T-Cell Response against Varicella Zoster Virus in Patients with Multiple Sclerosis during Relapse and Remission
Abstract
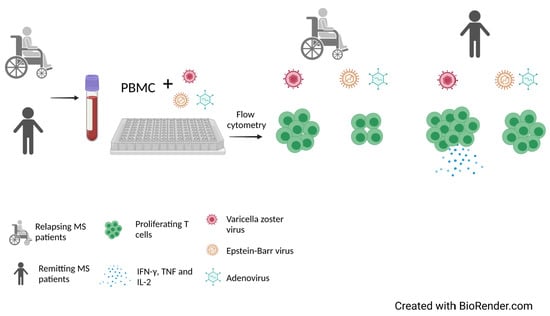
:1. Introduction
2. Results
2.1. Study Groups
2.2. T Cell Response to Stimulation with VZV
2.3. T Cell Response to Stimulation with AV
2.4. T Cell Response to Stimulation with EBV
2.5. Cytokine Cuantification in Stimulated Culture Cells
2.6. Specific IgG and IgM Serum Antibodies against VZV and EBV
3. Discussion
4. Materials and Methods
4.1. Patients and Control Subjects
4.2. Blood Samples
4.3. Isolation of PBMC
4.4. Total Proteins Quantification in Virus Sources
4.5. Proliferation Assays
4.6. Flow Cytometry
4.7. Quantification of Cytokine Secretion
4.8. IgG and IgM Anti-VZV and EBV
4.9. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nylander, A.; Hafler, D.A. Multiple sclerosis. J. Clin. Investig. 2012, 122, 1180–1188. [Google Scholar] [CrossRef] [PubMed]
- Dendrou, C.A.; Fugger, L.; Friese, M.A. Immunopathology of multiple sclerosis. Nat. Rev. Immunol. 2015, 15, 545–558. [Google Scholar] [CrossRef]
- Reich, D.S.; Lucchinetti, C.F.; Calabresi, P.A. Multiple Sclerosis. N. Engl. J. Med. 2018, 378, 169–180. [Google Scholar] [CrossRef] [PubMed]
- Steinman, L. Immunology of Relapse and Remission in Multiple Sclerosis. Annu. Rev. Immunol. 2014, 32, 257–281. [Google Scholar] [CrossRef]
- van Langelaar, J.; Rijvers, L.; Smolders, J.; van Luijn, M.M. B and T Cells Driving Multiple Sclerosis: Identity, Mechanisms and Potential Triggers. Front. Immunol. 2020, 11, 760. [Google Scholar] [CrossRef] [PubMed]
- Severson, C.; Hafler, D.A. T-Cells in Multiple Sclerosis. Results Probl. Cell Differ. 2010, 51, 75–98. [Google Scholar] [CrossRef]
- Yadav, S.K.; Mindur, J.E.; Ito, K.; Dhib-Jalbut, S. Advances in the immunopathogenesis of multiple sclerosis. Curr. Opin. Neurol. 2015, 28, 206–219. [Google Scholar] [CrossRef] [PubMed]
- Kaskow, B.J.; Baecher-Allan, C. Effector T Cells in Multiple Sclerosis. Cold Spring Harb. Perspect. Med. 2018, 8, a029025. [Google Scholar] [CrossRef]
- Jones, A.P.; Kermode, A.G.; Lucas, R.M.; Carroll, W.M.; Nolan, D.; Hart, P.H. Circulating immune cells in multiple sclerosis. Clin. Exp. Immunol. 2017, 187, 193–203. [Google Scholar] [CrossRef] [Green Version]
- Bai, Z.L.; Chen, D.D.; Wang, L.Y.; Zhao, Y.; Liu, T.T.; Yu, Y.; Yan, T.Y.; Cheng, Y. Cerebrospinal Fluid and Blood Cytokines as Biomarkers for Multiple Sclerosis: A Systematic Review and Meta-Analysis of 226 Studies With 13,526 Multiple Sclerosis Patients. Front. Neurosci. 2019, 13, 1026. [Google Scholar] [CrossRef]
- Luchtman, D.W.; Ellwardt, E.; Larochelle, C.; Zipp, F. IL-17 and related cytokines involved in the pathology and immunotherapy of multiple sclerosis: Current and future developments. Cytokine Growth Factor Rev. 2014, 25, 403–413. [Google Scholar] [CrossRef]
- Sospedra, M.; Martin, R. Immunology of Multiple Sclerosis. Semin. Neurol. 2016, 36, 115–127. [Google Scholar] [CrossRef] [PubMed]
