental-derived fetal growth restriction. Am. J. Obstet. Gynecol. 2018, 218, S745–S761. [Google Scholar] [CrossRef] [Green Version]
- Resnik, R. Intrauterine growth restriction. Obstet. Gynecol. 2002, 99, 490–496. [Google Scholar] [CrossRef]
- Maršál, K.; Persson, P.-H.; Larsen, T.; Lilja, H.; Selbing, A.; Sultan, B. Intrauterine growth curves based on ultrasonically estimated foetal weights. Acta Paediatr. 1996, 85, 843–848. [Google Scholar] [CrossRef]
- Zhang, S.; Regnault, T.R.; Barker, P.L.; Botting, K.J.; McMillen, I.C.; McMillan, C.M.; Roberts, C.T.; Morrison, J.L. Placental Adaptations in Growth Restriction. Nutrients 2015, 7, 360–389. [Google Scholar] [CrossRef] [Green Version]
- Villar, J.; Carroli, G.; Wojdyla, D.; Abalos, E.; Giordano, D.; Ba’Aqeel, H.; Farnot, U.; Bergsjø, P.; Bakketeig, L.; Lumbiganon, P.; et al. Preeclampsia, gestational hypertension and intrauterine growth restriction, related or independent conditions? Am. J. Obstet. Gynecol. 2006, 194, 921–931. [Google Scholar] [CrossRef]
- Crispi, F.; Llurba, E.; Domínguez, C.; Martín-Gallán, P.; Cabero, L.; Gratacós, E. Predictive value of angiogenic factors and uterine artery Doppler for early- versus late-onset pre-eclampsia and intrauterine growth restriction. Ultrasound Obstet. Gynecol. 2008, 31, 303–309. [Google Scholar] [CrossRef]
- Egbor, M.; Ansari, T.; Morris, N.H.; Green, C.J.; Sibbons, P.D. Maternal medicine: Morphometric placental villous and vascular abnormalities in early- and late-onset pre-eclampsia with and without fetal growth restriction. BJOG Int. J. Obstet. Gynaecol. 2006, 113, 580–589. [Google Scholar] [CrossRef]
- Ness, R.B.; Sibai, B.M. Shared and disparate components of the pathophysiologies of fetal growth restriction and preeclampsia. Am. J. Obstet. Gynecol. 2006, 195, 40–49. [Google Scholar] [CrossRef] [PubMed]
- Maršál, K. Preeclampsia and intrauterine growth restriction: Placental disorders still not fully understood. J. Périnat. Med. 2017, 45, 775–777. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Proenca, R.; Maffei, M.; Barone, M.; Leopold, L.; Friedman, J.M. Positional cloning of the mouse obese gene and its human homologue. Nat. Cell Biol. 1994, 372, 425–432. [Google Scholar] [CrossRef] [PubMed]
- Münzberg, H.; Morrison, C.D. Structure, production and signaling of leptin. Metabolism 2015, 64, 13–23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fasshauer, M.; Blüher, M. Adipokines in health and disease. Trends Pharmacol. Sci. 2015, 36, 461–470. [Google Scholar] [CrossRef]
- Nieuwenhuis, D.; Pujol-Gualdo, N.; Arnoldussen, I.A.; Kiliaan, A.J. Adipokines: A gear shift in puberty. Obes. Rev. 2020, 21, e13005. [Google Scholar] [CrossRef] [Green Version]
- Childs, G.V.; Odle, A.K.; MacNicol, M.C.; MacNicol, A.M. The Importance of Leptin to Reproduction. Endocrinology 2021, 162. [Google Scholar] [CrossRef]
- Sopjani, M.; Morina, R.; Uka, V.; Xuan, N.T.; Dërmaku-Sopjani, M. JAK2-mediated intracellular signaling. Curr. Mol. Med. 2020, 20, 1–9. [Google Scholar] [CrossRef]
