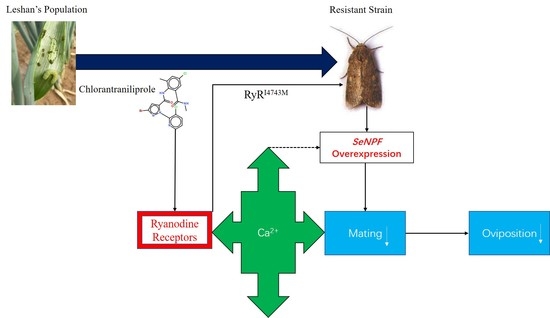
Fitness Costs of Chlorantraniliprole Resistance Related to the SeNPF Overexpression in the Spodoptera exigua (Lepidoptera: Noctuidae)
Abstract
:1. Introduction
2. Results
2.1. Comparison of the Reproductive Capacity and Egg Hatchability between the SE-Sus and SE-Sel Strains
2.2. Comparison of the Ovarian Index between the SE-Sus and SE-Sel Strains
2.3. Gene Expression Pattern of SeVg in the SE-Sus and SE-Sel Strains
2.4. Illumina Sequencing, Read Assembly, and Annotation
2.5. Analysis of Gene Expression and Cluster Analysis of DEGs
2.6. GO and KEGG Enrichment of DEGs
2.7. Screening the Candidate Genes and Quantitative PCR (RT-qPCR)
2.8. Neuropeptide Diversity Analysis
2.9. Analysis of the Effect of SeNPF Overexpression on Mating and Oviposition
2.10. Statistics and Annotation of SNPs and Sequence Analysis of the RyR Genes
3. Discussion
4. Methods and Methods
4.1. Insects
4.2. Insecticide and Reagents
4.3. Mating Behavior and Reproductive Bioassays
4.4. Ovary Solution Plane
4.5. Gene Expression Pattern of SeVg in the SE-Sus and SE-Sel Strains
4.6. Transcriptome Analysis
4.7. Quantitative PCR (qRT-PCR)
4.8. Diversity and Collinearity of Neuropeptide Genes
4.9. Statistics and Annotation of Single Erroneously Paired Nucleotides and Sequence Analysis of RyR Genes
4.10. RNAi
4.11. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Moulton, J.K.; Pepper, D.A.; Jansson, R.K.; Dennehy, T.J. Pro-active management of beet armyworm (Lepidoptera: Noctuidae) resistance to tebufenozide and methoxyfenozide: Baseline monitoring, risk assessment, and isolation of resistance. J. Econ. Entomol. 2002, 95, 414–424. [Google Scholar] [CrossRef]
- Wu, G.; Guo, J.Y.; Wan, F.H.; Xiao, N.W. Responses of three successive generations of beet armyworm, Spodoptera exigua, fed exclusively on different levels of gossypol in cotton leaves. J. Insect Sci. 2010, 10, 764–800. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Liu, Y.Q.; Yu, W.L.; Deng, Z.R.; Gao, M.; Liu, F.; Mu, W. Resistance of Spodoptera exigua to ten insecticides in Shandong, China. Phytoparasitica 2011, 39, 315–324. [Google Scholar] [CrossRef]
- Zheng, X.L.; Pan, W.; Cheng, W.J.; Wang, X.P.; Lei, C.L. Projecting overwintering regions of the Beet Armyworm, Spodoptera exigua, in China using the CLIMEX Model. J. Insect Sci. 2012, 12, 13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mu, W.; Wu, K.M.; Zhang, W.J.; Guo, Y.Y. The interaction resistance and population fitness of the near isogenic line of the beta cypermethrin resistant in Spodoptera exigua (Hübner). Sci. Agric. Sin. 2005, 38, 2007–2013. [Google Scholar]
- Lai, T.C.; Li, J.; Su, J.Y. Monitoring of beet armyworm Spodoptera exigua (Lepidoptera: Noctuidae) resistance to chlorantraniliprole in China. Pestic. Biochem. Physiol. 2011, 101, 198–205. [Google Scholar] [CrossRef]
- Che, W.N.; Shi, T.; Wu, Y.D.; Yang, Y.H. Insecticide resistance status of field populations of Spodoptera exigua (Lepidoptera: Noctuidae) from China. J. Econ. Entomol. 2013, 106, 1855–1862. [Google Scholar] [CrossRef] [PubMed]
