Probiotics, Photobiomodulation, and Disease Management: Controversies and Challenges
Abstract
:1. Introduction
2. Historical Background
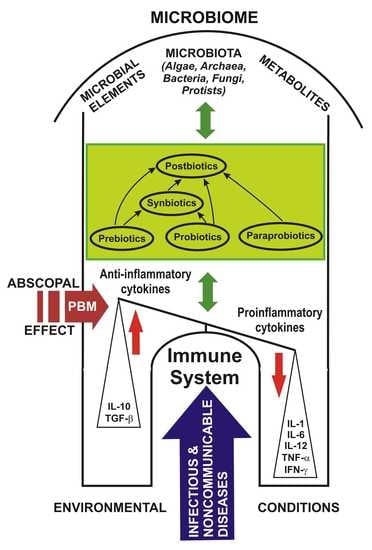
3. Microbiome and the Immune System
4. Prebiotics, Probiotics, Paraprobiotics, Postbiotics and Synbiotics: Challenges and Controversies
5. Probiotics in the Management of Various Pathologies: Perspectives in COVID-19
5.1. Probiotics in Digestive Tract Pathology
5.2. Probiotics in Pulmonary Viral Infections
5.3. Probiotics and COVID-19
6. Photobiomodulation Applied on the Gut–Lung–Brain Axis
- -
- absorption of photons by the first absorbing chromophores, cytochrome c oxidase in mitochondria and non-mitochondrial receptors, such as the ion channels and NADPH oxidase in cell membranes, also with a direct influence on the cellular cytoskeleton [264].
- -
- increased production of ATP, nitric oxide, a sudden outbreak of reactive oxygen species and the modulation of calcium levels.
- -
- initiation of intense generation of transcription factors, synthesis of new proteins, enhanced cell survival, multiplication, and migration.
7. Photobiomodulation and COVID-19
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Turnbaugh, P.J.; Ley, R.E.; Hamady, M.; Fraser-Liggett, C.M.; Knight, R.; Gordon, J.I. The human microbiome project. Nature 2007, 449, 804–810. [Google Scholar] [CrossRef]
- Gilbert, J.A.; Blaser, M.J.; Caporaso, J.G.; Jansson, J.K.; Lynch, S.V.; Knight, R. Current understanding of the human microbiome. Nat. Med. 2018, 24, 392–400. [Google Scholar] [CrossRef]
- Whipps, J.M.; Lewis, K.; Cooke, R.C. Mycoparasitism and plant disease control. In Fungi in Biological Control Systems; Burge, M., Ed.; Manchester University Press: Manchester, UK, 1988; pp. 161–187. [Google Scholar]
- Dubos, R.J. Louis Pasteur: Free Lance of Science, 1st ed.; Victor Gollancz Ltd.: London, UK, 1951; p. 418. [Google Scholar]
- Proctor, L. Priorities for the next 10 years of human microbiome research. Nature 2019, 569, 623–625. [Google Scholar] [CrossRef] [Green Version]
- Baquero, F.; Nombela, C. The microbiome as a human organ. Clin. Microbiol. Infect. 2012, 18, 2–4. [Google Scholar] [CrossRef] [Green Version]
- D’Argenio, V.; Salvatore, F. The role of the gut microbiome in the healthy adult status. Clin. Chim. Acta 2015, 451, 97–102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Microbiome. Definition of Microbiome. Merriam-Webster Dictionary. Available online: https://www.merriam-webster.com/dictionary/microbiome (accessed on 18 January 2021).
- Zimmer, C. Our Microbiomes, Ourselves. The New York Times. 4 December 2011, p. 12. Available online: https://carlzimmer.com/our-microbiomes-ourselves-282/ (accessed on 18 January 2021).
- Marchesi, J.R.; Ravel, J. The vocabulary of microbiome research: A proposal. Microbiome 2015, 3, 31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berg, G.; Rybakova, D.; Fischer, D.; Cernava, T.; Vergès, M.C.C.; Charles, T.; Chen, X.; Cocolin, L.; Eversole, K.; Corral, G.H.; et al. Microbiome definition re-visited: Old concepts and new challenges. Microbiome 2020, 8, 103. [Google Scholar] [CrossRef] [PubMed]
- Dupré, J.O.; O’Malley, M.A. Varieties of living things: Life at the intersection of lineage and metabolism. In Vitalism and the Scientific Image in Post-Enlightenment Life Science; History, Philosophy and Theory of the Life Sciences; Normandin, S., Wolfe, C., Eds.; Springer: Dordrecht, The Netherlands, 2009; pp. 1800–2010. [Google Scholar]
- Lederberg, J.; McCray, A.T. “Ome Sweet” Omics—A Genealogical Treasury of Words. Scientist 2001, 15, 8. [Google Scholar]
- What’s the Difference between Microbiome and Microbiota? Available online: https://atlasbiomed.com/blog/whats-the-difference-between-microbiome-and-microbiota/ (accessed on 18 January 2021).
- Ruthsatz, M.; Voisin, E.; Lima, N.; D’Hondt, K. Human Microbiomes in Health and Disease: Strategic Options for Regulatory Science and Healthcare Policy. Regulatory Focus. News Articles. July 2020. Regulatory Affairs Professionals Society. Available online: https://www.raps.org/news-and-articles/news-articles/2020/7/human-microbiomes-in-health-and-disease-strategic (accessed on 18 January 2021).
- Sender, R.; Fuchs, S.; Milo, R. Revised Estimates for the Number of Human and Bacteria Cells in the Body. PLoS Biol. 2016, 14, e1002533. [Google Scholar] [CrossRef] [Green Version]
- Żółkiewicz, J.; Marzec, A.; Ruszczyński, M.; Feleszko, W. Postbiotics—A Step Beyond Pre- and Probiotics. Nutrients 2020, 12, 2189. [Google Scholar] [CrossRef]
- Rinninella, E.; Raoul, P.; Cintoni, M.; Franceschi, F.; Miggiano, G.A.D.; Gasbarrini, A.; Mele, M.C. What is the Healthy Gut Microbiota Composition? A Changing Ecosystem across Age, Environment, Diet, and Diseases. Microorganisms 2019, 10, 14. [Google Scholar] [CrossRef] [Green Version]
- Lynch, S.V.; Pedersen, O. The Human Intestinal Microbiome in Health and Disease. N. Engl. J. Med. 2016, 375, 2369–2379. [Google Scholar] [CrossRef] [Green Version]
- Mohammadkhah, A.I.; Simpson, E.B.; Patterson, S.G.; Ferguson, J.F. Development of the Gut Microbiome in Children, and Lifetime Implications for Obesity and Cardiometabolic Disease. Children 2018, 5, 160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yatsunenko, T.; Rey, F.E.; Manary, M.J.; Trehan, I.; Dominguez-Bello, M.G.; Contreras, M.; Magris, M.; Hidalgo, G.; Baldassano, R.N.; Anokhin, A.P.; et al. Human gut microbiome viewed across age and geography. Nature 2012, 486, 222–227. [Google Scholar] [CrossRef] [PubMed]
- Lavin, T.; Preen, D.B. Investigating Caesarean Section Birth as a Risk Factor for Childhood Overweight. Child. Obes. 2018, 14, 131–138. [Google Scholar] [CrossRef]
- Jakobsson, H.E.; Abrahamsson, T.R.; Jenmalm, M.C.; Harris, K.; Quince, C.; Jernberg, C.; Björkstén, B.; Engstrand, L.; Andersson, A.F. Decreased gut microbiota diversity, delayed Bacteroidetes colonisation and reduced Th1 responses in infants delivered by caesarean section. Gut 2014, 63, 559–566. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sjögren, Y.M.; Tomicic, S.; Lundberg, A.; Böttcher, M.F.; Björkstén, B.; Sverremark-Ekström, E.; Jenmalm, M.C. Influence of early gut microbiota on the maturation of childhood mucosal and systemic immune responses. Clin. Exp. Allergy 2009, 39, 1842–1851. [Google Scholar] [CrossRef] [Green Version]
- Sordillo, J.E.; Korrick, S.; Laranjo, N.; Carey, V.; Weinstock, G.M.; Gold, D.R.; O’Connor, G.; Sandel, M.; Bacharier, L.B.; Beigelman, A.; et al. Association of the Infant Gut Microbiome with Early Childhood Neurodevelopmental Outcomes: An Ancillary Study to the VDAART Randomized Clinical Trial. JAMA Netw. Open 2019, 2, e190905. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Unger, S.; Stintzi, A.; Shah, P.; Mack, D.; O’Connor, D.L. Gut microbiota of the very-low-birth-weight infant. Pediatr. Res. 2015, 77, 205–213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pammi, M.; Cope, J.; Tarr, P.I.; Warner, B.B.; Morrow, A.L.; Mai, V.; Gregory, K.E.; Kroll, J.S.; McMurtry, V.; Ferris, M.J.; et al. Intestinal dysbiosis in preterm infants preceding necrotizing enterocolitis: A systematic review and meta-analysis. Microbiome 2017, 5, 31. [Google Scholar] [CrossRef] [Green Version]
- Rampelli, S.; Turroni, S.; Mallol, C.; Hernandez, C.; Galván, B.; Sistiaga, A.; Biagi, E.; Astolfi, A.; Brigidi, P.; Benazzi, S.; et al. Components of a Neanderthal gut microbiome recovered from fecal sediments from El Salt. Commun. Biol. 2021, 4, 169. [Google Scholar] [CrossRef]
- Ayeni, F.A.; Biagi, E.; Rampelli, S.; Fiori, J.; Soverini, M.; Audu, H.J.; Cristino, S.; Caporali, L.; Schnorr, S.L.; Carelli, V.; et al. Infant and Adult Gut Microbiome and Metabolome in Rural Bassa and Urban Settlers from Nigeria. Cell Rep. 2018, 23, 3056–3067. [Google Scholar] [CrossRef]
- Duvallet, C.; Gibbons, S.M.; Gurry, T.; Irizarry, R.A.; Alm, E.J. Meta-Analysis of gut microbiome studies identifies disease-specific and shared responses. Nat. Commun. 2017, 8, 1784. [Google Scholar] [CrossRef] [Green Version]
- Mariat, D.; Firmesse, O.; Levenez, F.; Guimarăes, V.; Sokol, H.; Doré, J.; Corthier, G.; Furet, J.P. The Firmicutes/Bacteroidetes ratio of the human microbiota changes with age. BMC Microbiol. 2009, 9, 123. [Google Scholar] [CrossRef] [PubMed]
- Belkaid, Y.; Harrison, O.J. Homeostatic Immunity and the Microbiota. Immunity 2017, 46, 562–576. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McGuckin, M.A.; Lindén, S.K.; Sutton, P.; Florin, T.H. Mucin dynamics and enteric pathogens. Nat. Rev. Microbiol. 2011, 9, 265–278. [Google Scholar] [CrossRef]
- Bested, A.C.; Logan, A.C.; Selhub, E.M. Intestinal microbiota, probiotics and mental health: From Metchnikoff to modern advances: Part I—Autointoxication revisited. Gut Pathog. 2013, 5, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Metchnikoff, E. Intestinal poisons and arteriosclerosis. Ann. Inst. Pasteur 1910, 24, 753–770. [Google Scholar]
- Metchnikoff, E.; Williams, H.S. Why not live forever? Cosmopolitan 1912, 53, 436–446. [Google Scholar]
- Pelton, B. Postbiotic Metabolites: The New Frontier in Microbiome Science. Townsend Letter. Available online: https://www.townsendletter.com/article/431-postbiotic-metabolites-the-new-frontier-in-microbiome-science/ (accessed on 28 January 2021).
- Editors, T. Arteriosclerosis and intestinal poisons. JAMA 1910, 55, 2311–2312. [Google Scholar]
- Metchnikoff, E. The Prolongation of Life: Optimistic Studies, 1st ed.; Springer: New York, NY, USA, 2004; 360p, ISBN 13 978-0826118769. [Google Scholar]
- Joint FAO/WHO Working Group Report on Drafting Guidelines for the Evaluation of Probiotics in Food; London Ontario (CA), 30 April and 1 May 2002. Available online: https://www.who.int/foodsafety/fs_management/en/probiotic_guidelines.pdf (accessed on 28 January 2021).
- Food and Agriculture Organization (FAO) of the United Nations. Food Safety and Quality: Probiotics. Available online: http://www.fao.org/food/food-safety-quality/a-z-index/probiotics/en/ (accessed on 19 April 2021).
- Žuntar, I.; Petric, Z.; Bursać Kovačević, D.; Putnik, P. Safety of Probiotics: Functional Fruit Beverages and Nutraceuticals. Foods 2020, 9, 947. [Google Scholar] [CrossRef]
- Illumina Acquires BlueBee to Accelerate Processing, Analysis and Sharing of Next Generation Sequencing Data at Scale. Available online: https://www.illumina.com/company/news-center/press-releases/press-release-details.html?newsid=76a97c76-a723-4233-acb3-030736719d80 (accessed on 28 January 2021).
- Proctor, L. The NIH Human Microbiome Project: Catalyst for an Emerging Field in Biomedical Research. 2018. Available online: https://www.genome.gov/Pages/About/NACHGR/February2018AgendaDocuments/HMP_talk_Feb_Council_final_020618.pdf (accessed on 28 January 2021).
- Breitbart, M.; Hewson, I.; Felts, B.; Mahaffy, J.M.; Nulton, J.; Salamon, P.; Rohwer, F. Metagenomic analyses of an uncultured viral community from human feces. J. Bacteriol. 2003, 185, 6220–6223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ley, R.E.; Peterson, D.A.; Gordon, J.I. Ecological and evolutionary forces shaping microbial diversity in the human intestine. Cell 2006, 124, 837–848. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hooper, L.V.; Macpherson, A.J. Immune adaptations that maintain homeostasis with the intestinal microbiota. Nat. Rev. Immunol. 2010, 10, 159–169. [Google Scholar] [CrossRef]
- Abt, M.C.; Osborne, L.C.; Monticelli, L.A.; Doering, T.A.; Alenghat, T.; Sonnenberg, G.F.; Paley, M.A.; Antenus, M.; Williams, K.L.; Erikson, J.; et al. Commensal bacteria calibrate the activation threshold of innate antiviral immunity. Immunity 2012, 37, 158–170. [Google Scholar] [CrossRef] [Green Version]
- Ai, T.L.; Solomon, B.D.; Hsieh, C.S. T-cell selection and intestinal homeostasis. Immunol. Rev. 2014, 259, 60–74. [Google Scholar] [CrossRef] [Green Version]
- Belkaid, Y.; Hand, T.W. Role of the microbiota in immunity and inflammation. Cell 2014, 157, 121–141. [Google Scholar] [CrossRef] [Green Version]
- Kumar, B.V.; Connors, T.J.; Farber, D.L. Human T Cell Development, Localization, and Function throughout Life. Immunity 2018, 48, 202–213. [Google Scholar] [CrossRef] [Green Version]
- Sun, H.; Sun, C.; Xiao, W.; Sun, R. Tissue-Resident lymphocytes: From adaptive to innate immunity. Cell Mol. Immunol. 2019, 16, 205–215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Booth, J.S.; Toapanta, F.R. B and T Cell Immunity in Tissues and Across the Ages. Vaccines 2021, 9, 24. [Google Scholar] [CrossRef]
- Soderholm, A.T.; Pedicord, V.A. Intestinal epithelial cells: At the interface of the microbiota and mucosal immunity. Immunology 2019, 158, 267–280. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mahapatro, M.; Erkert, L.; Becker, C. Cytokine-Mediated Crosstalk between Immune Cells and Epithelial Cells in the Gut. Cells 2021, 10, 111. [Google Scholar] [CrossRef] [PubMed]
- Pan American Health Organization (PAHO): Noncommunicable Disease Prevention and Control. Available online: https://www.paho.org/salud-en-las-americas-2017/?p=1391 (accessed on 24 January 2021).
- Gibson, G.R.; Hutkins, R.; Sanders, M.E.; Prescott, S.L.; Reimer, R.A.; Salminen, S.J.; Scott, K.; Stanton, C.; Swanson, K.S.; Cani, P.D.; et al. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 491–502. [Google Scholar] [CrossRef] [Green Version]
- 2018 Annual Meeting—International Scientific Association for Probiotics and Prebiotics (ISAPP). Minimum Criteria for Probiotics. Available online: https://isappscience.org/2018-annual-meeting/ (accessed on 31 January 2021).