- Hegyi, K.; Fülöp, K.; Kovacs, K.; Tóth, S.; Falus, A. Leptin-induced signal transduction pathways. Cell Biol. Int. 2004, 28, 159–169. [Google Scholar] [CrossRef]
- Aung, Z.K.; Grattan, D.; Ladyman, S. Pregnancy-induced adaptation of central sensitivity to leptin and insulin. Mol. Cell. Endocrinol. 2020, 516, 110933. [Google Scholar] [CrossRef] [PubMed]
- Von Versen-Höynck, F.; Rajakumar, A.; Parrott, M.; Powers, R. Leptin Affects System A Amino Acid Transport Activity in the Human Placenta: Evidence for STAT3 Dependent Mechanisms. Placenta 2009, 30, 361–367. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lepercq, J.; Challier, J.-C.; Guerre-Millo, M.; Cauzac, M.; Vidal, H.; Mouzon, S.H.-D. Prenatal Leptin Production: Evidence That Fetal Adipose Tissue Produces Leptin. J. Clin. Endocrinol. Metab. 2001, 86, 2409–2413. [Google Scholar] [CrossRef] [PubMed]
- Briffa, J.F.; McAinch, A.J.; Romano, T.; Wlodek, M.E.; Hryciw, D.H. Leptin in pregnancy and development: A contributor to adulthood disease? Am. J. Physiol. Metab. 2015, 308, E335–E350. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anim-Nyame, N.; Sooranna, S.R.; Steer, P.J.; Johnson, M.R. Longitudinal analysis of maternal plasma leptin concentrations during normal pregnancy and pre-eclampsia. Hum. Reprod. 2000, 15, 2033–2036. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Linnemann, K.; Malek, A.; Sager, R.; Blum, W.F.; Schneider, H.; Fusch, C. Leptin Production and Release in the Duallyin VitroPerfused Human Placenta1. J. Clin. Endocrinol. Metab. 2000, 85, 4298–4301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jaquet, D.; Leger, J.; Levy-Marchal, C.; Oury, J.F.; Czernichow, P. Ontogeny of Leptin in Human Fetuses and Newborns: Effect of Intrauterine Growth Retardation on Serum Leptin Concentrations. J. Clin. Endocrinol. Metab. 1998, 83, 1243–1246. [Google Scholar] [CrossRef]
- Lausten-Thomsen, U.; Christiansen, M.; Hedley, P.L.; Holm, J.-C.; Schmiegelow, K. Adipokines in umbilical cord blood from children born large for gestational age. J. Pediatr. Endocrinol. Metab. 2015, 29, 33–37. [Google Scholar] [CrossRef]
- Cetin, I.; Morpurgo, P.S.; Radaelli, T.; Taricco, E.; Cortelazzi, D.; Bellotti, M.; Pardi, G.; Beck-Peccoz, P. Fetal Plasma Leptin Concentrations: Relationship with Different Intrauterine Growth Patterns from 19 Weeks to Term. Pediatr. Res. 2000, 48, 646–651. [Google Scholar] [CrossRef] [Green Version]
- Karakosta, P.; Georgiou, V.; Fthenou, E.; Papadopoulou, E.; Roumeliotaki, T.; Margioris, A.; Castanas, E.; Kampa, M.; Kogevinas, M.; Chatzi, L. Maternal Weight Status, Cord Blood Leptin and Fetal Growth: A Prospective Mother-Child Cohort Study (Rhea Study). Paediatr. Périnat. Epidemiol. 2013, 27, 461–471. [Google Scholar] [CrossRef]
- Helland, I.B.; Reseland, J.E.; Saugstad, O.D.; Drevon, C.A. Leptin Levels in Pregnant Women and Newborn Infants: Gender Differences and Reduction During the Neonatal Period. Pediatrics 1998, 101, e12. [Google Scholar] [CrossRef] [Green Version]