- Su, J.Y.; Sun, X.X. High level of metaflumizone resistance and multiple insecticide resistance in field populations of Spodoptera exigua (Lepidoptera: Noctuidae) in Guangdong Province, China. Crop. Prot. 2014, 61, 58–63. [Google Scholar] [CrossRef]
- Zhang, P.; Gao, M.; Mu, W.; Zhou, C.; Li, X.H. Resistant levels of Spodoptera exigua to eight various insecticides in Shandong, China. J. Pest. Sci. 2014, 39, 7–13. [Google Scholar] [CrossRef] [Green Version]
- Truong, K.M.; Pessah, I.N. Comparison of chlorantraniliprole and flubendiamide activity toward wild-type and malignant hyperthermia-susceptible ryanodine receptors and heat stress intolerance. Toxicol. Sci. 2019, 167, 509–523. [Google Scholar] [CrossRef]
- Teixeira, L.A.; Andaloro, J.T. Diamide insecticides: Global efforts to address insect resistance stewardship challenges. Pestic. Biochem. Physiol. 2013, 106, 76–78. [Google Scholar] [CrossRef]
- Sun, L.; Qiu, G.S.; Li, C.; Ma, C.S.; Yuan, H.Z. Molecular characterization of a ryanodine receptor gene from Spodoptera exigua and its upregulation by chlorantraniliprole. Pestic. Biochem. Physiol. 2015, 123, 56–63. [Google Scholar] [CrossRef] [PubMed]
- James, M.M.; Edward, E.C.; Sonia, O.M.; Dixon, J.W.; Susan, D.K.; Brian, M.S.; José, R.L. Functional ryanodine receptors in the membranes of neurohypophysial secretory granules. J. Gen. Physiol. 2014, 143, 693–702. [Google Scholar]
- McKinney, D.A.; Eum, J.H.; Dhara, A.; Strand, M.R.; Brown, M.R. Calcium influx enhances neuropeptide activation of ecdysteroid hormone production by mosquito ovaries. Insect Biochem. Mol. Biol. 2016, 70, 160–169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lahm, G.P.; Selby, T.P.; Freudenberger, J.H.; Stevenson, T.M.; Myers, B.J.; Seburyamo, G.; Smith, B.K.; Flexner, L.; Clark, C.E.; Cordova, D. Insecticidal anthranilic diamides: A new class of potent ryanodine receptor activators. Bioorg. Med. Chem. Lett. 2005, 15, 4898–4906. [Google Scholar] [CrossRef]
- David, B.S.; Daniel, C.; Timothy, R.C. Insect ryanodine receptors: Molecular targets for novel pest control chemicals. Invert. Neurosci. 2008, 8, 107–119. [Google Scholar]
- Gao, C.; Yao, R.; Zhang, Z.; Wu, M.; Zhang, S.; Su, J. Susceptibility baseline and chlorantraniliprole resistance monitoring in Chilo suppressalis (Lepidoptera: Pyralidae). J. Econ. Entomol. 2013, 106, 2190–2194. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Liang, P.; Zhou, X.; Gao, X.W. Novel mutations and mutation combinations of ryanodine receptor in a chlorantraniliprole resistant population of Plutella xylostella (L.). Sci. Rep. 2014, 4, 6924. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.; Wu, Y. High levels of resistance to chlorantraniliprole evolved in field populations of Plutella xylostella. J. Econ. Entomol. 2012, 105, 1019–1023. [Google Scholar] [CrossRef]
- Wang, X.G.; Xiang, X.; Yu, H.L.; Liu, S.H.; Yin, Y.; Cui, P.; Wu, Y.; Yang, J.; Jiang, C.X.; Yang, Q.F. Monitoring and biochemical characterization of beta-cypermethrin resistance in Spodoptera exigua (Lepidoptera: Noctuidae) in Sichuan Province, China. Pestic. Biochem. Physiol. 2018, 146, 71–79. [Google Scholar] [CrossRef]