- Behnsen, J.; Deriu, E.; Sassone-Corsi, M.; Raffatellu, M. Probiotics: Properties, examples, and specific applications. Cold Spring Harb. Perspect. Med. 2013, 3, a010074. [Google Scholar] [CrossRef] [Green Version]
- Toscano, M.; De Grandi, R.; Pastorelli, L.; Vecchi, M.; Drago, L. A consumer’s guide for probiotics: 10 golden rules for correct use. Dig. Liver Dis. 2017, 49, 1177–1184. [Google Scholar] [CrossRef] [PubMed]
- Johansen, E. Future access and improvement of industrial lactic acid bacteria cultures. Microb. Cell Fact. 2017, 16, 230. [Google Scholar] [CrossRef] [Green Version]
- Binda, S.; Hill, C.; Johansen, E.; Obis, D.; Pot, B.; Sanders, M.E.; Tremblay, A.; Ouwehand, A.C. Criteria to Qualify Microorganisms as “Probiotic” in Foods and Dietary Supplements. Front. Microbiol. 2020, 11, 1662. [Google Scholar] [CrossRef]
- Brüssow, H. Probiotics and prebiotics in clinical tests: An update. F1000Research 2019, 8, 1157. [Google Scholar] [CrossRef] [Green Version]
- Yadav, A.K.; Tyagi, A.; Kumar, A.; Panwar, S.; Grover, S.; Saklani, A.C.; Hemalatha, R.; Batish, V.K. Adhesion of lactobacilli and their anti-infectivity potential. Crit. Rev. Food Sci. Nutr. 2017, 57, 2042–2056. [Google Scholar] [CrossRef]
- D’Amelio, P.; Sassi, F. Gut microbiota, immune system, and bone. Calcif. Tissue Int. 2017, 102, 415–425. [Google Scholar] [CrossRef]
- Gomez-Llorente, C.; Munoz, S.; Gil, A. Role of toll-like receptors in the development of immunotolerance mediated by probiotics. Proc. Nutr. Soc. 2010, 69, 381–389. [Google Scholar] [CrossRef]
- Ahmed, F.E.; Ahmed, N.C. Anti-Inflammatory probiotic biomarkers in Fermented foods. J. Clin. Nephrol. 2019, 3, 19–41. [Google Scholar] [CrossRef] [Green Version]
- Vincenzi, A.; Goettert, M.I.; Volken de Souza, C.F. An evaluation of the effects of probiotics on tumoral necrosis factor (TNF-α) signaling and gene expression. Cytokine Growth Factor Rev. 2020, 57, 27–38. [Google Scholar] [CrossRef] [PubMed]
- Lehtoranta, L.; Kalima, K.; He, L.; Lappalainen, M.; Roivainen, M.; Närkiö, M.; Mäkelä, M.; Siitonen, S.; Korpela, R.; Pitkäranta, A. Specific probiotics and virological findings in symptomatic conscripts attending military service in Finland. J. Clin. Virol. 2014, 60, 276–281. [Google Scholar] [CrossRef] [PubMed]
- Nataraj, B.H.; Ali, S.A.; Behare, P.V.; Yadav, H. Postbiotics-Parabiotics: The new horizons in microbial biotherapy and functional foods. Microb. Cell Fact. 2020, 19, 168. [Google Scholar] [CrossRef]
- Davani-Davari, D.; Negahdaripour, M.; Karimzadeh, I.; Seifan, M.; Mohkam, M.; Masoumi, S.J.; Berenjian, A.; Ghasemi, Y. Prebiotics: Definition, Types, Sources, Mechanisms, and Clinical Applications. Foods 2019, 8, 92. [Google Scholar] [CrossRef] [Green Version]
- FAO; WHO. Guidelines for the Evaluation of Probiotics in Food, Paris; FAO: Rome, Italy, 2002; pp. 1–11. Available online: http://www.fda.gov/ohrms/dockets/dockets/95s0316/95s-0316-rpt0282-tab-03-ref-19-joint-faowho-vol219.pdf (accessed on 10 February 2021).
- Marco, M.L.; Sanders, M.E.; Gänzle, M.; Arrieta, M.C.; Cotter, P.D.; De Vuyst, L.; Hill, C.; Holzapfel, W.; Lebeer, S.; Merenstein, D.; et al. Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on fermented foods. Nat. Rev. Gastroenterol. Hepatol. 2021. [Google Scholar] [CrossRef]
- Cuevas-González, P.F.; Liceaga, A.M.; Aguilar-Toalá, J.E. Postbiotics and paraprobiotics: From concepts to applications. Food Res. Int. 2020, 136, 109502. [Google Scholar] [CrossRef]
- Aguilar-Toalá, J.E.; Garcia-Varela, R.; Garcia, H.S.; Mata-Haro, V.; González-Córdova, A.F.; Vallejo-Cordoba, B.; Hernández-Mendoza, A. Postbiotics: An evolving term within the functional foods field. Trends Food Sci. Technol. 2018, 75, 105–114. [Google Scholar] [CrossRef]
- Plaza-Díaz, J.; Ruiz-Ojeda, F.J.; Vilchez-Padial, L.M.; Gil, A. Evidence of the Anti-Inflammatory Effects of Probiotics and Synbiotics in Intestinal Chronic Diseases. Nutrients 2017, 9, 555. [Google Scholar] [CrossRef] [Green Version]
- Varzakas, T.; Kandylis, P.; Dimitrellou, D.; Salamoura, C.; Zakynthinos, G.; Proestos, C. Innovative and fortified food: Probiotics, prebiotics, gmos, and superfood. In Preparation and Processing of Religious and Cultural Foods; Elsevier: London, UK, 2018; pp. 67–129. [Google Scholar]
- Sources. Probiotics Have Been with Us for a Long Time. Available online: https://internationalprobiotics.org/resources/sources/ (accessed on 8 February 2021).
- Akter, S.; Park, J.H.; Jung, H.K. Potential Health-Promoting Benefits of Paraprobiotics, Inactivated Probiotic Cells. J. Microbiol. Biotechnol. 2020, 30, 477–481. [Google Scholar] [CrossRef]
- Wang, Y.; Qin, S.; Jia, J.; Huang, L.; Li, F.; Jin, F.; Ren, Z.; Wang, Y. Intestinal microbiota-associated metabolites: Crucial factors in the effectiveness of herbal medicines and diet therapies. Front. Physiol. 2019, 10, 1343. [Google Scholar] [CrossRef] [PubMed]
- Vandenplas, Y.; Zakharova, I.; Dmitrieva, Y. Oligosaccharides in infant formula: More evidence to validate the role of prebiotics. Br. J. Nutr. 2015, 113, 1339–1344. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salmerón, I. Fermented cereal beverages: From probiotic, prebiotic and synbiotic towards Nanoscience designed healthy drinks. Lett. Appl. Microbiol. 2017, 65, 114–124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kanyer, A.J.; Bornhorst, G.M.; Marco, M.L.; Bamforth, C.W. Is beer a source of prebiotics? J. Inst. Brew. 2017, 123, 361–365. [Google Scholar] [CrossRef]
- Apolinar-Valiente, R.; Romero-Cascales, I.; Williams, P.; Gómez-Plaza, E.; López-Roca, J.M.; Ros-García, J.M.; Doco, T. Oligosaccharides of Cabernet Sauvignon, Syrah and Monastrell red wines. Food Chem. 2015, 179, 311–317. [Google Scholar] [CrossRef]
- Dueñas, M.; Cueva, C.; Muñoz-González, I.; Jiménez-Girón, A.; Sánchez-Patán, F.; Santos-Buelga, C.; Moreno-Arribas, M.V.; Bartolomé, B. Studies on Modulation of Gut Microbiota by Wine Polyphenols: From Isolated Cultures to Omic Approaches. Antioxidants 2015, 4, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Thompson, R. Four Popular Types of Probiotics. Available online: https://www.healthcareglobal.com/technology-and-ai-3/four-popular-types-probiotics (accessed on 8 February 2021).
- Teame, T.; Wang, A.; Xie, M.; Zhang, Z.; Yang, Y.; Ding, Q.; Gao, C.; Olsen, R.E.; Ran, C.; Zhou, Z. Paraprobiotics and Postbiotics of Probiotic Lactobacilli, Their Positive Effects on the Host and Action Mechanisms: A Review. Front. Nutr. 2020, 7, 570344. [Google Scholar] [CrossRef]
- Scott, K.P.; Martin, J.C.; Duncan, S.H.; Flint, H.J. Prebiotic stimulation of human colonic butyrate-producing bacteria and bifidobacteria, in vitro. FEMS Microbiol. Ecol. 2014, 87, 30–40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guarino, M.; Altomare, A.; Emerenziani, S.; Di Rosa, C.; Ribolsi, M.; Balestrieri, P.; Iovino, P.; Rocchi, G.; Cicala, M. Mechanisms of Action of Prebiotics and Their Effects on Gastro-Intestinal Disorders in Adults. Nutrients 2020, 12, 1037. [Google Scholar] [CrossRef] [Green Version]
- Shimizu, H.; Masujima, Y.; Ushiroda, C.; Mizushima, R.; Taira, S.; Ohue-Kitano, R.; Kimura, I. Dietary Short-Chain Fatty Acid Intake Improves the Hepatic Metabolic Condition Via Ffar3. Sci. Rep. 2019, 9, 16574. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Subedi, L.; Venkatesan, R.; Kim, S.Y. Neuroprotective and Anti-Inflammatory Activities of Allyl Isothiocyanate through Attenuation of JNK/NF-κB/TNF-α Signaling. Int. J. Mol. Sci. 2017, 18, 1423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, Y.; Zhai, Q.; Li, D.; Mao, B.; Liu, X.; Zhao, J.; Mao, B.; Liu, X.; Zhao, J.; Zhang, H.; et al. Restoration of cefixime-induced gut microbiota changes by Lactobacillus cocktails and fructooligosaccharides in a mouse model. Microbiol. Res. 2017, 200, 14–24. [Google Scholar] [CrossRef] [PubMed]
- Bermudez-Brito, M.; Plaza-Diaz, J.; Munoz-Quezada, S.; Gomez-Llorente, C.; Gil, A. Probiotic mechanisms of action. Ann. Nutr. Metab. 2012, 61, 160–174. [Google Scholar] [CrossRef] [PubMed]
- Aliakbarpour, H.R.; Chamani, M.; Rahimi, G.; Sadeghi, A.A.; Qujeq, D. The Bacillus subtilis and Lactic Acid Bacteria Probiotics Influences Intestinal Mucin Gene Expression, Histomorphology and Growth Performance in Broilers. Asian Australas. J. Anim. Sci. 2012, 25, 1285–1293. [Google Scholar] [CrossRef] [Green Version]
- Monteagudo-Mera, A.; Rastall, R.A.; Gibson, G.R.; Charalampopoulos, D.; Chatzifragkou, A. Adhesion mechanisms mediated by probiotics and prebiotics and their potential impact on human health. Appl. Microbiol. Biotechnol. 2019, 103, 6463–6472. [Google Scholar] [CrossRef] [Green Version]
- Castilho, N.P.A.; Colombo, M.; Oliveira, L.L.; de Todorov, S.D.; Nero, L.A. Lactobacillus curvatus UFV-NPAC1 and other lactic acid bacteria isolated from calabresa, a fermented meat product, present high bacteriocinogenic activity against Listeria monocytogenes. BMC Microbiol. 2019, 19, 63. [Google Scholar] [CrossRef] [Green Version]
- Parada Venegas, D.; De la Fuente, M.K.; Landskron, G.; González, M.J.; Quera, R.; Dijkstra, G.; Harmsen, H.J.M.; Faber, K.N.; Hermoso, M.A. Short Chain Fatty Acids (SCFAs)-Mediated Gut Epithelial and Immune Regulation and Its Relevance for Inflammatory Bowel Diseases. Front. Immunol. 2019, 10, 277, Erratum in Front. Immunol. 2019, 10, 1486. [Google Scholar] [CrossRef] [Green Version]
- Bermúdez-Humarán, L.G.; Salinas, E.; Ortiz, G.G.; Ramirez-Jirano, L.J.; Morales, J.A.; Bitzer-Quintero, O.K. From Probiotics to Psychobiotics: Live Beneficial Bacteria Which Act on the Brain-Gut Axis. Nutrients 2019, 11, 890. [Google Scholar] [CrossRef] [Green Version]
- Zheng, D.; Liwinski, T.; Elinav, E. Interaction between microbiota and immunity in health and disease. Cell Res. 2020, 30, 492–506. [Google Scholar] [CrossRef]
- Abd El-Ghany, W.A. Paraprobiotics and postbiotics: Contemporary and promising natural antibiotics alternatives and their applications in the poultry field. Open Vet. J. 2020, 10, 323–330. [Google Scholar] [CrossRef]
- Taverniti, V.; Guglielmetti, S. The immunomodulatory properties of probiotic microorganisms beyond their viability (ghost probiotics: Proposal of paraprobiotic concept). Genes Nutr. 2011, 6, 261–274. [Google Scholar] [CrossRef] [Green Version]
- Hirose, Y.; Murosaki, S.; Yamamoto, Y.; Yoshikai, Y.; Tsuru, T. Daily intake of heat-killed Lactobacillus plantarum L-137 augments acquired immunity in healthy adults. J. Nutr. 2006, 136, 3069–3073. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haileselassie, Y.; Navis, M.; Vu, N.; Qazi, K.R.; Rethi, B.; Sverremark-Ekstrom, E. Postbiotic modulation of retinoic acid imprinted mucosal-like dendritic cells by probiotic Lactobacillus reuteri 17938 in vitro. Front. Immunol. 2016, 7, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cousin, F.; Jouan-Lanhouet, S.; Dimanche-Boitrel, M.T.; Corcos, L.; Jan, G. Milk Fermented by Propionibacterium freudenreichii Induces Apoptosis of HGT-1 Human Gastric Cancer Cells. PLoS ONE 2012, 7, e31892. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malagón-Rojas, J.N.; Mantziari, A.; Salminen, S.; Szajewska, H. Postbiotics for Preventing and Treating Common Infectious Diseases in Children: A Systematic Review. Nutrients 2020, 12, 389. [Google Scholar] [CrossRef] [Green Version]
- Uchida, M.; Ishii, I.; Inoue, C.; Akisato, Y.; Watanabe, K.; Hosoyama, S.; Toida, T.; Ariyoshi, N.; Kitada, M. Kefiran reduces atherosclerosis in rabbits fed a high cholesterol diet. J. Atheroscler. Thromb. 2010, 17, 980–988. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andreux, P.A.; Blanco-Bose, W.; Ryu, D.; Burdet, F.; Ibberson, M.; Aebischer, P.; Auwerx, J.; Singh, A.; Rinsch, C. The mitophagy activator urolithin A is safe and induces a molecular signature of improved mitochondrial and cellular health in humans. Nat. Metab. 2019, 1, 595–603. [Google Scholar] [CrossRef]
- Knackstedt, R.; Knackstedt, T.; Gatherwright, J. The role of topical probiotics on wound healing: A review of animal and human studies. Int. Wound J. 2020, 17, 1687–1694. [Google Scholar] [CrossRef]
- Maftei, M.N. Probiotic, Prebiotic and Synbiotic Products in Human Health. 2019. Available online: https://www.intechopen.com/books/frontiers-and-new-trends-in-the-science-of-fermented-food-and-beverages/probiotic-prebiotic-and-synbiotic-products-in-human-health (accessed on 14 February 2021). [CrossRef] [Green Version]
- Kassaian, N.; Aminorroaya, A.; Feizi, A.; Jafari, P.; Amini, M. The effects of probiotic and synbiotic supplementation on metabolic syndrome indices in adults at risk of type 2 diabetes: Study protocol for a randomized controlled trial. Trials 2017, 18, 148. [Google Scholar] [CrossRef] [Green Version]
- Sergeev, I.N.; Aljutaily, T.; Walton, G.; Huarte, E. Effects of Synbiotic Supplement on Human Gut Microbiota, Body Composition and Weight Loss in Obesity. Nutrients 2020, 12, 222. [Google Scholar] [CrossRef] [Green Version]
- Barengolts, E. Gut microbiota, prebiotics, probiotics, and synbiotics in management of obesity and prediabetes: Review of randomized controlled trials. Endocr. Pract. 2016, 22, 1224–1234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harris, L.A.; Baffy, N. Modulation of the gut microbiota: A focus on treatments for irritable bowel syndrome Postgrad. Med. 2017, 129, 872–888. [Google Scholar] [CrossRef] [PubMed]
- van der Aa, L.B.; van Aalderen, W.M.; Heymans, H.S.; Henk Sillevis Smitt, J.; Nauta, A.J.; Knippels, L.M.; Ben Amor, K.; Sprikkelman, A.B.; Synbad Study Group. Synbiotics prevent asthma-like symptoms in infants with atopic dermatitis. Allergy 2011, 66, 170–177. [Google Scholar] [CrossRef]
- Piqué, N.; Berlanga, M.; Miñana-Galbis, D. Health Benefits of Heat-Killed (Tyndallized) Probiotics: An Overview. Int. J. Mol. Sci. 2019, 20, 2534. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van den Nieuwboer, M.; Claassen, E. Dealing with the remaining controversies of probiotic safety. Benef. Microbes 2019, 10, 605–616. [Google Scholar] [CrossRef] [PubMed]
- Swanson, K.S.; Gibson, G.R.; Hutkins, R.; Reimer, R.A.; Reid, G.; Verbeke, K.; Karen, P.; Scott, K.P.; Holscher, H.D.; Azad, M.B.; et al. The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of synbiotics. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 687–701. [Google Scholar] [CrossRef]
- UNICEF; WHO; World Bank; UN-DESA Population Division. Levels and Trends in Child Mortality Report 2019. Estimates Developed by the UN Inter-Agency Group for Child Mortality Estimation. Available online: https://www.who.int/maternal_child_adolescent/documents/levels_trends_child_mortality_2019/en/ (accessed on 12 February 2021).