- Wabitsch, M.; Blum, W.F.; Muche, R.; Braun, M.; Hube, F.; Rascher, W.; Heinze, E.; Teller, W.; Hauner, H. Contribution of androgens to the gender difference in leptin production in obese children and adolescents. J. Clin. Investig. 1997, 100, 808–813. [Google Scholar] [CrossRef] [Green Version]
- Matsuda, J.; Yokota, I.; Iida, M.; Murakami, T.; Naito, E.; Ito, M.; Shima, K.; Kuroda, Y. Serum Leptin Concentration in Cord Blood: Relationship to Birth Weight and Gender. J. Clin. Endocrinol. Metab. 1997, 82, 1642. [Google Scholar] [CrossRef]
- Yura, S.; Sagawa, N.; Mise, H.; Mori, T.; Masuzaki, H.; Ogawa, Y.; Nakao, K. A positive umbilical venous-arterial difference of leptin level and its rapid decline after birth. Am. J. Obstet. Gynecol. 1998, 178, 926–930. [Google Scholar] [CrossRef]
- Laml, T.; Hartmann, B.W.; Ruecklinger, E.; Preyer, O.; Soeregi, G.; Wagenbichler, P. Maternal Serum Leptin Concentrations Do Not Correlate with Cord Blood Leptin Concentrations in Normal Pregnancy. J. Soc. Gynecol. Investig. 2001, 8, 43–47. [Google Scholar] [CrossRef]
- Andersson-Hall, U.K.; Pivodic, A.; De Maré, H.K.; Svedin, J.P.; Mallard, E.C.; Albertsson-Wikland, K.G.; Holmäng, A.B. Infant body composition relationship to maternal adipokines and fat mass: The PONCH study. Pediatr. Res. 2020, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Walsh, J.M.; Byrne, J.; Mahony, R.M.; Foley, M.E.; McAuliffe, F.M. Leptin, fetal growth and insulin resistance in non-diabetic pregnancies. Early Hum. Dev. 2014, 90, 271–274. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Pérez, A.; Sánchez-Jiménez, F.; Maymó, J.; Dueñas, J.L.; Varone, C.; Sánchez-Margalet, V. Role of leptin in female reproduction. Clin. Chem. Lab. Med. 2015, 53, 15–28. [Google Scholar] [CrossRef] [PubMed]
- Challier, J.; Galtier, M.; Bintein, T.; Cortez, A.; Lepercq, J.; Mouzon, S.H.-D. Placental leptin receptor isoforms in normal and pathological pregnancies. Placenta 2003, 24, 92–99. [Google Scholar] [CrossRef]
- Dotsch, J.; Nüsken, K.-D.; Knerr, I.; Kirschbaum, M.; Repp, R.; Rascher, W. Leptin and Neuropeptide Y Gene Expression in Human Placenta: Ontogeny and Evidence for Similarities to Hypothalamic Regulation. J. Clin. Endocrinol. Metab. 1999, 84, 2755–2758. [Google Scholar] [CrossRef] [Green Version]
- Gutaj, P.; Sibiak, R.; Jankowski, M.; Awdi, K.; Bryl, R.; Mozdziak, P.; Kempisty, B.; Wender-Ozegowska, E. The Role of the Adipokines in the Most Common Gestational Complications. Int. J. Mol. Sci. 2020, 21, 9408. [Google Scholar] [CrossRef]
- Henson, M.C.; Castracane, V.D. Leptin in Pregnancy: An Update1. Biol. Reprod. 2006, 74, 218–229. [Google Scholar] [CrossRef] [Green Version]
- Hendler, I.; Blackwell, S.C.; Mehta, S.H.; Whitty, J.E.; Russell, E.; Sorokin, Y.; Cotton, D.B. The levels of leptin, adiponectin, and resistin in normal weight, overweight, and obese pregnant women with and without preeclampsia. Am. J. Obstet. Gynecol. 2005, 193, 979–983. [Google Scholar] [CrossRef] [PubMed]