- Kliot, A.; Ghanim, M. Fitness costs associated with insecticide resistance. Pest Manag. Sci. 2012, 68, 1431–1437. [Google Scholar] [CrossRef] [PubMed]
- Abbas, N.; Shah, R.M.; Shad, S.A.; Azher, F. Dominant fitness costs of resistance to fipronil in Musca domestica Linnaeus (Diptera: Muscidae). Vet. Parasitol. 2016, 226, 78–82. [Google Scholar] [CrossRef]
- Cao, G.C.; Han, Z.J. Tebufenozide resistance selected in Plutella xylostella and its cross-resistance and fitness cost. Pest Manag. Sci. 2006, 62, 746–751. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, L.M.S.; Wanderley-Teixeira, V.; Ferreira, H.N.; Teixeira, A.A.C.; Siqueira, H.A.A. Fitness costs associated with field-evolved resistance to chlorantraniliprole in Plutella xylostella (Lepidoptera: Plutellidae). Bull. Entomol. Res. 2013, 104, 88. [Google Scholar] [CrossRef]
- Abbas, N.; Shad, S.A.; Razaq, M.; Waheed, A.; Aslam, M. Resistance of Spodoptera litura (Lepidoptera: Noctuidae) to profenofos: Relative fitness and cross resistance. Crop. Prot. 2014, 58, 49–54. [Google Scholar] [CrossRef]
- Passos, D.A.; Silva-Torres, C.S.A.; Siqueira, H.A.A. Behavioral response and adaptive cost in resistant and susceptible Plutella xylostella to chlorantraniliprole. Bull. Entomol. Res. 2019, 110, 96–105. [Google Scholar] [CrossRef]
- Ma, K.S.; Tang, Q.L.; Xia, J.; Lv, N.N.; Gao, X.W. Fitness costs of sulfoxaflor resistance in the cotton aphid, Aphis gossypii Glover. Pestic. Biochem. Physiol. 2019, 158, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Wahli, W. Evolution and expression of vitellogenin genes. Trends Genet. 1988, 4, 227–232. [Google Scholar] [CrossRef]
- Giorgi, F.; Bradley, J.T.; Nordin, J.H. Differential vitellin polypeptide processing in insect embryos. Micron 1999, 30, 579–596. [Google Scholar] [CrossRef]
- Tufail, M.; Nagaba, Y.; Elgendy, A.M.; Takeda, M. Regulation of vitellogenin genes in insects. Entomol. Sci. 2014, 17, 269–282. [Google Scholar] [CrossRef]
- Zuo, Y.Y.; Ma, H.H.; Lu, W.J.; Wang, X.L.; Wu, S.W.; Nauen, R.; Wu, Y.D.; Yang, Y.H. Identification of the ryanodine receptor mutation I4743M and its contribution to diamide insecticide resistance in Spodoptera exigua (Lepidoptera: Noctuidae). Insect Sci. 2020, 27, 791–800. [Google Scholar] [CrossRef]
- Peng, W.; Shen, H.Z.; Wu, J.P.; Guo, W.Q.; Pan, X.J.; Wang, R.W.; Chen, S.R.W.; Yan, N. Structural basis for the gating mechanism of the type 2 ryanodine receptor RyR2. Science 2016, 354, 53241–532410. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.L.; Mao, K.K.; Liao, X.; He, B.Y.; Jin, R.H.; Tang, T.; Wan, H.; Li, J.H. Fitness cost of nitenpyram resistance in the brown planthopper Nilaparvata lugens. J. Pest Sci. 2018, 91, 1145–1151. [Google Scholar] [CrossRef]
- Castellanos, N.L.; Haddi, K.; Carvalho, G.A.; de Paulo, P.D.; Hirose, E.; Guedes, R.N.C.; Smagghe, G.; Oliveira, E.E. Imidacloprid resistance in the Neotropical brown stink bug Euschistus heros: Selection and fitness costs. J Pest. Sci. 2019, 92, 847–860. [Google Scholar] [CrossRef]