- Baranowski, J.R.; Claud, E.C. Necrotizing enterocolitis and the preterm infant microbiome. Adv. Exp. Med. Biol. 2019, 1125, 25–36. [Google Scholar] [CrossRef] [PubMed]
- Troeger, C.; Blacker, B.F.; Khalil, I.A.; Rao, P.C.; Cao, S.; Zimsen, S.R.; Albertson, S.B.; Stanaway, J.D.; Deshpande, A.; Abebe, Z.; et al. Estimates of the global, regional, and national morbidity, mortality, and aetiologies of diarrhoea in 195 countries: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Infect. Dis. 2018, 18, 1211–1228. [Google Scholar] [CrossRef] [Green Version]
- Maguire, M.; Maguire, G. Gut dysbiosis, leaky gut, and intestinal epithelial proliferation in neurological disorders: Towards the development of a new therapeutic using amino acids, prebiotics, probiotics, and postbiotics. Rev. Neurosci. 2019, 30, 179–201. [Google Scholar] [CrossRef]
- Klemashevich, C.; Wu, C.; Howsmon, D.; Alaniz, R.C.; Lee, K.; Jayaraman, A. Rational identification of diet-derived postbiotics for improving intestinal microbiota function. Curr. Opin. Biotechnol. 2014, 26, 85–90. [Google Scholar] [CrossRef]
- Collado, M.C.; Vinderola, G.; Salminen, S. Postbiotics: Facts and open questions. A position paper on the need for a consensus definition. Benef. Microbes 2019, 10, 711–719. [Google Scholar] [CrossRef] [PubMed]
- Zawistowska-Rojek, A.; Tyski, S. Are Probiotic Really Safe for Humans? Pol. J. Microbiol. 2018, 67, 251–258. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vuotto, C.; Longo, F.; Donelli, G. Probiotics to counteract biofilm-associated infections: Promising and conflicting data. Int. J. Oral Sci. 2014, 6, 189–194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wen, K.; Li, G.; Bui, T.; Liu, F.; Li, Y.; Kocher, J.; Lin, L.; Yang, X.; Yuan, L. High dose and low dose Lactobacillus acidophilus exerted differential immune modulating effects on T cell immune responses induced by an oral human rotavirus vaccine in gnotobiotic pigs. Vaccine 2012, 30, 1198–1207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schauber, J.; Gallo, R.L. Antimicrobial peptides and the skin immune defense system. J. Allergy Clin. Immunol. 2009, 124, R13–R18. [Google Scholar] [CrossRef]
- Embleton, N.D.; Zalewski, S.; Berrington, J.E. Probiotics for prevention of necrotizing enterocolitis and sepsis in preterm infants. Curr. Opin. Infect. Dis. 2016, 29, 256–261. [Google Scholar] [CrossRef]
- Doron, S.; Snydman, D.R. Risk and safety of probiotics. Clin. Infect. Dis. 2015, 60, S129–S134. [Google Scholar] [CrossRef] [Green Version]
- Vahabnezhad, E.; Mochon, A.B.; Wozniak, L.J.; Ziring, D.A. Lactobacillus bacteremia associated with probiotic use in a pediatric patient with ulcerative colitis. J. Clin. Gastroenterol. 2013, 47, 437–439. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meini, S.; Laureano, R.; Fani, L.; Tascini, C.; Galano, A.; Antonelli, A.; Rossolini, G.M. Breakthrough Lactobacillus rhamnosus GG bacteremia associated with probiotic use in an adult patient with severe active ulcerative colitis: Case report and review of the literature. Infection 2015, 43, 777–781. [Google Scholar] [CrossRef] [PubMed]
- Salminen, M.K.; Rautelin, H.; Tynkkynen, S.; Poussa, T.; Saxelin, M.; Valtonen, V.; Järvinen, A. Lactobacillus bacteremia, clinical significance, and patient outcome, with special focus on probiotic L. rhamnosus GG. Clin. Infect. Dis. 2004, 38, 62–69. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salminen, M.K.; Tynkkynen, S.; Rautelin, H.; Saxelin, M.; Vaara, M.; Ruutu, P.; Sarna, S.; Valtonen, V.; Järvinen, A. Lactobacillus bacteremia during a rapid increase in probiotic use of Lactobacillus rhamnosus GG in Finland. Clin. Infect. Dis. 2002, 35, 1155–1160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santino, I.; Alari, A.; Bono, S.; Teti, E.; Marangi, M.; Bernardini, A.; Magrini, L.; Di Somma, S.; Teggi, A. Saccharomyces cerevisiae fungemia, a possible consequence of the treatment of Clostridium difficile colitis with a probioticum. Int. J. Immunopathol. Pharmacol. 2014, 27, 143–146. [Google Scholar] [CrossRef]
- Thygesen, J.B.; Glerup, H.; Tarp, B. Saccharomyces boulardii fungemia caused by treatment with a probioticum. BMJ Case Rep. 2012, bcr0620114412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cherifi, S.; Robberecht, J.; Miendje, Y. Saccharomyces cerevisiae fungemia in an elderly patient with Clostridium difficile colitis. Acta Clin. Belg. 2004, 59, 223–224. [Google Scholar] [CrossRef]
- Henry, S.; D’Hondt, L.; André, M.; Holemans, X.; Canon, J.L. Saccharomyces cerevisiae fungemia in a head and neck cancer patient: A case report and review of the literature. Acta Clin. Belg. 2004, 59, 220–222. [Google Scholar] [CrossRef]
- Cesaro, S.; Chinello, P.; Rossi, L.; Zanesco, L. Saccharomyces cerevisiae fungemia in a neutropenic patient treated with Saccharomyces boulardii. Support. Care Cancer 2000, 8, 504–505. [Google Scholar] [CrossRef]
- Hennequin, C.; Kauffmann-Lacroix, C.; Jobert, A.; Viard, J.P.; Ricour, C.; Jacquemin, J.L.; Berche, P. Possible role of catheters in Saccharomyces boulardii fungemia. Eur. J. Clin. Microbiol. Infect. Dis. 2000, 19, 16–20. [Google Scholar] [CrossRef] [PubMed]
- Lherm, T.; Monet, C.; Nougière, B.; Soulier, M.; Larbi, D.; Le Gall, C.; Caen, D.; Malbrunot, C. Seven Cases of fungemia with Saccharomyces boulardii in critically ill patients. Intensive Care Med. 2002, 28, 797–801. [Google Scholar] [CrossRef]
- Muñoz, P.; Bouza, E.; Cuenca-Estrella, M.; Eiros, J.M.; Pérez, M.J.; Sánchez-Somolinos, M.; Rincón, C.; Hortal, J.; Peláez, T. Saccharomyces cerevisiae fungemia: An emerging infectious disease. Clin. Infect. Dis. 2005, 40, 1625–1634. [Google Scholar] [CrossRef]
- Kara, I.; Yıldırım, F.; Özgen, Ö.; Erganiş, S.; Aydoğdu, M.; Dizbay, M.; Gürsel, G.; Kalkanci, A. Saccharomyces cerevisiae fungemia after probiotic treatment in an intensive care unit patient. J. Mycol. Med. 2018, 28, 218–221. [Google Scholar] [CrossRef] [PubMed]
- Martin, I.W.; Tonner, R.; Trivedi, J.; Miller, H.; Lee, R.; Liang, X.; Rotello, L.; Isenbergh, E.; Anderson, J.; Perl, T. Saccharomyces boulardii probiotic-associated fungemia: Questioning the safety of this preventive probiotic’s use. Diagn. Microbiol. Infect. Dis. 2017, 87, 286–288. [Google Scholar] [CrossRef] [PubMed]
- Roy, U.; Jessani, L.; Rudramurthy, S.M.; Gopalakrishnan, R.; Dutta, S.; Chakravarty, C.; Jillwin, J.; Chakrabarti, A. Seven cases of Saccharomyces fungaemia related to use of probiotics. Mycoses 2017, 60, 375–380. [Google Scholar] [CrossRef] [PubMed]
- Eren, Z.; Gurol, Y.; Sonmezoglu, M.; Eren, H.S.; Celik, G.; Kantarci, G. Probiyotik tedavisinden sonra yaşlı bir hastada gelişen Saccharomyces cerevisiae fungemisi [Saccharomyces cerevisiae fungemia in an elderly patient following probiotic treatment]. Mikrobiyol. Bul. 2014, 48, 351–355. [Google Scholar] [CrossRef]
- Costa, R.L.; Moreira, J.; Lorenzo, A.; Lamas, C.C. Infectious complications following probiotic ingestion: A potentially underestimated problem? A systematic review of reports and case series. BMC Complement. Altern. Med. 2018, 18, 329. [Google Scholar] [CrossRef] [Green Version]
- Landaburu, M.F.; López Daneri, G.A.; Relloso, S.; Zarlenga, L.J.; Vinante, M.A.; Mujica, M.T. Fungemia following probiotic treatment in an elderly patient. Rev. Argent. Microbiol. 2020, 52, 27–30. [Google Scholar] [CrossRef]
- Sadowska-Krawczenko, I.; Paprzycka, M.; Korbal, P.; Wiatrzyk, A.; Krysztopa-Grzybowska, K.; Polak, M.; Czajka, U.; Lutyńska, A. Lactobacillus rhamnosus GG suspected infection in a newborn with intrauterine growth restriction. Benef. Microbes 2014, 5, 397–402. [Google Scholar] [CrossRef]
- Luong, M.L.; Sareyyupoglu, B.; Nguyen, M.H.; Silveira, F.P.; Shields, R.K.; Potoski, B.A.; Pasculle, W.A.; Clancy, C.J.; Toyoda, Y. Lactobacillus probiotic use in cardiothoracic transplant recipients: A link to invasive Lactobacillus infection? Transpl. Infect. Dis. 2010, 12, 561–564. [Google Scholar] [CrossRef]
- Uusitalo, U.; Andren Aronsson, C.; Liu, X.; Kurppa, K.; Yang, J.; Liu, E.; Skidmore, J.; Winkler, C.; Rewers, M.J.; Hagopian, W.A.; et al. Early Probiotic Supplementation and the Risk of Celiac Disease in Children at Genetic Risk. Nutrients 2019, 11, 1790. [Google Scholar] [CrossRef] [Green Version]
- Stavropoulou, E.; Bezirtzoglou, E. Probiotics as a Weapon in the Fight against COVID-19. Front. Nutr. 2020, 7, 614986. [Google Scholar] [CrossRef]
- Rowan, N.J.; Deans, K.; Anderson, J.G.; Gemmell, C.G.; Hunter, I.S.; Chaithong, T. Putative virulence factor expression by clinical and food isolates of Bacillus spp. after growth in reconstituted infant milk formulae. Appl. Environ. Microbiol. 2001, 67, 3873–3881. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wong, A.; Ngu, D.Y.S.; Dan, L.A.; Ooi, A.; Lim, R.L.H. Detection of antibiotic resistance in probiotics of dietary supplements. Nutr. J. 2015, 14, 95. [Google Scholar] [CrossRef] [PubMed]
- Aceti, A.; Beghetti, I.; Maggio, L.; Martini, S.; Faldella, G.; Corvaglia, L. Filling the Gaps: Current Research Directions for a Rational Use of Probiotics in Preterm Infants. Nutrients 2018, 10, 1472. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Courvalin, P. Antibiotic resistance: The pros and cons of probiotics. Dig. Liver Dis. 2006, 38, S261–S265. [Google Scholar] [CrossRef]
- Zheng, M.; Zhang, R.; Tian, X.; Zhou, X.; Pan, X.; Wong, A. Assessing the Risk of Probiotic Dietary Supplements in the Context of Antibiotic Resistance. Front. Microbiol. 2017, 8, 908. [Google Scholar] [CrossRef]
- Homayouni-Rad, A.; Aghebati Maleki, L.; Samadi Kafil, H.; Fathi Zavoshti, H.; Abbasi, A. Postbiotics as novel health-promoting ingredients in functional foods. Health Promot. Perspect. 2020, 10, 3–4. [Google Scholar] [CrossRef] [Green Version]
- Moore, R.E.; Townsend, S.D. Temporal development of the infant gut microbiome. Open Biol. 2019, 9, 190128. [Google Scholar] [CrossRef] [Green Version]
- Homayouni-Rad, A.; Akbarzadeh, F.; Vaghef-Mehrabany, E. Which are more important: Prebiotics or probiotics? Nutrition 2012, 28, 1196–1197. [Google Scholar] [CrossRef]
- Martinez, K.B.; Leone, V.; Chang, E.B. Microbial metabolites in health and disease: Navigating the unknown in search of function. J. Biol Chem. 2017, 292, 8553–8559. [Google Scholar] [CrossRef] [Green Version]
- National Research Council (US) Committee on Metagenomics: Challenges and Functional Applications. The New Science of Metagenomics: Revealing the Secrets of Our Microbial Planet; National Academies Press: Washington, DC, USA, 2007. [Google Scholar]
- Rad, A.H.; Abbasi, A.; Kafil, H.S.; Ganbarov, K. Potential Pharmaceutical and Food Applications of Postbiotics: A Review. Curr. Pharm. Biotechnol. 2020, 21, 1576–1587. [Google Scholar] [CrossRef]
- Mayer, E. The Mind-Gut Connection: How the Mind Communicates with the Gut, 1st ed.; Harper Collins Publishers: New York, NY, USA, 2016; Chapter 2; pp. 29–651. ISBN 978-0-06-237655-8. [Google Scholar]
- Mikelsaar, M.; Zilmer, M. Lactobacillus fermentum ME-3: An anti-microbial and anti-oxidative probiotic. Micro Ecol. Health Dis. 2009, 21, 1–27. [Google Scholar]
- Hill, M.J. Intestinal flora and endogenous vitamin synthesis. Eur. J. Cancer Prev. 1997, 6, S43–S45. [Google Scholar] [CrossRef]
- Dobson, A.; Cotter, P.D.; Ross, R.P.; Hill, C. Bacteriocin production: A probiotic trait? Appl. Environ. Microbiol. 2012, 78, 1–6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cava, F.; Lam, H.; de Pedro, M.A.; Waldor, M.K. Emerging knowledge of regulatory roles of D-amino acids in bacteria. Cell Mol. Life Sci. 2011, 68, 817–831. [Google Scholar] [CrossRef] [Green Version]
- Hertzberger, R.; Arents, J.; Dekker, H.L.; Pridmore, R.D.; Gysler, C.; Kleerebezem, M.; de Mattos, M.J. H2O2 production in species of the Lactobacillus acidophilus group: A central role for a novel NADH-dependent flavin reductase. Appl. Environ. Microbiol. 2014, 80, 2229–2239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van Hylckama Vlieg, J.E.; Veiga, P.; Zhang, C.; Derrien, M.; Zhao, L. Impact of microbial transformation of food on health—From fermented foods to fermentation in the gastro-intestinal tract. Curr. Opin. Biotechnol. 2011, 22, 211–219. [Google Scholar] [CrossRef]
- Paul, D.; Manna, S.; Mandal, S.M. Antibiotics Associated Disorders and Post-biotics Induced Rescue in Gut Health. Curr. Pharm. Des. 2018, 24, 821–829. [Google Scholar] [CrossRef] [PubMed]
- Frece, J.; Kos, B.; Svetec, I.K.; Zgaga, Z.; Mrsa, V.; Susković, J. Importance of S-layer proteins in probiotic activity of Lactobacillus acidophilus M92. J. Appl. Microbiol. 2005, 98, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Chapot-Chartier, M.P.; Vinogradov, E.; Sadovskaya, I.; Andre, G.; Mistou, M.Y.; Trieu-Cuot, P.; Furlan, S.; Bidnenko, E.; Courtin, P.; Péchoux, C.; et al. Cell Surface of Lactococcus lactis Is Covered by a Protective Polysaccharide Pellicle. J. Biol. Chem. 2010, 285, 10464–10471. [Google Scholar] [CrossRef] [Green Version]
- Mogensen, T.H. Pathogen Recognition and Inflammatory Signaling in Innate Immune Defenses. Clin. Microbiol. Rev. 2009, 22, 240–273. [Google Scholar] [CrossRef] [Green Version]
- Mantziari, A.; Salminen, S.; Szajewska, H.; Malagón-Rojas, J.N. Postbiotics against Pathogens Commonly Involved in Pediatric Infectious Diseases. Microorganisms 2020, 8, 1510. [Google Scholar] [CrossRef]
- Jayamani, E.; Mylonakis, E. Effector triggered manipulation of host immune response elicited by different pathotypes of Escherichia coli. Virulence 2014, 5, 733–739. [Google Scholar] [CrossRef] [Green Version]
- Kawai, T.; Akira, S. The roles of TLRs, RLRs and NLRs in pathogen recognition. Int. Immunol. 2009, 21, 317–337. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kummer, J.A.; Broekhuizen, R.; Everett, H.; Agostini, L.; Kuijk, L.; Martinon, F.; van Bruggen, R.; Tschopp, J. Inflammasome Components NALP 1 and 3 Show Distinct but Separate Expression Profiles in Human Tissues Suggesting a Site-specific Role in the Inflammatory Response. J. Histochem. Cytochem. 2007, 55, 443–452. [Google Scholar] [CrossRef] [Green Version]
- Feerick, C.L.; McKernan, D.P. Understanding the regulation of pattern recognition receptors in inflammatory diseases—A ‘Nod’ in the right direction. Immunology 2017, 150, 237–247. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fenini, G.; Contassot, E.; French, L.E. Potential of IL-1, IL-18 and Inflammasome Inhibition for the Treatment of Inflammatory Skin Diseases. Front. Pharmacol. 2017, 8, 278. [Google Scholar] [CrossRef] [PubMed]
- Petes, C.; Odoardi, N.; Gee, K. The Toll for Trafficking: Toll-Like Receptor 7 Delivery to the Endosome. Front. Immunol. 2017, 8, 1075. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, S.; Gao, N. Compartmentalizing Intestinal Epithelial Cell Toll-like Receptors for Immune Surveillance. Cell. Mol. Life Sci. CMLS 2015, 72, 3343–3353. [Google Scholar] [CrossRef] [Green Version]