- Laivuori, H.; Gallaher, M.; Collura, L.; Crombleholme, W.; Markovic, N.; Rajakumar, A.; Hubel, C.; Roberts, J.; Powers, R. Relationships between maternal plasma leptin, placental leptin mRNA and protein in normal pregnancy, pre-eclampsia and intrauterine growth restriction without pre-eclampsia. Mol. Hum. Reprod. 2006, 12, 551–556. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mise, H.; Yura, S.; Itoh, H.; Nuamah, M.A.; Takemura, M.; Sagawa, N.; Fujii, S. The Relationship between Maternal Plasma Leptin Levels and Fetal Growth Restriction. Endocr. J. 2007, 54, 945–951. [Google Scholar] [CrossRef] [Green Version]
- Masuyama, H.; Segawa, T.; Sumida, Y.; Masumoto, A.; Inoue, S.; Akahori, Y.; Hiramatsu, Y. Different profiles of circulating angiogenic factors and adipocytokines between early- and late-onset pre-eclampsia. BJOG Int. J. Obstet. Gynaecol. 2009, 117, 314–320. [Google Scholar] [CrossRef] [PubMed]
- El Shahat, A.M.; Ahmed, A.B.; Ahmed, M.R.; Mohamed, H.S. Maternal serum leptin as a marker of preeclampsia. Arch. Gynecol. Obstet. 2013, 288, 1317–1322. [Google Scholar] [CrossRef] [PubMed]
- Salimi, S.; Farajian-Mashhadi, F.; Naghavi, A.; Mokhtari, M.; Shahrakipour, M.; Saravani, M.; Yaghmaei, M. Different Profile of Serum Leptin between Early Onset and Late Onset Preeclampsia. Dis. Markers 2014, 2014, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Eleuterio, N.M.; Palei, A.C.T.; Machado, J.S.R.; Tanus-Santos, J.E.; Cavalli, R.C.; Sandrim, V.C. Correlations between circulating levels of adipokines and anti-angiogenic factors in women with BMI <30 and a Late-Onset Preeclampsia. Hypertens. Pregnancy 2014, 33, 72–80. [Google Scholar] [CrossRef]
- Song, Y.; Gao, J.; Qu, Y.; Wang, S.; Wang, X.; Liu, J. Serum levels of leptin, adiponectin and resistin in relation to clinical characteristics in normal pregnancy and preeclampsia. Clin. Chim. Acta 2016, 458, 133–137. [Google Scholar] [CrossRef]
- Kharb, S.; Bala, J.; Nanda, S. Markers of obesity and growth in preeclamptic and normotensive pregnant women. J. Obstet. Gynaecol. 2017, 37, 610–615. [Google Scholar] [CrossRef] [PubMed]
- Kharb, S.; Nanda, S. Patterns of Biomarkers in Cord Blood During Pregnancy and Preeclampsia. Curr. Hypertens. Rev. 2017, 13, 1. [Google Scholar] [CrossRef]
- Laml, T.; Preyer, O.; Hartmann, B.W.; Ruecklinger, E.; Soeregi, G.; Wagenbichler, P. Decreased Maternal Serum Leptin in Pregnancies Complicated by Preeclampsia. J. Soc. Gynecol. Investig. 2001, 8, 89–93. [Google Scholar] [CrossRef]
- Kafulafula, G.E.; Moodley, J.; Ojwang, P.J.; Kagoro, H. Leptin and pre-eclampsia in Black African parturients. BJOG Int. J. Obstet. Gynaecol. 2002, 109, 1256–1261. [Google Scholar] [CrossRef]
- Doster, Y.; Demir, B.C.; Atalay, M.A.; Durusoy, E.E.; Kucukkomurcu, S. The possible role of serum leptin in preeclampsia. Clin. Exp. Obstet. Gynecol. 2016, 43, 98–102. [Google Scholar]