- Amdam, G.V.; Page, R.E.; Fondrk, M.K.; Brent, C.S. Hormone response to bidirectional selection on social behavior. Evol. Dev. 2010, 12, 428–436. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, J.; Sun, Y.; Xiao, L.B.; Tan, Y.A.; Bai, L.X. Molecular characterization and expression of vitellogenin gene from Spodoptera exigua exposed to cadmium stress. Gene 2016, 593, 179–184. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Lu, M.X.; Han, G.J.; Du, Y.Z.; Wang, J.J. Sublethal effects of chlorantraniliprole on development, reproduction, and vitellogenin gene (CsVg) expression in the rice stem borer, Chilo. suppressalis. Pest Manag. Sci. 2016, 72, 2280–2286. [Google Scholar] [CrossRef]
- Zhao, J.; Sun, Y.; Xiao, L.; Tan, Y.; Jiang, Y.; Bai, L. Vitellogenin and vitellogenin receptor gene expression profiles in Spodoptera exigua are related to host plant suitability. Pest Manag. Sci. 2018, 74, 950–958. [Google Scholar] [CrossRef] [PubMed]
- Zhen, C.A.; Miao, L.; Gao, X.W. Sublethal effects of sulfoxaflor on biological characteristics and vitellogenin gene (AlVg) expression in the mirid bug, Apolygus. lucorum (Meyer-Dür). Pestic. Biochem. Physiol. 2018, 144, 57–63. [Google Scholar] [CrossRef]
- Shu, Y.H.; Wang, J.W.; Lu, K.; Zhou, J.L.; Zhou, Q.; Zhang, G.R. The first vitellogenin receptor from a Lepidopteran insect: Molecular characterization, expression patterns and RNA interference analysis. Insect Mol. Biol. 2011, 20, 61–73. [Google Scholar] [CrossRef]
- Hansen, I.A.; Attardo, G.M.; Roy, S.G.; Raikhel, A.S. Target of rapamycin-dependent activation of S6 kinase is a central step in the transduction of nutritional signals during egg development in a mosquito. J. Biol. Chem. 2005, 280, 20565–20572. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Subala, P.R.; Shivakumar, M.S. Circadian variation affects the biology and digestive profiles of a nocturnal insect Spodoptera litura (Insecta: Lepidoptera). Biol. Rhythm. Res. 2017, 48, 207–226. [Google Scholar] [CrossRef]
- Parthasarathy, R.; Sun, Z.Y.; Bai, H.; Palli, S.R. Juvenile hormone regulation of vitellogenin synthesis in the red flour beetle, Tribolium castaneum. Insect Biochem. Mol. Biol. 2010, 40, 405–414. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Romain, L.; Miguel, C.; Franziska, W.; Dihego, O.A.; Jose, E.S.; Laurent, K. Interplay between insulin signaling, juvenile hormone, and vitellogenin regulates maternal effects on polyphenism in ants. Proc. Natl. Acad. Sci. USA 2013, 10, 11050–11055. [Google Scholar]
- Maxwell, R.A.; Welch, W.H.; Horodyski, F.M.; Schegg, K.M.; Schooley, D.A. Juvenile hormone diol kinase. II. Sequencing, cloning, and molecular modeling of juvenile hormone-selective diol kinase from Manduca sexta. J. Biol. Chem. 2002, 277, 21882–21890. [Google Scholar] [CrossRef] [Green Version]
- Stay, B.; Tobe, S.S. The role of allatostatins in juvenile hormone synthesis in insects and crustaceans. Annu. Rev. Entomol. 2007, 52, 277–299. [Google Scholar] [CrossRef]