- Byun, M.S.; Yu, O.K.; Cha, Y.S.; Park, T.S. Korean traditional Chungkookjang improves body composition, lipid profiles and atherogenic indices in overweight/obese subjects: A double-blind, randomized, crossover, placebo-controlled clinical trial. Eur. J. Clin. Nutr. 2016, 70, 1116–1122. [Google Scholar] [CrossRef] [PubMed]
- Barczyńska, R.; Litwin, M.; Sliżewska, K.; Szalecki, M.; Berdowska, A.; Bandurska, K.; Libudzisz, Z.; Kapuśniak, J. Bacterial Microbiota and Fatty Acids in the Faeces of Overweight and Obese Children. Pol. J. Microbiol. 2018, 67, 339–345. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kwon, D.Y.; Daily, J.W., 3rd; Kim, H.J.; Park, S. Antidiabetic effects of fermented soybean products on type 2 diabetes. Nutr. Res. 2010, 30, 1–13. [Google Scholar] [CrossRef]
- Selhub, E.M.; Logan, A.C.; Bested, A.C. Fermented foods, microbiota, and mental health: Ancient practice meets nutritional psychiatry. J. Physiol. Anthropol. 2014, 33, 2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lim, J.H.; Jung, E.S.; Choi, E.K.; Jeong, D.Y.; Jo, S.W.; Jin, J.H.; Lee, J.M.; Park, B.H.; Chae, S.W. Supplementation with Aspergillus oryzae-fermented kochujang lowers serum cholesterol in subjects with hyperlipidemia. Clin. Nutr. 2015, 34, 383–387. [Google Scholar] [CrossRef]
- Tu, M.Y.; Chen, H.L.; Tung, Y.T.; Kao, C.C.; Hu, F.C.; Chen, C.M. Short-Term Effects of Kefir-Fermented Milk Consumption on Bone Mineral Density and Bone Metabolism in a Randomized Clinical Trial of Osteoporotic Patients. PLoS ONE 2015, 10, e0144231. [Google Scholar] [CrossRef]
- Pekmez, C.T.; Dragsted, L.O.; Brahe, L.K. Gut microbiota alterations and dietary modulation in childhood malnutrition—The role of short chain fatty acids. Clin. Nutr. 2019, 38, 615–630. [Google Scholar] [CrossRef] [PubMed]
- Corsello, G.; Carta, M.; Marinello, R.; Picca, M.; De Marco, G.; Micillo, M.; Ferrara, D.; Vigneri, P.; Cecere, G.; Ferri, P.; et al. Preventive Effect of Cow’s Milk Fermented with Lactobacillus paracasei CBA L74 on Common Infectious Diseases in Children: A Multicenter Randomized Controlled Trial. Nutrients 2017, 9, 669. [Google Scholar] [CrossRef]
- Nocerino, R.; Paparo, L.; Terrin, G.; Pezzella, V.; Amoroso, A.; Cosenza, L.; Cecere, G.; Marco, G.D.; Micillo, M.; Albano, F.; et al. Cow’s milk and rice fermented with Lactobacillus paracasei CBA L74 prevent infectious diseases in children: A randomized controlled trial. Clin. Nutr. 2017, 36, 118–125. [Google Scholar] [CrossRef]
- Salazar-Lindo, E.; Figueroa-Quintanilla, D.; Caciano, M.; Reto-Valiente, V.; Chauviere, G.; Colin, P. Effectiveness and Safety of Lactobacillus LB in the Treatment of Mild Acute Diarrhea in Children. J. Pediatr. Gastroenterol. Nutr. 2007, 44, 571–576. [Google Scholar] [CrossRef] [PubMed]
- Gou, W.; Fu, Y.; Yue, L.; Chen, G.; Cai, X.; Shuai, M.; Xu, F.; Yi, X.; Chen, H.; Zhu, Y.; et al. Gut microbiota may underlie the predisposition of healthy individuals to COVID-19. medRxiv 2020. [Google Scholar] [CrossRef] [Green Version]
- Kalantar-Zadeh, K.; Ward, S.A.; Kalantar-Zadeh, K.; El-Omar, E.M. Considering the Effects of Microbiome and Diet on SARS-CoV-2 Infection: Nanotechnology Roles. ACS Nano 2020, 14, 5179–5182. [Google Scholar] [CrossRef]
- Maldonado, J.; Cañabate, F.; Sempere, L.; Vela, F.; Sánchez, A.; Narbona, E.; López-Huertas, E.; Geerlings, A.; Valero, A.; Olivares, M.; et al. Human Milk Probiotic Lactobacillus fermentum CECT5716 Reduces the Incidence of Gastrointestinal and Upper Respiratory Tract Infections in Infants. J. Pediatr. Gastroenterol. Nutr. 2012, 54, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Tsilingiri, K.; Barbosa, T.; Penna, G.; Caprioli, F.; Sonzogni, A.; Viale, G.; Rescigno, M. Probiotic and postbiotic activity in health and disease: Comparison on a novel polarised ex-vivo organ culture model. Gut 2012, 61, 1007–1015. [Google Scholar] [CrossRef] [Green Version]
- Wegh, C.A.M.; Geerlings, S.Y.; Knol, J.; Roeselers, G.; Belzer, C. Postbiotics and Their Potential Applications in Early Life Nutrition and Beyond. Int. J. Mol. Sci. 2019, 20, 4673. [Google Scholar] [CrossRef] [Green Version]
- Rossoni, R.D.; de Barros, P.P.; Mendonça, I.D.C.; Medina, R.P.; Silva, D.H.S.; Fuchs, B.B.; Junqueira, J.C.; Mylonakis, E. The Postbiotic Activity of Lactobacillus paracasei 28.4 against Candida auris. Front. Cell Infect. Microbiol. 2020, 10, 397. [Google Scholar] [CrossRef] [PubMed]
- Hackam, D.; Caplan, M. Necrotizing enterocolitis: Pathophysiology from a historical context. Semin. Pediatr. Surg. 2018, 27, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Mosca, F.; Gianni, M.L.; Rescigno, M. Can Postbiotics Represent a New Strategy for NEC? Adv. Exp. Med. Biol. 2019, 1125, 37–45. [Google Scholar] [CrossRef]
- Aguilar-Toalá, J.E.; Hall, F.G.; Urbizo-Reyes, U.C.; Garcia, H.S.; Vallejo-Cordoba, B.; González-Córdova, A.F.; Hernández-Mendoza, A.; Liceaga, A.M. In Silico Prediction and In Vitro Assessment of Multifunctional Properties of Postbiotics Obtained from Two Probiotic Bacteria. Probiotics Antimicrob. Proteins 2020, 12, 608–622. [Google Scholar] [CrossRef]
- Humam, A.M.; Loh, T.C.; Foo, H.L.; Samsudin, A.A.; Mustapha, N.M.; Zulkifli, I.; Izuddin, W.I. Effects of Feeding Different Postbiotics Produced by Lactobacillus plantarum on Growth Performance, Carcass Yield, Intestinal Morphology, Gut Microbiota Composition, Immune Status, and Growth Gene Expression in Broilers under Heat Stress. Animals 2019, 9, 644. [Google Scholar] [CrossRef] [Green Version]
- Liévin-Le Moal, V.; Servin, A.L. Anti-Infective activities of Lactobacillus strains in the human intestinal microbiota: From probiotics to gastrointestinal anti-infectious biotherapeutic agents. Clin. Microbiol. Rev. 2014, 27, 167–199. [Google Scholar] [CrossRef] [Green Version]
- Johnson, C.N.; Kogut, M.H.; Genovese, K.; He, H.; Kazemi, S.; Arsenault, R.J. Administration of a Postbiotic Causes Immunomodulatory Responses in Broiler Gut and Reduces Disease Pathogenesis Following Challenge. Microorganisms 2019, 7, 268. [Google Scholar] [CrossRef] [Green Version]
- Blaabjerg, S.; Artzi, D.M.; Aabenhus, R. Probiotics for the Prevention of Antibiotic-Associated Diarrhea in Outpatients—A Systematic Review and Meta-Analysis. Antibiotics 2017, 6, 21. [Google Scholar] [CrossRef] [Green Version]
- Vanderhoof, J.A.; Whitney, D.B.; Antonson, D.L.; Hanner, T.L.; Lupo, J.V.; Young, R.J. Lactobacillus GG in the prevention of antibiotic-associated diarrhea in children. J. Pediatr. 1999, 135, 564–568. [Google Scholar] [CrossRef]
- Guarino, A.; Guandalini, S.; Lo Vecchio, A. Probiotics for Prevention and Treatment of Diarrhea. J. Clin. Gastroenterol. 2015, 49, S37–S45. [Google Scholar] [CrossRef]
- Guo, Q.; Goldenberg, J.Z.; Humphrey, C.; El Dib, R.; Johnston, B.C. Probiotics for the prevention of pediatric antibiotic-associated diarrhea. Cochrane Database Syst. Rev. 2019, 4, CD004827. [Google Scholar] [CrossRef]
- Yan, T.; Goldman, D.R. Probiotics for antibiotic-associated diarrhea in children. Can. Fam. Physician 2020, 66, 37–39. [Google Scholar]
- Hilty, M.; Burke, C.; Pedro, H.; Cardenas, P.; Bush, A.; Bossley, C.; Davies, J.; Ervine, A.; Poulter, L.; Pachter, L.; et al. Disordered microbial communities in asthmatic airways. PLoS ONE 2010, 5, e8578. [Google Scholar] [CrossRef] [Green Version]
- Staley, J.T.; Konopka, A. Measurement of in situ activities of nonphotosynthetic microorganisms in aquatic and terrestrial habitats. Annu. Rev. Microbiol. 1985, 39, 321–346. [Google Scholar] [CrossRef]
- Dickson, R.P.; Erb-Downward, J.R.; Martinez, F.J.; Huffnagle, G.B. The Microbiome and the Respiratory Tract. Annu. Rev. Physiol. 2016, 78, 481–504. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, C.S.; Kao, C.Y. Current understanding of the gut microbiota shaping mechanisms. J. Biomed. Sci. 2019, 26, 59. [Google Scholar] [CrossRef] [PubMed]
- Sommariva, M.; Le Noci, V.; Bianchi, F.; Camelliti, S.; Balsari, A.; Tagliabue, E.; Sfondrini, L. The lung microbiota: Role in maintaining pulmonary immune homeostasis and its implications in cancer development and therapy. Cell. Mol. Life Sci. 2020, 77, 2739–2749. [Google Scholar] [CrossRef] [Green Version]
- O’Dwyer, D.N.; Dickson, R.P.; Moore, B.B. The Lung Microbiome, Immunity, and the Pathogenesis of Chronic Lung Disease. J. Immunol. 2016, 196, 4839–4847. [Google Scholar] [CrossRef] [Green Version]
- Frank, D.N.; Feazel, L.M.; Bessesen, M.T.; Price, C.S.; Janoff, E.N.; Pace, N.R. The human nasal microbiota and Staphylococcus aureus carriage. PLoS ONE 2010, 5, e10598. [Google Scholar] [CrossRef]
- Noverr, M.C.; Noggle, R.M.; Toews, G.B.; Huffnagle, G.B. Role of antibiotics and fungal microbiota in driving pulmonary allergic responses. Infect. Immun. 2004, 72, 4996–5003. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manuzak, J.A.; Hensley-McBain, T.; Zevin, A.S.; Miller, C.; Cubas, R.; Agricola, B.; Gile, J.; Richert-Spuhler, L.; Patilea, G.; Estes, J.D. Enhancement of Microbiota in Healthy Macaques Results in Beneficial Modulation of Mucosal and Systemic Immune Function. J. Immunol. 2016, 196, 2401–2409. [Google Scholar] [CrossRef] [Green Version]
- GBD Chronic Respiratory Disease Collaborators. Prevalence and attributable health burden of chronic respiratory diseases, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet Respir. Med. 2020, 8, 585–596. [Google Scholar] [CrossRef]
- Gohil, K.; Samson, R.; Dastager, S.; Dharne, M. Probiotics in the prophylaxis of COVID-19: Something is better than nothing. 3 Biotech 2021, 11, 1. [Google Scholar] [CrossRef] [PubMed]
- Maeda, N.; Nakamura, R.; Hirose, Y.; Murosaki, S.; Yamamoto, Y.; Kase, T.; Yoshikai, Y. Oral administration of heat-killed Lactobacillus plantarum L-137 enhances protection against influenza virus infection by stimulation of type I interferon production in mice. Int. Immunopharmacol. 2009, 9, 1122–1125. [Google Scholar] [CrossRef] [PubMed]
- Murosaki, S.; Yamamoto, Y.; Ito, K.; Inokuchi, T.; Kusaka, H.; Ikeda, H.; Yoshikai, Y. Heat-Killed Lactobacillus plantarum L-137 suppresses naturally fed antigen-specific IgE production by stimulation of IL-12 production in mice. J. Allergy Clin. Immunol. 1998, 102, 57–64. [Google Scholar] [CrossRef]
- Murosaki, S.; Muroyama, K.; Yamamoto, Y.; Kusaka, H.; Liu, T.; Yoshikai, Y. Immunopotentiating activity of nigerooligosaccharides for the T helper 1-like immune response in mice. Biosci. Biotechnol. Biochem. 1999, 63, 373–378. [Google Scholar] [CrossRef] [Green Version]
- Murosaki, S.; Muroyama, K.; Yamamoto, Y.; Yoshikai, Y. Antitumor effect of heat-killed Lactobacillus plantarum L-137 through restoration of impaired interleukin-12 production in tumor-bearing mice. Cancer Immunol. Immunother. 2000, 49, 157–164. [Google Scholar] [CrossRef]
- Hori, T.; Kiyoshima, J.; Shida, K.; Yasui, H. Effect of intranasal administration of Lactobacillus casei shirota on influenza virus infection of upper respiratory tract in mice. Clin. Diagn. Lab. Immunol. 2001, 8, 593–597. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lehtoranta, L.; Pitkäranta, A.; Korpela, R. Probiotics in respiratory virus infections. Eur. J. Clin. Microbiol. Infect. Dis. 2014, 33, 1289–1302. [Google Scholar] [CrossRef] [PubMed]
- Arshad, M.S.; Khan, U.; Sadiq, A.; Khalid, W.; Hussain, M.; Yasmeen, A.; Asghar, Z.; Rehana, H. Coronavirus Disease (COVID-19) and Immunity Booster Green Foods: A Mini Review. Food Sci. Nutr. 2020, 8, 3971–3976. [Google Scholar] [CrossRef]
- Dumas, A.; Bernard, L.; Poquet, Y.; Lugo-Villarino, G.; Neyrolles, O. The role of the lung microbiota and the gut-lung axis in respiratory infectious diseases. Cell Microbiol. 2018, 20, e12966. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yeoh, Y.K.; Zuo, T.; Lui, G.C.; Zhang, F.; Liu, Q.; Li, A.Y.L.; Chung, A.C.K.; Cheung, C.P.; Tso, E.Y.K.; Fung, K.S.C.; et al. Gut microbiota composition reflects disease severity and dysfunctional immune responses in patients with COVID-19. Gut 2021, 1–9. [Google Scholar] [CrossRef]
- Stavropoulou, E.; Bezirtzoglou, E. Probiotics in medicine: A long debate. Front. Immunol. 2020, 11, 2192. [Google Scholar] [CrossRef]
- Stevens, R.D.; Puybasset, L. The brain-lung-brain axis. Intensive Care Med. 2011, 37, 1054–1056. [Google Scholar] [CrossRef] [Green Version]
- Dhar, D.; Mohanty, A. Gut microbiota and Covid-19—Possible link and implications. Virus Res. 2020, 285, 198018. [Google Scholar] [CrossRef] [PubMed]
- Harata, G.; He, F.; Hiruta, N.; Kawase, M.; Kubota, A.; Hiramatsu, M.; Yausi, H. Intranasal administration of Lactobacillus rhamnosus GG protects mice from H1N1 influenza virus infection by regulating respiratory immune responses. Lett. Appl. Microbiol. 2010, 50, 597–602. [Google Scholar] [CrossRef]
- de Marcken, M.; Dhaliwal, K.; Danielsen, A.C.; Gautron, A.S.; Dominguez-Villar, M. TLR7 and TLR8 activate distinct pathways in monocytes during RNA virus infection. Sci. Signal. 2019, 12, eaaw1347. [Google Scholar] [CrossRef]
- Birra, D.; Benucci, M.; Landolfi, L.; Merchionda, A.; Loi, G.; Amato, P.; Licata, G.; Quartuccio, L.; Triggiani, M.; Moscato, P. COVID 19: A clue from innate immunity. Immunol. Res. 2020, 68, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Ahlawat, S.A.; Sharma, K.K. Immunological co-ordination between gut and lungs in SARS-CoV-2 infection. Virus Res. 2020, 286, 198103. [Google Scholar] [CrossRef]
- Jamilloux, Y.; Henry, T.; Belot, A.; Viel, S.; Fauter, M.; El Jammal, T.; Walzer, T.; François, B.; Sève, P. Should we stimulate or suppress immune responses in COVID-19? Cytokine and anti-cytokine interventions. Autoimmun. Rev. 2020, 19, 102567. [Google Scholar] [CrossRef]
- Patra, S.; Saxena, S.; Sahu, N.; Pradhan, B.; Roychowdhury, A. Systematic Network and Meta-analysis on the Antiviral Mechanisms of Probiotics: A Preventive and Treatment Strategy to Mitigate SARS-CoV-2 Infection. Probiotics Antimicrob. Proteins 2021, 1–19. [Google Scholar] [CrossRef]
- Kwon, H.K.; Lee, C.G.; So, J.S.; Chae, C.S.; Hwang, J.S.; Sahoo, A.; Nam, J.H.; Rhee, J.H.; Hwang, K.C.; Im, S.H. Generation of regulatory dendritic cells and CD4+Foxp3+ T cells by probiotics administration suppresses immune disorders. Proc. Natl. Acad. Sci. USA 2010, 107, 2159–2164. [Google Scholar] [CrossRef] [Green Version]
- Baud, D.; Agri, V.D.; Gibson, G.R.; Reid, G.; Giannoni, E. Using Probiotics to Flatten the Curve of Coronavirus Disease COVID-2019 Pandemic. Front. Public Health 2020, 8, 186. [Google Scholar] [CrossRef]
- Finsen, N.R. Biographical. NobelPrize.org. Nobel Media AB 2021. Tue. 2 February 2021. Available online: https://www.nobelprize.org/prizes/medicine/1903/finsen/biographical/ (accessed on 2 February 2021).