- Asnafi, N.; Sharbatdaran, M.; Hajian, K. Comparison of maternal and neonatal serum leptin levels in preeclampsia and normal pregnancy. Iran. J. Reprod. Med. 2011, 9, 131–134. [Google Scholar]
- Taylor, B.D.; Ness, R.B.; Olsen, J.; Hougaard, D.M.; Skogstrand, K.; Roberts, J.M.; Haggerty, C.L. Serum Leptin Measured in Early Pregnancy Is Higher in Women with Preeclampsia Compared with Normotensive Pregnant Women. Hypertension 2015, 65, 594–599. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hedley, P.L.; Placing, S.; Wøjdemann, K.; Carlsen, A.L.; Shalmi, A.-C.; Sundberg, K.; Tabor, A.; Christiansen, M. Free leptin index and PAPP-A: A first trimester maternal serum screening test for pre-eclampsia. Prenat. Diagn. 2009, 30, 103–109. [Google Scholar] [CrossRef]
- Papastefanou, I.; Samolis, S.; Panagopoulos, P.; Tagia, M.; Bale, C.; Kouskoukis, A.; Galazios, G. Correlation between maternal first trimester plasma leptin levels and birth weight among normotensive and preeclamptic women. J. Matern. Neonatal Med. 2010, 23, 1435–1443. [Google Scholar] [CrossRef]
- Thagaard, I.N.; Hedley, P.L.; Holm, J.-C.; Lange, T.; Larsen, T.; Krebs, L.; Christiansen, M. Leptin and Adiponectin as markers for preeclampsia in obese pregnant women, a cohort study. Pregnancy Hypertens. 2019, 15, 78–83. [Google Scholar] [CrossRef]
- Savvidou, M.D.; Sotiriadis, A.; Kaihura, C.; Nicolaides, K.H.; Sattar, N. Circulating levels of adiponectin and leptin at 23–25 weeks of pregnancy in women with impaired placentation and in those with established fetal growth restriction. Clin. Sci. 2008, 115, 219–224. [Google Scholar] [CrossRef] [Green Version]
- McCarthy, J.F.; Misra, D.N.; Roberts, J.M. Maternal plasma leptin is increased in preeclampsia and positively correlates with fetal cord concentration. Am. J. Obstet. Gynecol. 1999, 180, 731–736. [Google Scholar] [CrossRef]
- Mise, H.; Sagawa, N.; Matsumoto, T.; Yura, S.; Nanno, H.; Itoh, H.; Mori, T.; Masuzaki, H.; Hosoda, K.; Ogawa, Y.; et al. Augmented Placental Production of Leptin in Preeclampsia: Possible Involvement of Placental Hypoxia 1. J. Clin. Endocrinol. Metab. 1998, 83, 3225–3229. [Google Scholar] [CrossRef]
- Anato, V.; Garmendia, J.V.; Bianco, N.E.; De Sanctis, J.B. Serum leptin levels in different types of hypertension during pregnancy. Res. Commun. Mol. Pathol. Pharmacol. 2000, 108, 147–153. [Google Scholar]
- Dalamaga, M.; Srinivas, S.K.; Elovitz, M.A.; Chamberland, J.; Mantzoros, C.S. Serum adiponectin and leptin in relation to risk for preeclampsia: Results from a large case-control study. Metabolism 2011, 60, 1539–1544. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jenkins, L.D.; Powers, R.W.; Adotey, M.; Gallaher, M.J.; Markovic, N.; Ness, R.B.; Roberts, J.M. Maternal Leptin Concentrations are Similar in African Americans and Caucasians in Normal Pregnancy, Preeclampsia and Small-for-Gestational-Age Infants. Hypertens. Pregnancy 2007, 26, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Weedon-Fekjær, M.; Sheng, Y.; Sugulle, M.; Johnsen, G.; Herse, F.; Redman, C.; Lyle, R.; Dechend, R.; Staff, A. Placental miR-1301 is dysregulated in early-onset preeclampsia and inversely correlated with maternal circulating leptin. Placenta 2014, 35, 709–717. [Google Scholar] [CrossRef] [PubMed]