- Elekonich, M.M.; Horodyski, F.M. Insect allatotropins belong to a family of structurally myoactive peptides present in several invertebrate phyla. Peptides 2003, 24, 1623–1632. [Google Scholar] [CrossRef]
- Gospocic, J.; Shields, E.J.; Glastad, K.M.; Lin, Y.; Penick, C.A.; Yan, H.; Mikheyev, A.S.; Linksvayer, T.A.; Garcia, B.A.; Berger, S.L.; et al. The neuropeptide corazonin controls social behavior and caste identity in ants. Cell 2017, 170, 748–759.e12. [Google Scholar] [CrossRef]
- Oh, Y.; Yoon, S.E.; Zhang, Q.; Chae, H.S.; Daubnerova, I.; Shafer, O.T.; Choe, J.; Kim, Y.J. A homeostatic sleep-stabilizing pathway in Drosophila composed of the sex peptide receptor and its ligand, the myoinhibitory peptide. PLoS Biol. 2014, 12, e1001974. [Google Scholar] [CrossRef]
- Tsuda, M.; Aigaki, T. Evolution of sex-peptide in Drosophila. Fly 2016, 10, 172–177. [Google Scholar] [CrossRef] [PubMed]
- Hasemeyer, M.; Yapici, N.; Heberlein, U.; Dickson, B.J. Sensory neurons in the Drosophila genital tract regulate female reproductive behavior. Neuron 2009, 61, 511–518. [Google Scholar] [CrossRef] [Green Version]
- Yao, Z.; Macara, A.M.; Lelito, K.R.; Minosyan, T.Y.; Shafer, O.T. Analysis of functional neuronal connectivity in the Drosophila brain. J. Neurobiol. 2012, 108, 684–696. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shohat-Ophir, G.; Kaun, K.R.; Azanchi, R.; Mohammed, H.; Heberlein, U. Sexual deprivation increases ethanol intake in Drosophila. Science 2012, 335, 1351–1355. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, X.J.; Riabinina, O.; Li, J.; Potter, C.J.; Clandinin, T.R.; Luo, L. A transcriptional reporter of intracellular Ca2+ in Drosophila. Nat. Neurosci. 2015, 18, 917–925. [Google Scholar] [CrossRef] [Green Version]
- Liu, W.; Ganguly, A.; Huang, J.; Wang, Y.; Ni, J.D.; Gurav, A.S.; Aguilar, M.A.; Montell, C. Neuropeptide F regulates courtship in Drosophila through a male-specific neuronal circuit. eLife 2019, 12, 8. [Google Scholar] [CrossRef]
- Tuelher, E.; da Silva, É.H.; Freitas, H.; Namorato, F.; Serrão, J.; Guedes, R.; Oliveira, E. Chlorantraniliprole-mediated toxicity and changes in sexual fitness of the Neotropical brown stink bug Euschistus heros. J. Pest Sci. 2017, 90, 397–405. [Google Scholar] [CrossRef]
- Robinson, R.; Carpenter, D.; Shaw, M.A.; Halsall, J.; Hopkins, P. Mutations in RYR1 in malignant hyperthermia and central core disease. Hum. Mutat. 2006, 27, 977–989. [Google Scholar] [CrossRef]
- Angela, C.G.; Timothy, W.H.; Naohiro, Y. Malignant hyperthermia-associated mutations in S2-S3 cytoplasmic loop of type 1 ryanodine receptor calcium channel impair calcium-dependent inactivation. Am. J. Physiol. Cell Physiol. 2016, 311, 749–757. [Google Scholar]
- Gould, F.; Brown, Z.S.; Kuzma, J. Wicked evolution: Can we address the sociobiological dilemma of pesticide resistance. Science 2018, 360, 728–732. [Google Scholar] [CrossRef] [Green Version]
- Tao, Y.; Gutteridge, S.; Benner, E.A.; Wu, L.; Rhoades, D.F.; Sacher, M.D.; Rivera, M.A.; Desaeger, J.; Cordova, D. Identification of a critical region in the Drosophila ryanodine receptor that confers sensitivity to diamide insecticides. Insect Biochem. Mol. Biol. 2013, 43, 820–828. [Google Scholar] [CrossRef]