- Marrodan, M.; Alessandro, L.; Farez, M.F.; Correale, J.E. The role of infections in multiple sclerosis. Mult. Scler. J. 2019, 25, 891–901. [Google Scholar] [CrossRef] [PubMed]
- Libbey, J.E.; Cusick, M.F.; Fujinami, R.S. Role of Pathogens in Multiple Sclerosis. Int. Rev. Immunol. 2014, 33, 266–283. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cusick, M.F.; Libbey, J.E.; Fujinami, R.S. Multiple sclerosis: Autoimmunity and viruses. Curr. Opin. Rheumatol. 2013, 25, 496–501. [Google Scholar] [CrossRef] [Green Version]
- Tarrats, R.; Ordonez, G.; Rios, C.; Sotelo, J. Varicella, ephemeral breastfeeding and eczema as risk factors for multiple sclerosis in Mexicans. Acta Neurol. Scand. 2002, 105, 88–94. [Google Scholar] [CrossRef]
- Najafi, S.; Ghane, M.; Yousefzadeh-Chabok, S.; Amiri, M. The High Prevalence of the Varicella Zoster Virus in Patients with Relapsing-Remitting Multiple Sclerosis: A Case-Control Study in the North of Iran. Jundishapur J. Microbiol. 2016, 9, e34158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jacobs, B.M.; Giovannoni, G.; Cuzick, J.; Dobson, R. Systematic review and meta-analysis of the association between Epstein-Barr virus, multiple sclerosis and other risk factors. Mult. Scler. J. 2020, 26, 1281–1297. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.H.; Sheu, J.J.; Kao, S.; Lin, H.C. Increased Risk of Multiple Sclerosis Following Herpes Zoster: A Nationwide, Population-Based Study. J. Infect. Dis. 2011, 204, 188–192. [Google Scholar] [CrossRef] [PubMed]
- Virtanen, J.O.; Wohler, J.; Fenton, K.; Reich, D.S.; Jacobson, S. Oligoclonal bands in multiple sclerosis reactive against two herpesviruses and association with magnetic resonance imaging findings. Mult. Scler. J. 2014, 20, 27–34. [Google Scholar] [CrossRef] [Green Version]
- Sotelo, J.; Ordonez, G.; Pineda, B. Varicella-zoster virus at relapses of multiple sclerosis. J. Neurol. 2007, 254, 493–500. [Google Scholar] [CrossRef]
- Sotelo, J.; Martinez-Palomo, A.; Ordonez, G.; Pineda, B. Varicella-zoster virus in cerebrospinal fluid at relapses of multiple sclerosis. Ann. Neurol. 2008, 63, 303–311. [Google Scholar] [CrossRef]
- Sotelo, J.; Ordonez, G.; Pineda, B.; Flores, J. The participation of varicella zoster virus in relapses of multiple sclerosis. Clin. Neurol. Neurosurg. 2014, 119, 44–48. [Google Scholar] [CrossRef] [PubMed]
- Ribbons, K.A.; McElduff, P.; Boz, C.; Trojano, M.; Izquierdo, G.; Duquette, P.; Girard, M.; Grand’Maison, F.; Hupperts, R.; Grammond, P.; et al. Male Sex Is Independently Associated with Faster Disability Accumulation in Relapse-Onset MS but Not in Primary Progressive MS. PLoS ONE 2015, 10, e0122686. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Snyder, C.M.; Cho, K.S.; Bonnett, E.L.; van Dommelen, S.; Shellam, G.R.; Hill, A.B. Memory Inflation during Chronic Viral Infection Is Maintained by Continuous Production of Short-Lived, Functional T Cells. Immunity 2008, 29, 650–659. [Google Scholar] [CrossRef] [Green Version]