- Dı́az, E.; Halhali, A.; Luna, C.; Dı́az, L.; Avila, E.; Larrea, F. Newborn Birth Weight Correlates with Placental Zinc, Umbilical Insulin-Like Growth Factor I, and Leptin Levels in Preeclampsia. Arch. Med Res. 2002, 33, 40–47. [Google Scholar] [CrossRef]
- Lepercq, J.; Guerre-Millo, M.; André, J.; Caüzac, M.; Mouzon, S.H.-D. Leptin: A potential marker of placental insufficiency. Gynecol. Obstet. Investig. 2003, 55, 151–155. [Google Scholar] [CrossRef] [PubMed]
- Ødegård, R.A.; Vatten, L.J.; Nilsen, S.T.; Salvesen, K.Å.; Austgulen, R. Umbilical cord plasma leptin is increased in preeclampsia. Am. J. Obstet. Gynecol. 2002, 186, 427–432. [Google Scholar] [CrossRef] [PubMed]
- Nishizawa, H.; Pryor-Koishi, K.; Kato, T.; Kowa, H.; Kurahashi, H.; Udagawa, Y. Microarray Analysis of Differentially Expressed Fetal Genes in Placental Tissue Derived from Early and Late Onset Severe Pre-eclampsia. Placenta 2007, 28, 487–497. [Google Scholar] [CrossRef] [PubMed]
- Sitras, V.; Paulssen, R.; Grønaas, H.; Leirvik, J.; Hanssen, T.; Vårtun, Å.; Acharya, G. Differential Placental Gene Expression in Severe Preeclampsia. Placenta 2009, 30, 424–433. [Google Scholar] [CrossRef]
- Yildiz, L.; Avci, B.; Ingec, M. Umbilical Cord and Maternal Blood Leptin Concentrations in Intrauterine Growth Retardation. Clin. Chem. Lab. Med. 2002, 40, 1114–1117. [Google Scholar] [CrossRef] [PubMed]
- Pighetti, M.; Tommaselli, G.A.; D’Elia, A.; Di Carlo, C.; Mariano, A.; Di Carlo, A.; Nappi, C. Maternal serum and umbilical cord blood leptin concentrations with fetal growth restriction. Obstet. Gynecol. 2003, 102, 535–543. [Google Scholar] [CrossRef]
- Sagawa, N.; Yura, S.; Itoh, H.; Mise, H.; Kakui, K.; Korita, D.; Takemura, M.; Nuamah, M.; Ogawa, Y.; Masuzaki, H.; et al. Role of Leptin in Pregnancy—A Review. Placenta 2002, 23, S80–S86. [Google Scholar] [CrossRef]
- Krishna, R.G.; Bhat, B.V. Molecular mechanisms of intrauterine growth restriction. J. Matern. Neonatal Med. 2017, 31, 2634–2640. [Google Scholar] [CrossRef]
- Lea, R.G.; Howe, D.; Hannah, L.T.; Bonneau, O.; Hunter, L.; Hoggard, N. Placental leptin in normal, diabetic and fetal growth-retarded pregnancies. Mol. Hum. Reprod. 2000, 6, 763–769. [Google Scholar] [CrossRef]
- Wang, C.-N.; Chang, S.-D.; Peng, H.-H.; Lee, Y.-S.; Chang, Y.-L.; Cheng, P.-J.; Chao, A.-S.; Wang, T.-H.; Wang, H.-S. Change in Amniotic Fluid Levels of Multiple Anti-Angiogenic Proteins before Development of Preeclampsia and Intrauterine Growth Restriction. J. Clin. Endocrinol. Metab. 2010, 95, 1431–1441. [Google Scholar] [CrossRef] [Green Version]
- Scott-Finley, M.; Woo, J.G.; Habli, M.; Ramos-Gonzales, O.; Cnota, J.; Wang, Y.; Kamathrayne, B.D.; Hinton, A.C.; Polzin, W.; Crombleholme, T.M.; et al. Standardization of amniotic fluid leptin levels and utility in maternal overweight and fetal undergrowth. J. Perinatol. 2015, 35, 547–552. [Google Scholar] [CrossRef]