- Bolognia, J.L.; Jorizzo, J.L.; Rapini, R.P. Dermatology (2 Volume Set), 2nd ed.; Mosby: St. Louis, MO, USA, 2008; ISBN 13 978-141602999. [Google Scholar]
- The Nobel Prize in Physiology or Medicine 1903. Available online: https://www.nobelprize.org/prizes/medicine/1903/summary/ (accessed on 2 February 2021).
- Santana-Blank, L.A.; Rodríguez-Santana, E. Physiologic rhythms responding to low-level electromagnetic and mechanical signals: The Joule equivalence principle. Photomed. Laser Surg. 2008, 26, 405–406. [Google Scholar] [CrossRef]
- The Nobel Prize in Physiology or Medicine 2017. Available online: https://www.nobelprize.org/prizes/medicine/2017/summary/ (accessed on 2 February 2021).
- Foster, R.G.; Kreitzman, L. The rhythms of life: What your body clock means to you! Exp. Physiol. 2014, 99, 599–606. [Google Scholar] [CrossRef]
- Kuehn, B.M. Resetting the Circadian Clock Might Boost Metabolic Health. JAMA 2017, 317, 1303–1305. [Google Scholar] [CrossRef] [PubMed]
- Santana-Blank, L.; Rodríguez-Santana, E. Photobiomodulation in Light of Our Biological Clock’s Inner Workings. Photomed. Laser Surg. 2018, 36, 119–121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Llinás, R.R. The intrinsic electrophysiological properties of mammalian neurons: Insights into central nervous system function. Science 1988, 242, 1654–1664. [Google Scholar] [CrossRef]
- Lambert, G.W.; Reid, C.; Kaye, D.M.; Jennings, G.L.; Esler, M.D. Effect of sunlight and season on serotonin turnover in the brain. Lancet 2002, 360, 1840–1842. [Google Scholar] [CrossRef]
- Hamblin, M.R.; Huang, Y.Y.; Heiskanen, V. Non-Mammalian Hosts and Photobiomodulation: Do All Life-forms Respond to Light? Photochem. Photobiol. 2019, 95, 126–139. [Google Scholar] [CrossRef] [PubMed]
- Mole, R.H. Whole body irradiation; radiobiology or medicine? Br. J. Radiol. 1953, 26, 234–241. [Google Scholar] [CrossRef]
- Brix, N.; Tiefenthaller, A.; Anders, H.; Belka, C.; Lauber, K. Abscopal, immunological effects of radiotherapy: Narrowing the gap between clinical and preclinical experiences. Immunol. Rev. 2017, 280, 249–279. [Google Scholar] [CrossRef]
- Postow, M.A.; Callahan, M.K.; Barker, C.A.; Yamada, Y.; Yuan, J.; Kitano, S.; Mu, Z.; Rasalan, T.; Adamow, M.; Ritter, E.; et al. Immunologic correlates of the abscopal effect in a patient with melanoma. N. Engl. J. Med. 2012, 366, 925–931. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vanpouille-Box, C.; Diamond, J.M.; Pilones, K.A.; Zavadil, J.; Babb, J.S.; Formenti, S.C.; Barcellos-Hoff, M.H.; Demaria, S. TGFβ Is a Master Regulator of Radiation Therapy-Induced Antitumor Immunity. Cancer Res. 2015, 75, 2232–2242. [Google Scholar] [CrossRef] [Green Version]
- Rodriguez-Ruiz, M.E.; Rodriguez, I.; Garasa, S.; Barbes, B.; Solorzano, J.L.; Perez-Gracia, J.L.; Labiano, S.; Sanmamed, M.F.; Azpilikueta, A.; Bolaños, E.; et al. Abscopal Effects of Radiotherapy Are Enhanced by Combined Immunostimulatory mAbs and Are Dependent on CD8 T Cells and Crosspriming. Cancer Res. 2016, 76, 5994–6005. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Twyman-Saint Victor, C.; Rech, A.J.; Maity, A.; Rengan, R.; Pauken, K.E.; Stelekati, E.; Benci, J.L.; Xu, B.; Dada, H.; Odorizzi, P.M.; et al. Radiation and dual checkpoint blockade activate non-redundant immune mechanisms in cancer. Nature 2015, 520, 373–377. [Google Scholar] [CrossRef] [Green Version]
- Sender, R.; Fuchs, S.; Milo, R. Are We Really Vastly Outnumbered? Revisiting the Ratio of Bacterial to Host Cells in Humans. Cell 2016, 164, 337–340. [Google Scholar] [CrossRef] [Green Version]
- Gill, R.; Pop, M.; Deboy, R.T.; Eckburg, P.B.; Turnbaugh, P.J.; Samuel, B.S.; Gordon, J.I.; Relman, D.A.; Fraser-Liggett, C.M.; Nelson, K.E. Metagenomic analysis of the human distal gut microbiome. Science 2006, 312, 1355–1359. [Google Scholar] [CrossRef] [Green Version]
- Cryan, J.F.; O’Riordan, K.J.; Cowan, C.S.M.; Sandhu, K.V.; Bastiaanssen, T.F.S.; Boehme, M.; Codagnone, M.G.; Cussotto, S.; Fulling, C.; Golubeva, A.V.; et al. The Microbiota-Gut-Brain Axis. Physiol. Rev. 2019, 99, 1877–2013. [Google Scholar] [CrossRef]
- Karakan, T.; Ozkul, C.; Küpeli Akkol, E.; Bilici, S.; Sobarzo-Sánchez, E.; Capasso, R. Gut-Brain-Microbiota Axis: Antibiotics and Functional Gastrointestinal Disorders. Nutrients 2021, 13, 389. [Google Scholar] [CrossRef]
- de la Fuente-Nunez, C.; Meneguetti, B.T.; Franco, O.L.; Lu, T.K. Neuromicrobiology: How Microbes Influence the Brain. ACS Chem. Neurosci. 2018, 9, 141–150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salehpour, F.; Hamblin, M.R. Photobiomodulation for Parkinson’s Disease in Animal Models: A Systematic Review. Biomolecules 2020, 10, 610. [Google Scholar] [CrossRef] [Green Version]
- Hamblin, M.R. History of low-level laser (light) therapy. In Handbook of Low-Level Laser Therapy, 1st ed.; Hamblin, M.R., de Sousa, M.V.P., Agrawal, T., Eds.; Pan Stanford Publishing: Singapore, 2016; pp. 17–35. [Google Scholar] [CrossRef]
- Liebert, A.D.; Chow, R.T.; Bicknell, B.T.; Varigos, E. Neuroprotective Effects against POCD by Photobiomodulation: Evidence from Assembly/Disassembly of the Cytoskeleton. J. Exp. Neurosci. 2016, 10, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Hamblin, M.R. Mechanisms and applications of the anti-inflammatory effects of photobiomodulation. AIMS Biophys. 2017, 4, 337–361. [Google Scholar] [CrossRef] [PubMed]
- Bicknell, B.; Liebert, A.; Johnstone, D.; Kiat, H. Photobiomodulation of the microbiome: Implications for metabolic and inflammatory diseases. Lasers Med. Sci. 2019, 34, 317–327. [Google Scholar] [CrossRef] [PubMed]
- Tetel, M.J.; de Vries, G.J.; Melcangi, R.C.; Panzica, G.; O’Mahony, S.M. Steroids, stress and the gut microbiome-brain axis. J. Neuroendocrinol. 2018, 30. [Google Scholar] [CrossRef] [PubMed]
- Johnstone, D.M.; Moro, C.; Stone, J.; Benabid, A.L.; Mitrofanis, J. Turning on Lights to Stop Neurodegeneration: The Potential of Near Infrared Light Therapy in Alzheimer’s and Parkinson’s Disease. Front. Neurosci. 2016, 9, 500. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klingelhoefer, L.; Reichmann, H. Pathogenesis of Parkinson disease—The gut-brain axis and environmental factors. Nat. Rev. Neurol. 2015, 11, 625–636. [Google Scholar] [CrossRef]
- Liebert, A.; Bicknell, B.; Johnstone, D.M.; Gordon, L.C.; Kiat, H.; Hamblin, M.R. “Photobiomics”: Can Light, Including Photobiomodulation, Alter the Microbiome? Photobiomodul. Photomed. Laser Surg. 2019, 37, 681–693. [Google Scholar] [CrossRef] [Green Version]
- Stacy, A.; Andrade-Oliveira, V.; McCulloch, J.A.; Hild, B.; Oh, J.H.; Perez-Chaparro, P.J.; Sim, C.K.; Lim, A.I.; Link, V.M.; Enamorado, M.; et al. Infection trains the host for microbiota-enhanced resistance to pathogens. Cell 2021, 184, 615–627. [Google Scholar] [CrossRef] [PubMed]
- Sanmarco, L.M.; Wheeler, M.A.; Gutiérrez-Vázquez, C.; Polonio, C.M.; Linnerbauer, M.; Pinho-Ribeiro, F.A.; Li, Z.; Giovannoni, F.; Batterman, K.V.; Scalisi, G.; et al. Gut-licensed IFNγ+ NK cells drive LAMP1+TRAIL+ anti-inflammatory astrocytes. Nature 2021, 590, 473–479. [Google Scholar] [CrossRef] [PubMed]
- Chiu, L.; Bazin, T.; Truchetet, M.E.; Schaeverbeke, T.; Delhaes, L.; Pradeu, T. Protective microbiota: From localized to long-reaching co-immunity. Front. Immunol. 2017, 8, 1678. [Google Scholar] [CrossRef]
- Soret, P.; Vandenborght, L.E.; Francis, F.; Coron, N.; Enaud, R.; Avalos, M.; Schaeverbeke, T.; Berger, P.; Fayon, M.; Thiebaut, R.; et al. Respiratory mycobiome and suggestion of inter-kingdom network during acute pulmonary exacerbation in cystic fibrosis. Sci. Rep. 2020, 10, 3589. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grier, A.; McDavid, A.; Wang, B.; Qiu, X.; Java, J.; Bandyopadhyay, S.; Yang, H.; Holden-Wiltse, J.; Kessler, H.A.; Gill, A.L.; et al. Neonatal gut and respiratory microbiota: Coordinated development through time and space. Microbiome 2018, 6, 193. [Google Scholar] [CrossRef] [Green Version]
- Madan, J.C.; Koestler, D.C.; Stanton, B.A.; Davidson, L.; Moulton, L.A.; Housman, M.L.; Moore, J.H.; Guill, M.F.; Morrison, H.G.; Sogin, M.L.; et al. Serial analysis of the gut and respiratory microbiome in cystic fibrosis in infancy: Interaction between intestinal and respiratory tracts and impact of nutritional exposures. mBio 2012, 3, e00251-12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, T.; Yang, Z.; Zhang, X.; Han, N.; Yuan, J.; Cheng, Y. 16S rDNA analysis of the effect of fecal microbiota transplantation on pulmonary and intestinal flora. 3 Biotech 2017, 7, 370. [Google Scholar] [CrossRef]
- Trompette, A.; Gollwitzer, E.S.; Pattaroni, C.; Lopez-Mejia, I.C.; Riva, E.; Pernot, J.; Ubags, N.; Fajas, L.; Nicod, L.P.; Marsland, B.J. Dietary Fiber Confers Protection against Flu by Shaping Ly6c- Patrolling Monocyte Hematopoiesis and CD8+ T Cell Metabolism. Immunity 2018, 48, 992–1005. [Google Scholar] [CrossRef] [Green Version]
- Arrieta, M.C.; Arévalo, A.; Stiemsma, L.; Dimitriu, P.; Chico, M.E.; Loor, S.; Vaca, M.; Boutin, R.C.T.; Morien, E.; Jin, M.; et al. Associations between infant fungal and bacterial dysbiosis and childhood atopic wheeze in a nonindustrialized setting. J. Allergy Clin. Immunol. 2018, 142, 424–434. [Google Scholar] [CrossRef] [Green Version]
- Enaud, R.; Prevel, R.; Ciarlo, E.; Beaufils, F.; Wieërs, G.; Guery, B.; Delhaes, L. The Gut-Lung Axis in Health and Respiratory Diseases: A Place for Inter-Organ and Inter-Kingdom Crosstalks. Front. Cell Infect. Microbiol. 2020, 10, 9. [Google Scholar] [CrossRef] [Green Version]
- Acosta, P.L.; Byrne, A.B.; Hijano, D.R.; Talarico, L.B. Human Type I Interferon Antiviral Effects in Respiratory and Reemerging Viral Infections. J. Immunol. Res. 2020, 2020, 1372494. [Google Scholar] [CrossRef] [PubMed]
- Worldometers. Coronavirus. COVID-19 Coronavirus Pandemic. Available online: https://www.worldometers.info/coronavirus/ (accessed on 21 February 2021).