- Silva, J.E.; Ribeiro, L.M.S.; Vinasco, N.; Guedes, R.N.C.; Siqueira, H.A.A. Field-evolved resistance to chlorantraniliprole in the tomato pinworm Tuta absoluta: Inheritance, cross-resistance profile, and metabolism. J. Pest Sci. 2019, 92, 1421–1431. [Google Scholar] [CrossRef]
- Guo, L.; Wang, Y.; Zhou, X.G.; Li, Z.Y.; Liu, S.Z.; Pei, L.; Gao, X.W. Functional analysis of a point mutation in the ryanodine receptor of Plutella xylostella (L.) associated with resistance to chlorantraniliprole. Pest Manag. Sci. 2014, 70, 1083–1089. [Google Scholar] [CrossRef]
- Zuo, Y.Y.; Wang, H.; Xu, Y.J.; Huang, J.L.; Wu, S.W.; Wu, Y.D.; Yang, Y.H. CRISPR/Cas9 mediated G4946E substitution in the ryanodine receptor of Spodoptera exigua confers high levels of resistance to diamide insecticides. Insect Biochem. Mol. Biol. Biol. 2017, 89, 79–85. [Google Scholar] [CrossRef]
- Roditakis, E.A.; Steinbach, D.B.C.; Moritz, G.C.; Vasakis, E.A.; Stavrakaki, M.A.; Ilias, A.A.; García-Vidal, L.D.; Martínez-Aguirre, M.D.R.D.; Bielza, P.D.; Morou, E.D.; et al. Ryanodine receptor point mutations confer diamide insecticide resistance in tomato leafminer, Tuta absoluta (Lepidoptera: Gelechiidae). Insect Biochem. Mol. Biol. 2017, 80, 11–20. [Google Scholar] [CrossRef]
- Huang, Q.; Wang, X.G.; Yao, X.G.; Gong, C.W.; Shen, L.T. Effects of bistrifluron resistance on the biological traits of Spodoptera litura (Fab.) (Noctuidae Lepidoptera). Ecotoxicology 2019, 28, 323–332. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.G.; Chen, Y.Q.; Gong, C.W.; Yao, X.G.; Jiang, C.X.; Yang, Q.F. Molecular identification of four novel cytochrome P450 genes related to the development of resistance of Spodoptera exigua (Lepidoptera: Noctuidae) to chlorantraniliprole. Pest. Manag. Sci. 2018, 74, 1938–1952. [Google Scholar] [CrossRef]
- Mortazavi, A.; Williams, B.A.; McCue, K.; Schaeffer, L.; Wold, B. Mapping and quantifying mammalian transcriptomes by RNA-Seq. Nat. Methods. 2008, 5, 621–628. [Google Scholar] [CrossRef]
- McKenna, A.; Hanna, M.; Banks, E.; Sivachenko, A.; Cibulskis, K.; Kernytsky, A.; Garimella, K.; Altshuler, D.; Gabriel, S.; Daly, M.; et al. The genome analysis toolkit: A mapreduce framework for analyzing next-generation DNA sequencing data. Genome Res. 2010, 20, 1297–1303. [Google Scholar] [CrossRef] [Green Version]
- Cingolani, P.; Platts, A.; Wang, L.L.; Coon, M.; Nguyen, T.; Wang, L.; Land, S.J.; Lu, X.Y.; Ruden, D.M. A program for annotating and predicting the effects of single erroneously paired nucleotides, SnpEff: SNPs in the genome of Drosophila melanogaster strain w1118; iso-2; iso-3. Fly 2012, 6, 80–92. [Google Scholar] [CrossRef] [Green Version]
- Ma, Z.Z.; Zhou, H.; Wei, Y.L.; Yan, S.; Shen, J.A. Novel plasmid-Escherichia coli system produces large batch dsRNAs for insect gene silencing. Pest. Manag. Sci. 2020, 76, 2505–2512. [Google Scholar] [CrossRef]