- White, V.; González, E.; Capobianco, E.; Pustovrh, C.; Martínez, N.; Higa, R.; Baier, M.; Jawerbaum, A. Leptin modulates nitric oxide production and lipid metabolism in human placenta. Reprod. Fertil. Dev. 2006, 18, 425–432. [Google Scholar] [CrossRef] [PubMed]
- Iwagaki, S.; Yokoyama, Y.; Tang, L.; Takahashi, Y.; Nakagawa, Y.; Tamaya, T. Augmentation of leptin and hypoxia-inducible factor 1α? mRNAs in the pre-eclamptic placenta. Gynecol. Endocrinol. 2004, 18, 263–268. [Google Scholar] [CrossRef] [PubMed]
- Rolfo, A.; Many, A.; Racano, A.; Tal, R.; Tagliaferro, A.; Ietta, F.; Wang, J.; Post, M.; Caniggia, I. Abnormalities in Oxygen Sensing Define Early and Late Onset Preeclampsia as Distinct Pathologies. PLoS ONE 2010, 5, e13288. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moore, L.E.; Wallace, K.L.; Alexander, B.T.; May, W.L.; Thigpen, B.D.; Bennett, W.A. Reduced Placental Perfusion Causes an Increase in Maternal Serum Leptin. Placenta 2003, 24, 877–881. [Google Scholar] [CrossRef]
- Islami, D.; Bischof, P.; Chardonnens, D. Modulation of placental vascular endothelial growth factor by leptin and hCG. Mol. Hum. Reprod. 2003, 9, 395–398. [Google Scholar] [CrossRef] [Green Version]
- Nezar, M.A.-S.; El-Baky, A.M.A.; Soliman, O.A.-S.; Abdel-Hady, H.A.-S.; Hammad, A.M.; Al-Haggar, M.S. Endothelin-1 and leptin as markers of intrauterine growth restriction. Indian J. Pediatr. 2009, 76, 485–488. [Google Scholar] [CrossRef] [PubMed]
- Özkan, S.; Erel, C.T.; Madazli, R.; Aydınlı, K. Serum leptin levels in hypertensive disorder of pregnancy. Eur. J. Obstet. Gynecol. Reprod. Biol. 2005, 120, 158–163. [Google Scholar] [CrossRef]
- Briana, D.D.; Malamitsi-Puchner, A. Reviews: Adipocytokines in Normal and Complicated Pregnancies. Reprod. Sci. 2009, 16, 921–937. [Google Scholar] [CrossRef]
- Laivuori, H.; Kaaja, R.; Koistinen, H.; Karonen, S.-L.; Andersson, S.; Koivisto, V.; Ylikorkala, O. Leptin during and after preeclamptic or normal pregnancy: Its relation to serum insulin and insulin sensitivity. Metabolism 2000, 49, 259–263. [Google Scholar] [CrossRef]
- Spradley, F.T. Metabolic abnormalities and obesity’s impact on the risk for developing preeclampsia. Am. J. Physiol. Integr. Comp. Physiol. 2017, 312, R5–R12. [Google Scholar] [CrossRef] [Green Version]
- Anderson, C.M.; Ren, J. Leptin, leptin resistance and endothelial dysfunction in pre-eclampsia. Cell. Mol. Boil. 2002, 48, OL323-9. [Google Scholar]
- Chrelias, G.; Makris, G.-M.; Papanota, A.-M.; Spathis, A.; Salamalekis, G.; Sergentanis, T.N.; Rizos, D.; Karakitsos, P.; Chrelias, C. Serum inhibin and leptin: Risk factors for pre-eclampsia? Clin. Chim. Acta 2016, 463, 84–87. [Google Scholar] [CrossRef]
- Farley, D.; Choi, J.; Dudley, D.; Li, C.; Jenkins, S.; Myatt, L.; Nathanielsz, P. Placental Amino Acid Transport and Placental Leptin Resistance in Pregnancies Complicated by Maternal Obesity. Placenta 2010, 31, 718–724. [Google Scholar] [CrossRef]