- Fanos, V.; Pintus, M.C.; Pintus, R.; Marcialis, M.A. Lung microbiota in the acute respiratory disease: From coronavirus to metabolomics. J. Pediatr. Neonatal Individ. Med. (JPNIM) 2020, 9, e090139. [Google Scholar] [CrossRef]
- Gómez-Rial, J.; Rivero-Calle, I.; Salas, A.; Martinón-Torres, F. Role of Monocytes/Macrophages in Covid-19 Pathogenesis: Implications for Therapy. Infect. Drug Resist. 2020, 13, 2485–2493. [Google Scholar] [CrossRef] [PubMed]
- Grifoni, E.; Valoriani, A.; Cei, F.; Lamanna, R.; Gelli, A.M.G.; Ciambotti, B.; Vannucchi, V.; Moroni, F.; Pelagatti, L.; Tarquini, R.; et al. Interleukin-6 as prognosticator in patients with COVID-19. J. Infect. 2020, 81, 452–482. [Google Scholar] [CrossRef]
- Mehani, S.H.M. Immunomodulatory effects of two different physical therapy modalities in patients with chronic obstructive pulmonary disease. J. Phys. Ther. Sci. 2017, 29, 1527–1533. [Google Scholar] [CrossRef] [Green Version]
- Diao, B.; Wang, C.; Tan, Y.; Chen, X.; Liu, Y.; Ning, L.; Chen, L.; Li, M.; Liu, Y.; Wang, G.; et al. Reduction and Functional Exhaustion of T Cells in Patients with Coronavirus Disease 2019 (COVID-19). Front. Immunol. 2020, 11, 827. [Google Scholar] [CrossRef] [PubMed]
- Cury, V.; de Lima, T.M.; Prado, C.M.; Pinheiro, N.; Ariga, S.K.; Barbeiro, D.F.; Moretti, A.I.; Souza, H.P. Low level laser therapy reduces acute lung inflammation without impairing lung function. J. Biophotonics 2016, 9, 1199–1207. [Google Scholar] [CrossRef]
- Oliveira, M.C., Jr.; Greiffo, F.R.; Rigonato-Oliveira, N.C.; Custódio, R.W.A.; Silva, V.R.; Damaceno-Rodrigues, N.R.; Almeida, F.M.; Albertini, R.; Lopes-Martins, R.Á.B.; de Oliveira, L.V.F.; et al. Low-level laser therapy reduces acute lung inflammation in a model of pulmonary and extrapulmonary LPS-induced ARDS. J. Photochem. Photobiol. B. 2014, 134, 57–63. [Google Scholar] [CrossRef]
- Nejatifard, M.; Asefi, S.; Jamali, R.; Hamblin, M.R.; Fekrazad, R. Probable positive effects of the photobiomodulation as an adjunctive treatment in COVID-19: A systematic review. Cytokine 2021, 137, 155312. [Google Scholar] [CrossRef]
- Maldaner, D.R.; Azzolin, V.F.; Barbisan, F.; Mastela, M.H.; Teixeira, C.F.; Dihel, A.; Duarte, T.; Pellenz, N.L.; Lemos, L.F.C.; Negretto, C.M.U.; et al. In vitro effect of low-level laser therapy on the proliferative, apoptosis modulation, and oxi-inflammatory markers of premature-senescent hydrogen peroxide-induced dermal fibroblasts. Lasers Med. Sci. 2019, 34, 1333–1343. [Google Scholar] [CrossRef]
- Atri, C.; Guerfali, F.Z.; Laouini, D. Role of Human Macrophage Polarization in Inflammation during Infectious Diseases. Int. J. Mol. Sci. 2018, 19, 1801. [Google Scholar] [CrossRef] [Green Version]
- Tam, S.Y.; Tam, V.C.W.; Ramkumar, S.; Khaw, M.L.; Law, H.K.W.; Lee, S.W.Y. Review on the Cellular Mechanisms of Low-Level Laser Therapy Use in Oncology. Front. Oncol. 2020, 10, 1255. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, J.L.; Britto, A.; Souza, N.H.; Ligeiro de Oliveira, A.P.; Anatriello, E.; Albertini, R.; Aimbire, F. The M1/M2 Pattern and the Oxidative Stress are Modulated by Low-Level Laser in Human Macrophage. J. Clin. Cell Immunol. 2016, 7, 1. [Google Scholar] [CrossRef]
- Pestka, S.; Krause, C.D.; Walter, M.R. Interferons, interferon-like cytokines, and their receptors. Immunol. Rev. 2004, 202, 8–32. [Google Scholar] [CrossRef] [PubMed]
- Kawai, T.; Akira, S. Toll-like receptors and their crosstalk with other innate receptors in infection and immunity. Immunity 2011, 5, 637–650. [Google Scholar] [CrossRef] [Green Version]
- Baxter, V.K.; Griffin, D.E. Interferon-Gamma Modulation of the Local T Cell Response to Alphavirus Encephalomyelitis. Viruses 2020, 12, 113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Samuel, C.E. Antiviral actions of interferons. Clin. Microbiol. Rev. 2001, 14, 778–809. [Google Scholar] [CrossRef] [Green Version]
- Tolentino, M.; Cho, C.C.; Lyons, J.A. Photobiomodulation therapy (PBMT) regulates the production of IL-10 and IFN-Ɣ by peripheral blood mononuclear cells (PBMC) and CD4+ T cells isolated from subjects with Multiple Sclerosis (MS). J. Immunol. 2019, 202, 193.16. [Google Scholar]
- Soheilifar, S.; Fathi, H.; Naghdi, N. Photobiomodulation therapy as a high potential treatment modality for COVID-19. Lasers Med. Sci. 2020, 1–4. [Google Scholar] [CrossRef]
- Guimarães, L.L.; De Brito, A.A.; Santos, T.G.; Cereta, A.D.; De Oliveira, L.V.; De Oliveira, A.P.L.; Da Palma, R.K. Low-level laser boosts extracellular matrix cues and enhances acellular lung scaffold recellularization. Eur. Respir. J. 2020, 56, 561. [Google Scholar] [CrossRef]
- Eguchi, K.; Fujitani, N.; Nakagawa, H.; Miyazaki, T. Prevention of respiratory syncytial virus infection with probiotic lactic acid bacterium Lactobacillus gasseri SBT2055. Sci. Rep. 2019, 9, 4812. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, Y.; Saruta, J.; Takahashi, T.; To, M.; Shimizu, T.; Hayashi, T.; Morozumi, T.; Kubota, N.; Kamata, Y.; Makino, S.; et al. Effect of ingesting yogurt fermented with Lactobacillus delbrueckii ssp. bulgaricus OLL1073R-1 on influenza virus-bound salivary IgA in elderly residents of nursing homes: A randomized controlled trial. Acta Odontol. Scand. 2019, 77, 517–524. [Google Scholar] [CrossRef]
- Chong, H.X.; Yusoff, N.A.A.; Hor, Y.Y.; Lew, L.C.; Jaafar, M.H.; Choi, S.B.; Yusoff, M.S.B.; Wahid, N.; Abdullah, M.F.I.L.; Zakaria, N.; et al. Lactobacillus plantarum DR7 improved upper respiratory tract infections via enhancing immune and inflammatory parameters: A randomized, double-blind, placebo-controlled study. J. Dairy Sci. 2019, 102, 4783–4797. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Yeh, C.; Jin, Z.; Ding, L.; Liu, B.Y.; Zhang, L.; Dannelly, H.K. Prospective study of probiotic supplementation results in immune stimulation and improvement of upper respiratory infection rate. Synth. Syst. Biotechnol. 2018, 3, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Pu, F.; Guo, Y.; Li, M.; Zhu, H.; Wang, S.; Shen, X.; He, M.; Huang, C.; He, F. Yogurt supplemented with probiotics can protect the healthy elderly from respiratory infections: A randomized controlled open-label trial. Clin. Interv. Aging 2017, 12, 1223–1231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohamed, M.S.M.; Elshaghabee, F.M.F.; Alharbi, S.A.; El-Hussein, A. The Prospective Beneficial Effects of Red Laser Exposure on Lactocaseibacillus casei Fermentation of Skim Milk. Biology 2020, 9, 256. [Google Scholar] [CrossRef]
- Chen, Q.; Wu, J.; Dong, X.; Yin, H.; Shi, X.; Su, S.; Che, B.; Li, Y.; Yang, J. Gut flora-targeted photobiomodulation therapy improves senile dementia in an Aß-induced Alzheimer’s disease animal model. J. Photochem. Photobiol. B 2021, 216, 112152. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Yang, J.; Dong, C.; Fu, Y.; Liu, H. Gut microbiome-mediated changes in bone metabolism upon infrared light exposure in rats. J. Photochem. Photobiol. B. 2021, 217, 112156. [Google Scholar] [CrossRef]
- Zupin, L.; Caracciolo, I.; Tricarico, P.M.; Ottaviani, G.; D’Agaro, P.; Crovella, S. Antiviral properties of blue laser in an in vitro model of HSV-1 infection. Microbiol. Immunol. 2018, 62, 477–479. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferri, G.; Tricarico, P.M.; Vincelli, I.; Gratton, R.; Ottaviani, G.; Boniotto, M.; Zupin, L.; Crovella, S. Photobiomodulation therapy is able to decrease IL1B gene expression in an in vitro cellular model of hidradenitis suppurativa. Lasers Med. Sci. 2020, 35, 1003–1005. [Google Scholar] [CrossRef] [PubMed]
- Zupin, L.; Gratton, R.; Fontana, F.; Clemente, L.; Pascolo, L.; Ruscio, M.; Crovella, S. Blue photobiomodulation LED therapy impacts SARS-CoV-2 by limiting its replication in Vero cells. J. Biophotonics 2021, 14, e202000496. [Google Scholar] [CrossRef] [PubMed]
- Vetrici, M.A.; Mokmeli, S.; Bohm, A.R.; Monici, M.; Sigman, S.A. Evaluation of Adjunctive Photobiomodulation (PBMT) for COVID-19 Pneumonia via Clinical Status and Pulmonary Severity Indices in a Preliminary Trial. J. Inflamm. Res. 2021, 14, 965–979. [Google Scholar] [CrossRef] [PubMed]
- Weber, H.M.; Mehran, Y.Z.; Orthaber, A.; Saadat, H.H.; Weber, R.; Wojcik, M. Anti-viral Photodynamic Therapy in Covid-19 Management: A Novel Approach in Treating Patients in Early Infection Stages. Akupunkt. Aurikulomed. 2021, 47, 29–34. [Google Scholar] [CrossRef]

| Prebiotics | Probiotics | Paraprobiotics | Postbiotics | Synbiotics | |
|---|---|---|---|---|---|
| Definition | Prebiotics are a group of nutrients that are degraded by gut microbiota “dietary prebiotics” as “a selectively fermented ingredient that results in specific changes in the composition and/or activity of gastrointestinal microbiota, thus conferring benefit upon host health” [71]. | Live micro-organisms that, when administered in adequate amounts, confer a health benefit on the host, probiotic fermented food [72]. Food fermented by or containing probiotic(s) with strain-specific/without strain-specific evidence [73]. | They are also called phantom or inactivated probiotics and are in fact “non-viable microbial cells (either intact or broken), or crude cell extracts that, when administered (either orally or locally) in appropriate amounts, confer a benefit to the human consumer or animal” [70]. (or metabolic byproducts) secreted by viable bacteria or released after their lysis” [74]. As “inactivated probiotics” and “ghost probiotics” [75,104,105,106,107,108,109,110,111,112,113]. | They are “non-viable bacterial products or metabolic products” from micro-organisms that have biological activity in the host. [75,104,105,106,107,108,109,110,111,112,113]. | Probiotics and prebiotics that are used in combination, are known as “synbiotics” [76]. |
| Sources | Asparagus, sugar beet, garlic, chicory, onion, Jerusalem artichoke, wheat, honey, banana, barley, tomato, rye, soybean, human’s and cow’s milk, peas, beans, etc., and recently, seaweeds and microalgae [77]. | Fermented natural or industrial products with one or more types of bacteria such as: Lactobacillus acidophilus, LGG, Lactobacillus casei Shirota, Lactobacillus gasseri, and Bifidobacterium bifidum Yogurt; Frozen yogurt; Kefir; Buttermilk; Acidophilus milk; Lebne; Viili; Lassi; Aged cheeses; fermented cabbage; Pickles and olives produced by traditional methods [78]. | Different species of bacterial cultures: Lactobacillus spp. Bifidobacterium spp. proved their efficacy after inactivation, especially with heat. Bioactive compounds: Bifidobacterium lactis Bb12: peptides and proteins Lactic acid bacteria: peptidoglycans, lipopolysaccharides, and DNA Saccharomyces cerevisiae: β-glucan Lactobacillus strains: lipoteichoic acids LGG: lipoteichoic acid and peptidoglycans Lactococcus lactis H61 teichoic acid and lipoteichoic acid [79]. | Metabolic byproducts of live probiotic bacteria such as cell-free supernatant, vitamins, organic acids, short-chain fatty acids, secreted proteins/peptides, bacteriocins, neurotransmitters, secreted biosurfactants, amino acids, flavonoids derived postbiotics (desaminotyrosine, equol daidzein, daidzein, norathyriol), terpenoids derived postbiotics (genipin, paeoniflorin, paeoni lactone glycosides, paeonimetabolin I, II, III), phenolic-derived postbiotics (equol, urolithins, valerolactones, enterolactone, enterodiol, 8-prenylnaringenin) etc. [80]. | Lactobacillus spp., Bifidobacterium spp., S. boulardii, B. coagulans are probiotic strains that are used in synbiotic formulations; whereas the prebiotics used are as: oligosaccharides (fructooligosaccharide (FOS), galacto-oligosaccharide GOS and xyloseoligosaccharide (XOS), and inulin (from natural sources such as chicory and yacon roots)). |
| Types | Fructans Galacto-Oligosaccharides Starch and Glucose-Derived Oligosaccharides Other Oligosaccharides Non-Carbohydrate Oligosaccharides (e.g., cocoa-derived flavanols) [81]. Fermented grain foods or vegetables as well as beer and wine that contain β-glucans, oligosaccharides and polyphenols compounds. [82,83,85]. | Fermented natural or industrial products. The most popular types of probiotics are: Lactobacillus, or Döderlein’s bacillus; L. casei; Bifidobacterium bifidus; Saccharomyces boulardii [86]. | Paraprobiotics consist of a wide range of molecules including peptidoglycans, surface proteins, cell wall polysaccharides [87]. | Non-viable probiotics Biosurfactants Exopolysaccharides Cell surface proteins Teichoic acids Peptidoglycans Cell-free supernatant and soluble factors Bacteriocins Short-chain fatty acids Vitamins | Combinations of probiotic and prebiotic types. |
| Prebiotics | Probiotics | Paraprobiotics | Postbiotics | Synbiotics | |
|---|---|---|---|---|---|
| Mechanisms of Action | Could change the composition and population of the intestinal microbiota [30,31,32,33,34,35,36,37,38,39,40,88]. Anti-inflammatory effects by increasing short-chain fatty acids (SCFAs) [acetate, propionate, and butyrate] [89]. Influences glucose and lipid metabolism [90]. Important role in cell proliferation, differentiation, and apoptosis [91]. Improve immunity functions by increasing the population of protective micro-organisms by: Oligofructose and inulin mixture Fructo-oligosaccharides (FOS), Galacto-oligosaccharides (GOS) Trans-galacto-oligosaccharides (TOS) Can affect the brain by the vagus nerve. It affects the brain through three routes, including neural, endocrine, and immune pathways [71]. | a. Colonization and regulation of dysbiotic intestinal microbiota [92]. b. Protection of the epithelial barrier by maintaining tight junction integrity [74,93]. c. Induction of mucin production and B-cell-secreting IgA [94]. d. Ability to increase adhesion to the intestinal mucosa and to inhibit adhesion of the pathogens through competition [95]. e. Administration of antimicrobial products such as acetic and lactic acids and bacteriocins, which have strong inhibitory effects against Gram-negative bacteria [96]. f. Produce of SCFAs with anti-inflammatory and immune modeling effects. Participates in the differentiation, proliferation cell and release of immune pathway signaling molecules. SCFAs increase the expression of the anti-inflammatory cytokine IL-10 and suppress pro-inflammatory responses [65,67,97]. h. Gut–brain axis interaction with the production of metabolites as well g-aminobutyric acid (GABA) [98]. i. Adjusting the innate and / or adaptive immune response of the host [99]. | Colonization and regulation of dysbiotic intestinal microbiota. Protection of the epithelial barrier. Ability to increase adhesion to the intestinal mucosa. Produce of SCFAs with anti-inflammatory and immune modeling effects. Immunomodulatory, adjusting the innate and / or adaptive immune response. Inhibition of the NF-kB signaling pathway. Antiviral Antihypertensive Hypocholesterolemic Antiproliferative Antioxidant Immunomodulatory [100]. Anti-inflammatory [101]. Antimicrobial and maintaining of gut health [102]. Antitumor activity Antimicrobial properties Antagonistic effects against pathogens | Immunomodulator, influenced by retinoic acid-acting mucosal dendritic cells and their subsequent effects on regulatory T-cells, with increased IL10 production [103]. Anti-inflammatory action: increases IL-10 secretion, inhibits TNF-α secretion, reduces IL-12, increases IL-8 levels, blocks NF-κB activation. Antioxidant activity Antitumor effects [104]. Infection prevention [105]. Anti-atherosclerotic [106]. Autophagy [107]. Accelerated wound healing [108]. | Elevated levels of lactobacilli and bifidobacteria, balance the intestinal microbiota. Prevention of bacterial translocation and the incidence of nosocomial infections in surgical patients. Improving liver function in patients with cirrhosis. Improving immunomodulatory capacity [109]. |
| Clinical Applications | Irritable Bowel Syndrome Crohn’s Disease Colorectal Cancer Necrotizing Enterocolitis Memory, concentration, and learning; Mood; Autism Allergic skin diseases; atopic dermatitis. Cardiovascular diseases Calcium absorption [71,95]. | Prevention and amelioration of various diseases: Acute nosocomial diarrhea, secondary to antibiotic therapy Allergic manifestations (eczema, allergic rhinitis, conjunctivitis, wheezing) Diarrhea due to inflammatory bowel disease Type 2 diabetes Obesity Heart disease Cancer etc. [87,95]. | Anti-infective Anti-allergic Obesity Anti-cancer Anti-inflammatory bowel disease Effects on respiratory diseases Recovery of intestinal injuries [70,87]. | Treating or preventing multiple diseases: Alzheimer’s disease, Allergies, Inflammatory bowel disease, Multiple sclerosis, In addition, in particular, many diseases in children [70,74]. | Are considered important tools in maintaining human and animal health, and in the prevention and/or alternatives to reduce the risks associated with diseases. Improve metabolic dysfunction and prevent diabetes in prediabetes [110]. Obesity [111,112]. Irritable bowel syndrome [113]. Suppression allergy syndrome Prevent asthma [114]. Disease prevention (e.g., prophylaxis of various types of cancer) Manages health. Reducing healthcare costs. |
| Side Effects | Prebiotics have no life-threatening or severe side effects. In some cases, abdominal discomfort, bloating and gas may occur while the digestive system adjusts [71]. | “Probiotics” may theoretically be responsible for four types of side effects: 1. Systemic infections 2. Deleterious metabolic activities 3. Excessive immune stimulation in susceptible individuals 4. Gene transfer [40,41,72]. | Paraprobiotics have long shelf life, safety, and beneficial effects, such as modulation of immunity, modification of biological responses, reduction of cholesterol, anti-inflammatory, and antiproliferative properties. [79]. | (1) There is no risk of bacterial translocation from the intestinal lumen into the blood of vulnerable and immune-compromised subjects (2) There is no chance of acquiring and transferring genes that produce antibiotic resistance (3) Easier to extract, standardize, transport and store (4) Loss of viability through cell lysis can produce additional benefits (5) Improved interaction of each molecule released from cells disrupted with the epithelial cells [115]. | Prebiotics and probiotics together tested to date have a strong safety record [116], and synbiotics formulated with them might also be presumed safe for the same intended uses [117]. Mild side effects are gas, bloating, digestive problems such as diarrhea or constipation. |
| Side Effects | Probiotics | Disease | Brief Description of the Study | Reference |
|---|---|---|---|---|
| Bacteremia | LGG | Ulcerative colitis Lactobacillus bacteremia | A case of Lactobacillus bacteremia has been described in a 17-year-old boy with ulcerative colitis treated with systemic corticosteroids and infliximab, who had a fever of 102 °F, flushing and chills one week after the start of LGG probiotics. | [130] |