- Ruan, Y.W.; Wang, X.G.; Xiang, X.; Xu, X.; Guo, Y.Q.; Liu, Y.H.; Yin, Y.; Wu, Y.Q.; Cheng, Q.H.; Gong, C.W.; et al. Status of insecticide resistance and biochemical characterization of chlorpyrifos resistance in Sogatella furcifera (Hemiptera:Delphacidae) in Sichuan Province, China. Pestic. Biochem. Physiol. 2021, 171, 104723. [Google Scholar] [CrossRef] [PubMed]








| Gene | Primer | Sequence (5′–3′) | Length |
|---|---|---|---|
| CYP6AEW | 4495-F | AAAAAGAATGTACCGCACGCC | 148 |
| 4495-R | CCGTTCCGTAGTAAGCTCCA | ||
| SeABCG23 | 6176-F | TGGAAGCTACGGAGCACTTG | 124 |
| 6176-R | GGTCCAGCGAAGTCAGTCAA | ||
| Vitellogenin receptor | SeVgR-F | CCCAGGAAGAGAATGTTAGCGA | 116 |
| SeVgR-R | GGTAGATACCTTGAGGGGTGC | ||
| SeABCOK | 15443-F | GCTGGTAGTATGCTCGGTCC | 106 |
| 15443-R | TTCAATGGTCCCGTGGAAGG | ||
| SeJHBWDS3 | 16076-F | CTAGTAACGCAAGCCACGGT | 145 |
| 16076-R | GAACTTCGGTGCCAACCTCT | ||
| SeJHBAN | 18636-F | CAAGAACCCATCCGAAGCCT | 149 |
| 18636-R | CCACAGCCTCGTCTTTGTCT | ||
| SeGST15 | 19590-F | CCCGGAGGTGTAGCCAAAAT | 169 |
| 19590-R | TGTTGGGGTACGTTGCTTCA | ||
| SeGST1 | 20364-F | CCGTGCCATCAGCAGATACT | 182 |
| 20364-R | CATCGTCAGCTTTTGCTCCG | ||
| SeGSTZ2 | 21647-F | AGGGAACTCTGTGAGGTGGT | 124 |
| 21647-R | TTAAGCCACGGTCAGTCCAG | ||
| SeCarEs1 | 27390-F | TCGATGTGCTCGGCTTTCTT | 120 |
| 27390-R | GGTCACCACCGAAGTTAGCA | ||
| SeCYP6AB10 | 34483-F | TTCATCGGTGTCTGCGCATT | 131 |
| 34483-R | ATAATCTTCAGGGCACCGGC | ||
| SeNPFR | 35241-F | TAGGCGAGGCTTCAAACAGG | 132 |
| 35241-R | CGCCGCGTCATACCATTTAC | ||
| SeNPF | 38343-F | GAACGTTTCGACACTGCTGA | 130 |
| 38343-R | CTCTGAAGATCACGGAGGCA | ||
| Vitellogenin | Vg-F | CACTCTGCCGTATCTCGCAT | 130 |
| Vg-R | GTTGAACGTGGCTGTGAACC | ||
| Actin | Actin-F | AGGGAAATCGTGCGTGACAT | 120 |
| Actin-R | GACCGTCGGGAAGTTCGTAG |
| Gene | Primer | Sequence (5′–3′) |
|---|---|---|
| RyR | RyRs-F | GCAAGCTCAAGAGCGTATGG |
| RyRs-R | CGGTAGACCTCGGAGTCATC |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gong, C.; Yao, X.; Yang, Q.; Wang, X.; Zhang, Y.; Wang, Y.; Shen, L. Fitness Costs of Chlorantraniliprole Resistance Related to the SeNPF Overexpression in the Spodoptera exigua (Lepidoptera: Noctuidae). Int. J. Mol. Sci. 2021, 22, 5027. https://doi.org/10.3390/ijms22095027
Gong C, Yao X, Yang Q, Wang X, Zhang Y, Wang Y, Shen L. Fitness Costs of Chlorantraniliprole Resistance Related to the SeNPF Overexpression in the Spodoptera exigua (Lepidoptera: Noctuidae). International Journal of Molecular Sciences. 2021; 22(9):5027. https://doi.org/10.3390/ijms22095027
Chicago/Turabian StyleGong, Changwei, Xinge Yao, Qunfang Yang, Xuegui Wang, Yuming Zhang, Yumeng Wang, and Litao Shen. 2021. "Fitness Costs of Chlorantraniliprole Resistance Related to the SeNPF Overexpression in the Spodoptera exigua (Lepidoptera: Noctuidae)" International Journal of Molecular Sciences 22, no. 9: 5027. https://doi.org/10.3390/ijms22095027
APA StyleGong, C., Yao, X., Yang, Q., Wang, X., Zhang, Y., Wang, Y., & Shen, L. (2021). Fitness Costs of Chlorantraniliprole Resistance Related to the SeNPF Overexpression in the Spodoptera exigua (Lepidoptera: Noctuidae). International Journal of Molecular Sciences, 22(9), 5027. https://doi.org/10.3390/ijms22095027