- Howell, K.R.; Powell, T.L. Effects of maternal obesity on placental function and fetal development. Reproduction 2017, 153, R97–R108. [Google Scholar] [CrossRef] [PubMed]
- Olson, K.N.; Redman, L.M.; Sones, J.L. Obesity “complements” preeclampsia. Physiol. Genom. 2019, 51, 73–76. [Google Scholar] [CrossRef] [PubMed]
- Saito, S.; Shiozaki, A.; Nakashima, A.; Sakai, M.; Sasaki, Y. The role of the immune system in preeclampsia. Mol. Asp. Med. 2007, 28, 192–209. [Google Scholar] [CrossRef]
- Laresgoiti-Servitje, E. A leading role for the immune system in the pathophysiology of preeclampsia. J. Leukoc. Biol. 2013, 94, 247–257. [Google Scholar] [CrossRef]
- Matarese, G.; Moschos, S.; Mantzoros, C.S. Leptin in Immunology. J. Immunol. 2005, 174, 3137–3142. [Google Scholar] [CrossRef] [Green Version]
- Redman, C.W.G.; Sargent, I.L. Immunology of Pre-Eclampsia. Am. J. Reprod. Immunol. 2010, 63, 534–543. [Google Scholar] [CrossRef]
- Chardonnens, D.; Cameo, P.; Aubert, M.L.; Pralong, F.P.; Islami, D.; Campana, A.; Gaillard, R.C.; Bischof, P. Modulation of human cytotrophoblastic leptin secretion by interleukin-1alpha and 17beta-oestradiol and its effect on HCG secretion. Mol. Hum. Reprod. 1999, 5, 1077–1082. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharma, A.; Satyam, A.; Sharma, J.B. Leptin, IL-10 and Inflammatory Markers (TNF-Alpha, IL-6 and IL-8) in Pre-Eclamptic, Normotensive Pregnant and Healthy Non-Pregnant Women. Am. J. Reprod. Immunol. 2007, 58, 21–30. [Google Scholar] [CrossRef]
- Atamer, Y.; Erden, A.C.; Demir, B.; Koçyigit, Y.; Atamer, A. The relationship between plasma levels of leptin and androgen in healthy and preeclamptic pregnant women. Acta Obstet. Gynecol. Scand. 2004, 83, 425–430. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Knegt, V.E.; Hedley, P.L.; Kanters, J.K.; Thagaard, I.N.; Krebs, L.; Christiansen, M.; Lausten-Thomsen, U. The Role of Leptin in Fetal Growth during Pre-Eclampsia. Int. J. Mol. Sci. 2021, 22, 4569. https://doi.org/10.3390/ijms22094569
de Knegt VE, Hedley PL, Kanters JK, Thagaard IN, Krebs L, Christiansen M, Lausten-Thomsen U. The Role of Leptin in Fetal Growth during Pre-Eclampsia. International Journal of Molecular Sciences. 2021; 22(9):4569. https://doi.org/10.3390/ijms22094569
Chicago/Turabian Stylede Knegt, Victoria E., Paula L. Hedley, Jørgen K. Kanters, Ida N. Thagaard, Lone Krebs, Michael Christiansen, and Ulrik Lausten-Thomsen. 2021. "The Role of Leptin in Fetal Growth during Pre-Eclampsia" International Journal of Molecular Sciences 22, no. 9: 4569. https://doi.org/10.3390/ijms22094569
APA Stylede Knegt, V. E., Hedley, P. L., Kanters, J. K., Thagaard, I. N., Krebs, L., Christiansen, M., & Lausten-Thomsen, U. (2021). The Role of Leptin in Fetal Growth during Pre-Eclampsia. International Journal of Molecular Sciences, 22(9), 4569. https://doi.org/10.3390/ijms22094569