| LGG | Severe active ulcerative colitis in an adult patient | It was reported on a case of bacteremia caused by LGG in an adult patient affected by severe active ulcerative colitis under treatment with corticosteroids and mesalazine. | [131] | |
| LGG | 89 patients with Lactobacillus bacteremia; 82% of cases had severe or fatal comorbidities | Risk factors and outcome were analyzed for 89 patients with Lactobacillus bacteremia. Mortality was 26% at one month, and 48% at one year. Serious underlying diseases were a significant predictor of mortality, while in vitro effective antimicrobial treatment was associated with lower mortality. | [132] | |
| LGG and 7 different species | Collection of cases of Lactobacillus bacteremia, National Infectious Disease Register (NIDR), 1995–2000, Finland | 90 cases of Lactobacillus bacteremia were diagnosed, of which LGG was the most common species. Annual incidence of Lactobacillus bacteremia in the Finnish population was, on average, 0.29 cases/100,000 inhabitants/year. | [133] | |
| Fungemia | Saccharomyces boulardii | Clostridioides difficile acute diarrhea Saccharomyces boulardii fungemia | A case of Saccharomyces cerevisiae fungemia has been reported in a patient with Clostridioides difficile-associated diarrhea (CDAD) in oral treatment with S. boulardii and vancomycin. The identification of S. cerevisiae was confirmed by molecular technique. Fungemia is a rare but serious complication of probiotic treatment. The authors draw clinicians’ attention to the risk of toxic effects when prescribing probiotics, especially for immunocompromised patients. | [134] |
| Saccharomyces boulardii | Clostridioides difficile recurrent diarrhea. Rheumatoid arthritis. Anemia. Malnutrition Saccharomyces boulardii fungemia | The authors published the case of 79-year-old female with rheumatoid arthritis, who after a resection of the intestine developed S. boulardii fungemia. She had complications: anemia, malnutrition and several nosocomial infections, including recurrent diarrhea associated with C. difficile. Diarrhea was treated with Metronidazole, Vancomycin and Sachaflor (probiotic Saccharomyces cerevisiae, subtype S. boulardii). | [135] | |
| Saccharomyces boulardii | Clostridioides difficile colitis. Saccharomyces boulardii fungemia | A case of fungemia caused by Saccharomyces cerevisiae in an elderly patient treated orally with S. boulardii in combination with vancomycin for Clostridioides difficile colitis. It is not recommended the administration of this viable yeast, especially in debilitated patients with active colitis. | [136] | |
| Saccharomyces boulardii | Head and neck cancer Aseptic diarrhea Oral mucositis | 65-year-old man who developed Saccharomyces cerevisiae fungemia after completing a course of chemotherapy and radiation therapy for head and neck cancer. For grade IV oral mucositis and received Saccharomyces boulardii (Perenterol) orally as a treatment for aseptic diarrhea, just before the onset of fungemia. | [137] | |
| Saccharomyces boulardii | Myeloid leukemia Saccharomyces fungemia | Saccharomyces fungemia in an 8-month-old baby with acute myeloid leukemia during treatment with intensive chemotherapy. Patient received prophylaxis treatment with Saccharomyces boulardii (Codex) capsules to prevent diarrhea. Saccharomyces cerevisiae was isolated from blood culture, although the patient also received antifungal prophylaxis with fluconazole. | [138] | |
| Saccharomyces boulardii | Vascular catheter Saccharomyces fungemia | Four cases of Saccharomyces boulardii fungemia in patients who had a vascular catheter. To prevent catheter contamination, the authors recommend that packages or capsules of Saccharomyces boulardii be opened with gloves outside the patient’s room. | [139] | |
| Fungemia | Saccharomyces boulardii | Seven cases of fungal infection with Saccharomyces boulardii pathology in Intensive Care Unit | Seven cases of fungal infection with Saccharomyces boulardii (Sb) occurred in a 12-bed intensive care unit (ICU); 11 severely ill patients, mechanically ventilated, treated with broad-spectrum antibiotics with central venous catheter and previously treated with Sb. Explanation of the phenomenon was discussed: (1) a high-dose intestinal translocation in seriously ill patients; (2) a contamination of the central venous catheter and (3) a massive colonization of patients with this yeast. | [140] |
| Saccharomyces boulardii | Pathology in Intensive Care Unit S. cerevisiae fungemia | 3 patients were identified with S. cerevisiae fungemia in an intensive care unit (ICU) after receiving a probiotic containing Saccharomyces boulardii (Ultralevura) through a nasogastric tube for an average duration of 8.5 days. A literature review identified another 57 cases of S. cerevisiae fungemia, of which 60% of patients were in intensive care and 71% received enteral or parenteral nutrition. The use of probiotics was identified in 26 patients and 17 patients died. The administration of S. cerevisiae probiotics should be carefully reevaluated, especially in immunosuppressed patients or critically ill patients. | [141] | |
| Saccharomyces boulardii | Pathology in Intensive Care Unit S. cerevisiae fungemia | Two cases of fungemia in an intensive care unit after a probiotic treatment containing S. boulardii. The authors draw attention to the use of probiotics in patients with critical illnesses. | [142] | |
| Saccharomyces boulardii | Pathology in Intensive Care Unit Saccharomyces boulardii probiotic-associated fungemia | A case of fungemia in an immunocompetent patient after administration of probiotics containing Saccharomyces boulardii; the fungal infection was proved by genomic and proteomic modeling methods. Study calls into question the safety of this preventive biotherapy. | [143] | |
| Saccharomyces boulardii | Saccharomyces cerevisiae fungaemia | Seven patients with S. cerevisiae fungus were reported in two hospitals in India between July 2014 and September 2015. Two patients were premature newborns, and five adults were admitted to an intensive care unit and received probiotics containing S. boulardii. Five patients responded promptly to echinocandins or voriconazole. The authors recommend avoiding the probiotic containing S. boulardii in patients with critical conditions. | [144] | |
| Saccharomyces boulardii | Urosepsis superinfected with Klebsiella pneumoniae and Escherichia coli and diarrhea. Saccharomyces cerevisiae fungemia | An 88-year-old patient was admitted to the intensive care unit with a diagnosis of urosepsis superinfected with Klebsiella pneumoniae, Escherichia coli and diarrhea. He received empirical treatment with meropenem (2 × 500 mg) and linezolid (1 × 600 mg), through a central venous catheter (CVC); for the relief of diarrhea received S. boulardii (Reflor 250 mg capsules). Attention was drawn concerning the use of probiotics in immunocompetent patients. | [145] | |
| Saccharomyces spp., Lactobacillus spp., Bifidobacterium spp., Bacillus spp. | Fungemia Endocarditis Abscess Pneumonia, Pleural empyema Septic arthritis Saccharomyces Lactobacillus Bifidobacterium Bacillus | In a systematic review of adverse reactions to probiotics in the main international databases published by August 2018, a total of 93 patients were analyzed. Fungemia was the most common infectious complications in 37.6% cases. Genus Saccharomyces was the most frequent in 50.6% cases, followed by Lactobacillus, Bifidobacterium, Bacillus, Pedioccocus and Escherichia in 27.9%, 12.8%, 5.4%, 2.2% and 1.1% cases, respectively. Adults over 60 years of age, Clostridioides difficile colitis, antibiotic use and Saccharomyces infections were associated with overall mortality. HIV infections, immune-suppressive drugs, solid organ transplantation, deep intravenous lines, enteral or parenteral nutrition were not associated with mortality. Administration of probiotics cannot be considered risk-free. | [146] | |
| Saccharomyces cerevisiae var. boulardii | Clostridioides difficile-associated diarrhea | A case of fungemia in a patient suffering from Clostridioides difficile-associated diarrhea treated with metronidazole and a probiotic containing S. cerevisiae var. boulardii. Fluconazole 400 mg/day was started, and the probiotic was stopped. Potential benefit of S. cerevisiae var. boulardii should be accurately evaluated, especially in elderly patients. | [147] | |
| Disseminated infection | LGG ATCC 53103 | Disseminated LGG ATCC 53103 infection Intrauterine growth restriction | A disseminated LGG ATCC 53103 infection was suspected in a 6-day-old newborn with intrauterine growth restriction symptoms, treated empirically with antibiotics and given LGG with the aim of preventing antibiotic-associated gastrointestinal complications. | [148] |
| Empyema | LGG | Lactobacillus empyema Immunodeficiency virus-infected lung transplant | A case of Lactobacillus empyema in a patient infected with the human immunodeficiency virus who received a cardiothoracic transplant and a probiotic containing LGG. | [149] |
| Risk of celiac disease autoimmunity | Lactobacillus reuteri and LGG | Celiac Disease Autoimmunity | Aim of the study was to investigate the relationship between probiotic use in dietary supplements or infant formulas up to 1 year of age and the occurrence of celiac disease autoimmunity (CDA) and celiac-like disease among a cohort of 6520 genetically susceptible children. The use of probiotics in the first year of life was detected in 1460 children through the intake of probiotic food supplements, which were associated with a slightly increased risk of CDA, compared to children who did not receive probiotics. | [150] |
| Type of Study | Probiotics | Targets/Types of Respiratory Tract Infections | Results | Reference |
|---|---|---|---|---|
| Animal study | VSL#3 probiotic: Bifidobacterium breve, Bifidobacterium longum, Bifidobacterium infantis, Lactobacillus acidophilus, Lactobacillus plantarum, Lactobacillus paracasei, Lactobacillus bulgaricus, Streptococcus thermophiles | Biopsies of colon and jejunum tissues, and inguinal or axillary lymph nodes. Cellular and humoral immunity and inflammation in healthy macaques | Daily treatment with the VSL#3 probiotic (PBio) resulted in significantly increased frequencies of B-cells expressing IgA in the colon and lymph node (LN), likely because of significantly increased LN T follicular helper cell frequencies and LN follicles. Increased frequencies of IL-23 + Antigen-presenting cells (APCs) in the colon were found post-PBio treatment, which correlated with LN T follicular helper cells. VSL#3 significantly downmodulated the response of TLR2-, TLR3-, TLR4-, and TLR9-expressing HEK293 cells. Beneficial impact of PBio on mucosal health and the possibility of using probiotics in the context of vaccination or prevention against mucosal infections. | [217] |
| Systematic review | Bifidobacterium longum BB536 Lactobacillus plantarum L-137 L. plantarum DK 119 Lactobacillus paracasei L. rhamnosus CRL 1505 L. reuteri DSM 1793 L. gasseri TMC0356 B. animalis subsp. Lactis BB12 | Acute respiratory tract infections (pneumonia, influenza, enterovirus, adenovirus, and respiratory syncytial virus infections) caused by DNA/RNA viruses. COVID-19 | Purpose of this review was to summarize existing information on the gut mediated-pulmonary immunity conferred by probiotics. Recent evidence has shown an association between COVID-19 disease and intestinal dysbiosis. Due to the proved close relationship of the gastrointestinal and respiratory tract, the dysfunction of the first may trigger disease in the last. Probiotics could reshape the composition of the intestinal microbiota and consequently, regulate immune responses in the respiratory system. Probiotic strains and their metabolites, such as bacteriocins, have been studied as potential antiviral agents. Due to the high mutational rate of RNA viruses and a major challenge of restricted antibiotic efficacy, probiotic administration would help increase host immunity and, similar to other antiviral studies, could reduce the symptoms of the new coronavirus. Probiotics have become a nutraceutical and promising immunobiotic agent to possibly treat COVID-19 infection following the absence of a vaccine or a proven therapeutic intervention. | [219] |
| Animal study | Heat-killed Lactobacillus plantarum L-137 (HK-LP) | Influenza virus infection | C57BL/6 mice intranasally infected with influenza virus A/FM/1/47 (H1N1, a mouse-adapted strain) were administered orally HK-LP. Survival time was significantly prolonged, an appreciable level of interferon (IFN)-β was detected in the serum, and the viral titers in the lung were significantly lower in mice treated with HK-LP than controls. No IFN-β was detected in controls after influenza infection. HK-LP, a potent IFN-β inducer, could prevent against influenza infection. | [220] |
| Animal study | Lactobacillus casei strain Shirota (LcS) | Upper respiratory influenza virus (IFV) infection | Mice were intranasally administered Lactobacillus casei strain Shirota (LcS) and a strong production of interleukin 12, gamma interferon, and tumor necrosis factor alpha was proved in mediastinal lymph node cells, very important in excluding influenza virus (IFV). Titers of virus in the nasal wash of mice inoculated with 200 μg of LcS for three consecutive days (LcS 200 group) before infection were significantly (p < 0.01) lower than those of mice not inoculated with LcS (control group) (100.9 ± 0.6 versus 102.1 ± 1.0). The survival rate of the mice in the LcS 200 group was significantly (p < 0.05) greater than that of the mice in the control group (69% versus 15%). Decrease in the titer of virus in the upper respiratory tract to 1/10 of the control level was important in preventing death. Intranasal administration of LcS enhances cellular immunity in the respiratory tract and protects against influenza virus infection. | [224] |
| Lactobacillus pentosus strains, L. casei Shirota, L. plantarum strains, L. delbrueckii subsp. bulgaricus OLL1073R1, LGG, L. gasseri TMC0356, Lactococcus lactis subsp. cremoris FC, L. brevis KB, B. breve YIT4064 | Influenza virus infection in mice | Oral or intranasal administration of mentioned probiotics have reduced the infection, virus titer in the lungs or nasal washings, and increased mice survival. | ||
| Systematic review | L. plantarum NCIMB 8826 L. reuteri F275 | Pneumovirus infection in mice | Virus-induced inflammation was suppressed, and the mice were protected against lethal disease. | [225] |
| L. rhamnosus CRL1505 L. rhamnosus CRL1506 | Respiratory syncytial virus infection | Nasally administered probiotics differentially modulated immune responses and induced protection against respiratory syncytial virus infection. | ||
| In vivo and in vitro animal study on BALB/cCrSlc mice | Lactobacillus gasseri SBT2055 (LG2055) | Antiviral activity against respiratory syncytial virus (RSV) on HEp-2 human laryngeal epithelial cells and MLE12 mouse lung epithelial cells. Pro-inflammatory cytokines TNF-α, CCL2, IL-1β, and IL-6 in lung tissue. Proteomic analysis of a total of 1120 proteins | LG2055 inhibited RSV replication in vitro and in vivo and suppressed the inflammatory response in the lungs of mice. LG2055 enhanced IFN-β and IFN-γ expression at the gene level in the lungs of mice, decreased the expression of SRCAP, one of the most strongly LG2055-down-regulated protein, and inhibited the RSV replication. LG2055 is a promising probiotic useful for preventing RSV infection and relieving the associated symptoms. | [302] |
| Clinical study on elderly (randomized and controlled) | Lactobacillus delbrueckii subsp. bulgaricus OLL1073R-1. 100 g of 1073R-1-yogurt for 12 weeks. Control participants consumed yogurt fermented with a different Lactobacillus strain (control yogurt). | Influenza A virus subtype H3N2-bound | Consumption of 1073R-1-yogurt affected influenza A virus subtype H3N2-bound IgA levels in saliva. In addition, saliva flow rate and total IgA levels increased in response to the yogurt intake period in both the 1073R-1 and control yogurt group. Continuous daily ingestion of 1073R-1-yogurt may help prevent infection with influenza A virus subtype H3N2 in elderly with weakened immunity. | [303] |
| Clinical trial (double-blind randomized, placebo-controlled) | L. plantarum DR7, isolated from bovine milk; 9 log CFU/day for 12 weeks | Health conditions via monthly questionnaires, and cytokine concentrations, peroxidation, oxidative stress, and gene expression in T-cells and natural killer (NK) cells from blood samples were assessed for upper respiratory tract infections (URTI), during the 12-wk intervention period | DR7 reduced the duration of nasal symptoms and the frequency of URTI, compared to placebo. DR7 suppressed plasma pro-inflammatory cytokines (IFN-γ, TNF-α) and increased anti-inflammatory cytokines (IL-4, IL-10); it reduced plasma peroxidation and oxidative stress levels compared to placebo group. A higher expression of plasma CD44 and CD117, and a lower expression of plasma CD4 and CD8 compared with the placebo, indicating less T-cell activation. Enhanced presence of non-resting and mature NK cells compared to placebo. DR7 treatment alleviated the symptoms of URTI by improving inflammatory parameters and enhancing immunomodulatory properties and could be suitable for food or health applications. | [304] |
| Prospective clinical trial (double-blind randomized, placebo-controlled) | Daily probiotic drink (150 mL) that contained L. paracasei at 3 × 107 CFU/mL, L. casei 431 at 3 × 107 CFU/mL, and L. fermentum PCC at 3 × 106 CFU/mL; or placebo drink administered after lunch, for 12 weeks. | 136 adults diagnosed with common cold or influenza-like respiratory illness (collectively upper respiratory infections (URI)) at least four times in the previous year were enrolled. Blood and fecal samples were collected at two time points: at baseline, and at 12 weeks. Subject compliance was followed by daily questionnaires | Probiotics significantly reduced the incidence of URIs and influenza-like symptoms with an oral temperature higher than 38 °C compared to the placebo group. The probiotic group had a significantly higher level of IFN-γ in serum and sIgA in the intestine compared to the placebo group and compared to the results of the initial tests. In contrast, there were no significant differences in serum with respect to IL-4, IL-10, IgA, IgG or IgM between probiotics and placebo groups. Probiotics have been safe and effective in combating the common cold and flu-like respiratory infections by stimulating the immune system. | [305] |
| Clinical study on 205 volunteers aged ≥45 years (double-blind randomized, placebo-controlled) | 300 mL/day of yogurt supplemented with Lactobacillus paracasei N1115, 3.6 × 107 CFU/mL for 12 weeks. Control group, normal diet without any probiotic | Incidence of URTI, and changes in serum protein, immunoglobulins, and the profiles of the T-lymphocyte subsets (total T-cells [CD3+], T-helper cells [CD4+], and T-cytotoxic-suppressor cells [CD8+]) | The risk of URTI in the intervention group was assessed as 55% of that in the control group. The change in the percentage of CD3+ cells in the intervention group was significantly higher than in the control group, but no significant differences were observed in the total levels of protein, albumin, globulin and prealbumin in both groups. Therefore, N1115 may reduce the risk of acute URTI in the elderly. Improving natural T-cell-mediated immune defense could be one of the important mechanisms underlying probiotics to express their anti-infective effects. | [306] |
| Type of Study | PBM Parameters and Protocol | Performed Analysis | PBM Effects | Reference |
|---|---|---|---|---|
| Experimental study with red laser on L. casei NRRL-B-1922 | Red laser 632.7 nm, 40 mW; 3, 6, 12 J/cm2; exposure time 10, 20, 40 min, respectively. | PBM (red laser exposure) applied to L. casei NRRL-B-1922 before the fermentation of skim milk. | Exposure of L. casei NRRL-B-1922 to the dose of 12 J/cm2 before skimmed milk fermentation exhibited a significant improvement of the anti-oxidant capacity, β-galactosidase, antimicrobial, and proteolytic activities. It decreased the cholesterol and lactose levels of fermented skimmed milk, enhancing the fermentation process of skimmed milk prepared with L. casei NRRL-B-1922. It opens the perspective of red laser photobiomodulation of probiotic bacteria during the fermentation process of skimmed milk to improve the quality of fermented milk on an industrial scale, with significant economic benefits, too. | [307] |
| Animal study on BALB/c mice | Abdomen irradiated with red (660 nm), output power 75 mW, power density 93.75 mW/cm2; or, infrared (808 nm), output power = 83 mW, power density = 103.75 mW/cm2, either as single or multiple doses, over a two-week period. Spot size = 0.8 cm2 for both lasers, pulse frequency of 250 Hz. Each mouse received a total energy density of 10 J/cm2. Sham treatments were identical. | Genomic DNA extracted from fecal pellets was pyrosequenced for the 16S rRNA gene. | Allobaculum bacterium, associated with a healthy microbiome, significantly increased (p < 0.001) after infrared (but not red light) PBM by day 14. It is the first experiment proving that PBM can alter microbiome diversity in healthy mice and increase numbers of Allobaculum. If confirmed in humans, it opens avenues for PBMT to be applied as an auxiliary treatment in obesity, cardiovascular and neurodegenerative diseases, as well as other disorders. | [266] |
| Animal study on C57BL/6 N mice | PBM was performed on the abdomen of the mice at the wavelengths of 630 nm, 730 nm, and 850 nm. Irradiation time was 1000 s (16 min and 40 s), the power density was 10 mW/cm2, and the energy density was 100 J/cm2, once a day, 5 times a week, for 8 weeks. | Gut flora-targeted PBM (gf-targeted PBM) on Alzheimer’s disease (AD) animal model. Expression levels of 509 proteins, which involved the pathways of hormone synthesis, phagocytosis, and metabolism. The 16 s rRNA gene sequencing of fecal contents. | Gf-targeted PBM reversed the imbalance of intestinal flora and improved learning ability, amyloid plaque deposition, tau phosphorylation, and microglia inflammation of Aß-induced AD mice. Many proteins in the hippocampus responded to gf-targeted PBM, with mitochondrial respiratory chain complex enzymes as a possible key intermediate target. PBM significantly altered the diversity and abundance of intestinal flora, reversing the typical increase of Helicobacter and uncultured Bacteroidales, and the decreasing the Rikenella seen in AD mice. Gf-targeted PBM has the potential to be a noninvasive microflora regulation method for Alzheimer’s disease patients. Future studies will confirm the effect of gf-targeted PBM on the brain-gut axis, promoting PBM as a potential prevention and treatment method for AD. | [308] |
| Animal study on Sprague-Dawley (SD) rats | PBM with IR (830 nm, 100 mW/cm2) supplementary light irradiation was carried out from 14:00 to 14:30 every day, for three months. Illuminance in the feeding box was 1000 lx. | Concentration of bone metabolism markers, including 1,25-dihydroxyvitamin D3 (1,25-(OH)2-D3), bone-specific alkaline phosphatase (BALP), and tartrate-resistant acid phosphatase (TRACP), were detected from blood samples in four study groups. Whole body, femur and tibia of the rats were scanned with a dual-energy X-ray bone densitometer. Bacterial genomic DNA was extracted from the frozen stool samples with a DNA extraction kit. The V3-V4 region of the 16S rRNA (341F-805R), F: GATCCTACGGGAGGCAGCA; R: GCTTACCGCGGCTGCTGGC) was studied. An open-source R package, Tax4Fun, was first used to analyze the enrichment of functional genes of the microbiome of each group. | Analysis of the structure and function of gut microbiota in the rats after PBM infrared supplementation significantly reduced the abundance of Saccharibacteria and increased the abundance of Clostridiaceae 1 and Erysipelotrichaceae bacteria. Results proved that changes in the gut microbiome correlate well with bone mass and bone metabolism. Infrared supplementation can have a positive effect on rat bone metabolism by affecting gut microbiota. These findings could be used in the future design of healthy lighting environments that prevent or possibly ameliorate osteoporosis. | [309] |
| Animal study on C57BL/6 mice | PBM with laser at 660 nm and radiant exposure of 10 J/cm2, was applied six hours after intratracheal inflammation produced with instillation of lipopolysaccharide (LPS) (5 mg/kg) or phosphate buffer saline (PBS). | Inflammatory cells in perivascular and alveolar spaces, and inflammatory mediator secretion. | Increased expression and secretion of cytokines (TNF-α, IL-1β, IL-6,) and chemokine (MCP-1). PBM induced a significant decrease in both inflammatory cell influx and inflammatory mediator secretion. PBM did not affect the mechanical properties of the lungs, nor the strength of the tissue, nor the elasticity. PBM reduced the inflammatory reaction in the lungs exposed to LPS without affecting lung function and recovery. | [288] |
| Animal study on BALB/c mice | PBM (830 nm laser, 9 J/cm2, 35 mW, 80s per point, 3 points per application) was applied in direct contact with skin, 1 h after LPS administration. Mice were distributed in control (n = 6; PBS), ARDS IT (n = 7; LPS orotracheally 10 μg/mouse), ARDS IP (n = 7; LPS intra-peritoneally 100 μg/mouse), ARDS IT + Laser (n = 9; LPS intra-tracheally 10 μg/mouse), ARDS IP + Laser (n = 9; LPS intra-peritoneally 100 μg/mouse). | LPS-induced pulmonary and extrapulmonary acute respiratory distress syndrome (ARDS). 24 h after last LPS administration, mice were studied for pulmonary inflammation by total and differential cell count in bronchoalveolar lavage (BAL), cytokines (IL-1beta, IL-6, KC and TNF-alpha) levels in BAL fluid and by quantitative analysis of neutrophils number in the lung parenchyma. | PBM significantly reduced pulmonary and extrapulmonary inflammation in LPS-induced ARDS, reduced number of total cells and neutrophils in BAL, reduced levels of IL-1beta, IL-6, KC and TNF-alpha in BAL fluid and in serum, as well as the number of neutrophils in lung parenchyma. PBM was efficient in reducing pulmonary inflammation in both pulmonary and extrapulmonary model of LPS-induced ARDS. | [289] |
| In vitro study of dermal fibroblast cell line (HFF-1) with premature senescence H2O2-induced | PBMT: 660 nm, energy density = 3, 4, 5, 6, and 8 J/cm2; power density = 35 mW; time = 10 s, 14 s, 16 s, 20 s, and 28 s. Beam area = 0.035 cm2, beam diameter = 0.21 cm2, frequency 16 Hz, pulsed. Number of points 8. Area of the laser application 9.6 cm2. Contact/No contact—distance of 35 mm. Cellular mortality, proliferation, and the levels of oxidative, inflammatory cytokines, apoptotic markers, and of two growth signaling molecules (FGF-1 and KGF) were compared among treatments. | Protein quantification of the following markers: DNA 8-deoxyguanosine and cytokines involved in inflammatory response interleukin IL-1β, IL-6, IL-10, tumoral necrosis factor alfa (TNF-α), and interferon-gamma (IFN-γ). Caspase-1, caspase-3, and caspase-8 activities were determined by assay kits, fluorometric. | Interaction between H2O2 at 50 μM and PBM at 4 J (best dose) showed partially reversion of the higher levels of DNA oxidation, CASP 3, CASP 8, IL-1B, IL-6, and IFN-γ induced by H2O2 exposure. PBM also trigger increase of IL-10 anti-inflammatory cytokine, FGF-1 and KGF levels. PBM on the fibroblast without injury was relative safe and harmless, given its cytogenotoxic potential, oxy-inflammatory, and proliferative effects. However, in the injured H2O2 fibroblast, PBM had significant protection and proliferative effect, partially or totally reversing the negative effects triggered by H2O2. At certain dose ranges, PBM may trigger anti-aging properties. | [291] |
| In vitro model of human keratinocytes cell line (HaCaT) infected with Herpes Simplex Virus Type-1 (HSV-1) | HSV-1 were irradiated using a diode laser device (class IV) with the following two protocols: 445 nm, 0.3 W/cm2, 60 J/cm2, CW, or 445 nm, 0.15 W/cm2, 30 J/cm2, 5 Hz. | After 30 min the virus irradiated and not irradiated was transferred to a HaCaT cells culture and then, after another 24 h HSV-1 quantification was performed on the cell supernatants. Five experimental settings were used and the increase in cell vitality and the decrease in HSV-1 viral load in supernatants of previously irradiated virus-infected cells were measured comparatively with non-irradiated virus-infected cells. | Experimental results proved that the blue laser has antiviral activity against HSV-1, and it is more effective against virus irradiated alone, suggesting that PBMT inactivates the virus prior to cell entry. In contrast, when the virus is already inside the cells, the effect of PBMT is less evident and does not increase cells’ resistance to infection. Blue PBM had a direct inhibitory effect on the virus itself. Further studies are necessary to determine how blue PBM exerts its antiviral effect, the aim being to move from an in vitro to a clinical setting, thus promoting its use on HSV-1 infected patients. | [310] |
| In vitro cellular model of hidradenitis suppurativa (HS) on human keratinocyte cell line (HaCaT) | Two irradiation protocols with near-infrared (NIR) and Blue PBM: 970 nm, 0.3 W/cm2, 20 J/cm2, continuous wave (CW) and 445 nm, 0.2 W/cm2, 10 J/cm2, CW, using fluency at 10–30–50 J/cm2. | Effect of PBM on IL1B gene (encoding for interleukin-1β [IL-1β]) expression in immortalized human keratinocyte cell line using a wild-type line and a knockout cell model mimicking genetic-driven Hidradenitis Suppurativa (HS). | Based on the hypothesis that increased production of pro-inflammatory cytokines would promote a dysbiosis of resident skin microbes and so, the perpetuation of skin inflammation in HS, it was shown that PBM decreased IL1B gene expression, which could block the up-mentioned vicious mechanism. PBM could be a useful tool in the management of skin lesions in patients with HS. | [311] |
| In vitro model of SARS-CoV-2 infection | PBMT using LEDs at 450 nm with 12.5 J/cm2; 454 nm with 10 J/cm2; 470 nm with 20 J/cm2; irradiance of 40 mW/cm2, continuous waves. | Experiments were performed on Vero E6 epithelial normal cell line derived from the kidney of Cercopithecus aethiops (ATCC CRL-1586), with three experimental settings: SARS-CoV-2 was irradiated and then transferred to cells; already infected cells were irradiated; cells were irradiated prior to infection. | Results may support the possible exploitation of blue light to meet the challenges of SARS-CoV-2, as blue wavelengths have stopped SARS-CoV-2 replication. The antiviral activity of PBMT against SARS-CoV-2 on human cell lines is intended to propose translatability for this new approach to support individuals affected by COVID-19, also considering that PBMT is largely safe, with no side effects, and well tolerated by patients. | [312] |
| Systematic review | PBM using red to infrared light (λ = 600–1070 nm) has been analyzed in several pre-clinical models of Alzheimer’s and Parkinson’s disease, as an emerging putative neuroprotective therapy. | Tissue stressed by hypoxia, toxic insult, genetic mutation and mitochondrial dysfunction. | Analysis proved important reductions in β-amyloid plaques, neurofibrillary tangles of hyperphosphorylated tau protein, inflammation and oxidative stress, together with increased ATP levels and improved overall mitochondrial function as follows: increase (↑) Cell survival (striatal and cortical cells), ↑T-Helper + cells, ↑ATP content, ↑Complex IV-dependent respiration, decrease (↓) Oxidative stress, ↓Inflammation, ↑Mitochondrial function, ↑Heat shock proteins, ↓Amyloid aggregates, ↓Hyperphosphorylated tau. In addition, PBM reduced the characteristic cognitive deficits in transgenic mouse models. | [268] |
| Systematic review | A literature search was conducted for published reports on the effect of PBM [visible or near-infrared (NIR)] on the microbiome, red (630–680 nm) or in the NIR region (780–940 nm) and (980 and 1064 nm). Power densities: 10–100 mW/cm2, energy densities in the region of 4–50 J/cm2. | Subcellular, cellular (neurons, epithelial cells, keratinocytes, fibroblasts etc.) and tissue levels. Organ level: brain (oscillation patterns), gut etc. Microbiome. | The following conclusions can be drawn: A. Light can affect the microbiome indirectly through the daily circadian rhythm. B. Light has an indirect effect on the microbiome through vitamin D, produced by the action of sunlight on keratinocytes. C. PBM effects on cytochrome c oxidase (CCO): ↑CCO, ↑Mitochondrial membrane potential, ↑ATP production, brief burst of reactive oxygen species, ↑nitric oxide, ↑cyclic AMP, ↑movements in intracellular calcium, ↑transcription factors, ↑expression of a multitude of gene, ↑structural proteins, ↑enzymes, ↑cell division, ↑cell migration. D. PBM effects at cellular and tissue level: ↑nonvisual phototransduction cascades involving opsins. Blue (415 nm) and green (540 nm) light absorbed by opsins, trigger opening of transient receptor potential (TRP) calcium ion channels. E. PBM on inflammatory pathways: ↓pro-inflammatory cytokines (IL-6, TNF-α, IFN-γ). F. PBM on immune system: ↑circulating immune cells (mast cells, macrophages, etc.), transduce protective signals from distal tissues to sites of injury (brain, heart, or gut). E. PBM on microbiome: ↑Akkermansia muciniphila, ↑Bifidobacterium spp., ↑Faecalibacterium spp., and ↓Firmicutes/Bacteroidetes ratio. | [270] |
| Randomized clinical trial with COVID-19 pneumonia | Two laser sources (808 nm and 905 nm), working simultaneously and synchronously as follows: 1. Three GaAlAs laser diodes, 808 nm, peak power of 1 W, average power 500 mW each diode, in total 1.5 W, power density 75 mW/cm2, 1500 Hz, duty cycle of 50%, pulse duration of 330 µs, spot size of 19.6 cm2. 2. Three superpulsed GaAs laser diodes, 905 nm, peak power 75 W, average power 203 mW each diode, in total 610 mW, power density 31 mW/cm2, 1500 Hz (train pulses 90 kHz modulated at 1 Hz ÷ 2 kHz), pulse duration of 100 ns, spot size of 19.6 cm2. Each lung was scanned for 14 min, from apex to base, over an area of 250 cm2 of the posterior thorax, resulting in 28 min of PBMT with a dosage of 7.18 J/cm2 and a total energy of 3590 J. | PBMT group received standard medical care plus adjunctive PBMT, four daily sessions of near-infrared light treatment targeting the lung tissue. Control group received only standard medical care. Patient outcomes were measured via blood work, chest x-rays, pulse oximetry and validated scoring tools for pneumonia. | PBMT-treated patients showed rapid recovery, did not require ICU admission or mechanical ventilation, and reported no long-term sequelae at 5 months after treatment. In the control group, 60% of patients were admitted to the ICU for mechanical ventilation. The control group had an overall mortality of 40%. At a 5-month follow-up, 40% of the control group experienced long-term sequelae. PBMT is a safe and effective potential treatment for COVID-19 pneumonia and improves clinical status in COVID-19 pneumonia. | [313] |
| PDT clinical trial with COVID-19 in the early stage of infection | Laser light watch with 4 red laser diodes (658 nm), 2 blue (447 nm), 2 green (532 nm) and 2 yellow (589 nm) LEDs for systemic treatment of blood via the wrist arteries for 60 min; one nose treatment applicator with 1 blue LED (447 nm) and 1 UVA LED (375 nm), 10 min each nostril with blue and UVA light (sides switched after 10 min); one mouth treatment applicator with 14 blue LEDs (447 nm) and 14 UVA LEDs (375 nm) for 20 min inside the mouth and throat. As photosensitizer for photodynamic therapy (PDT): 2 capsules Riboflavin-5phosphate 100 mg/each treatment, as follows: one capsule for systemic application taken 1 h before starting the PDT, and the second one (100 mg) dissolved into a glass of 200 mL water (for local application in nose, mouth and throat). | Two groups with 20 patients each: one group receiving PDT and daily testing, and a control group receiving conventional care plus testing. All patients in both groups had positive Covid-19 test results at the beginning of the study being in an early infection stage with mild symptoms such as fever, dry cough, headache, hard breathing, fatigue etc. QPCR tests with CT-viral load were performed on day 1, 2, 3, 4, 5 and 7 in the PDT group, and on day 1, 3, 5 and 7 in the control group. | All 20 patients in the PDT group showed significant improvement in clinical symptoms and viral load assessment within the 5 days of PDT. 14 out of 20 patients had a negative QPCR test after 5 days of PDT, while the other 6 patients also showed significantly reduced viral load. All 20 patients in the control group were tested 3 times within 5 days and no significant improvement could be seen clinically or in the viral load assessment. | [314] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ailioaie, L.M.; Litscher, G. Probiotics, Photobiomodulation, and Disease Management: Controversies and Challenges. Int. J. Mol. Sci. 2021, 22, 4942. https://doi.org/10.3390/ijms22094942
Ailioaie LM, Litscher G. Probiotics, Photobiomodulation, and Disease Management: Controversies and Challenges. International Journal of Molecular Sciences. 2021; 22(9):4942. https://doi.org/10.3390/ijms22094942
Chicago/Turabian StyleAilioaie, Laura Marinela, and Gerhard Litscher. 2021. "Probiotics, Photobiomodulation, and Disease Management: Controversies and Challenges" International Journal of Molecular Sciences 22, no. 9: 4942. https://doi.org/10.3390/ijms22094942
APA StyleAilioaie, L. M., & Litscher, G. (2021). Probiotics, Photobiomodulation, and Disease Management: Controversies and Challenges. International Journal of Molecular Sciences, 22(9), 4942. https://doi.org/10.3390/ijms22094942