- Huber, S.R.; van Beek, J.; de Jonge, J.; Luytjes, W.; van Baarle, D. T cell responses to viral infections—Opportunities for peptide vaccination. Front. Immunol. 2014, 5, 171. [Google Scholar] [CrossRef]
- Jelcic, I.; Al Nimer, F.; Wang, J.; Lentsch, V.; Planas, R.; Madjovski, A.; Ruhrmann, S.; Faigle, W.; Frauenknecht, K.; Pinilla, C.; et al. Memory B Cells Activate Brain-Homing, Autoreactive CD4(+) T Cells in Multiple Sclerosis. Cell 2018, 175, 85–100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saeidi, A.; Zandi, K.; Cheok, Y.Y.; Saeidi, H.; Wong, W.F.; Lee, C.Y.Q.; Cheong, H.C.; Yong, Y.K.; Larsson, M.; Shankar, E.M. T-Cell Exhaustion in Chronic Infections: Reversing the State of Exhaustion and Reinvigorating Optimal Protective Immune Responses. Front. Immunol. 2018, 9, 2569. [Google Scholar] [CrossRef]
- Kahan, S.M.; Wherry, E.J.; Zajac, A.J. T cell exhaustion during persistent viral infections. Virology 2015, 479, 180–193. [Google Scholar] [CrossRef]
- Fransson, M.E.; Liljenfeldt, L.S.E.; Fagius, J.; Totterman, T.H.; Loskog, A.S.I. The T-cell pool is anergized in patients with multiple sclerosis in remission. Immunology 2009, 126, 92–101. [Google Scholar] [CrossRef]
- Andersen, O.; Lygner, P.E.; Bergstrom, T.; Andersson, M.; Vahlne, A. Viral-Infections Trigger Multiple-Sclerosis Relapses—A Prospective Seroepidemiological Study. J. Neurol. 1993, 240, 417–422. [Google Scholar] [CrossRef] [PubMed]
- Steelman, A.J. Infection as an environmental trigger of multiple sclerosis disease exacerbation. Front. Immunol. 2015, 6, 520. [Google Scholar] [CrossRef] [Green Version]
- General Direction of Epidemiology. Epidemiological Bulletin National Epidemiological Surveillance System Unique Information System. Available online: https://www.gob.mx/salud/acciones-y-programas/direccion-general-de-epidemiologia-boletin-epidemiologico (accessed on 28 July 2021).
- Laing, K.J.; Ouwendijk, W.J.D.; Koelle, D.M.; Verjans, G. Immunobiology of Varicella-Zoster Virus Infection. J. Infect. Dis. 2018, 218, S68–S74. [Google Scholar] [CrossRef] [Green Version]
- Arellano, G.; Acuna, E.; Reyes, L.I.; Ottum, P.A.; De Sarno, P.; Villarroel, L.; Ciampi, E.; Martin, R.U.S.; Carcamo, C.; Naves, R. Th1 and Th17 Cells and Associated Cytokines Discriminate among Clinically Isolated Syndrome and Multiple Sclerosis Phenotypes. Front. Immunol. 2017, 8, 753. [Google Scholar] [CrossRef]
- Martynova, E.; Goyal, M.; Johri, S.; Kumar, V.; Khaibullin, T.; Rizvanov, A.A.; Verma, S.; Khaiboullina, S.F.; Baranwal, M. Serum and Cerebrospinal Fluid Cytokine Biomarkers for Diagnosis of Multiple Sclerosis. Mediat. Inflamm. 2020, 2020, 2727042. [Google Scholar] [CrossRef]
- Rieckmann, P.; Albrecht, M.; Kitze, B.; Weber, T.; Tumani, H.; Broocks, A.; Luer, W.; Helwig, A.; Poser, S. Tumor-Necrosis-Factor-Alpha Messenger-RNA Expression in Patients with Relapsing-Remitting Multiple-Sclerosis Is Associated with Disease-Activity. Ann. Neurol. 1995, 37, 82–88. [Google Scholar] [CrossRef]
- Kattimani, Y.; Veerappa, A.M. Complex interaction between mutant HNRNPA1 and gE of varicella zoster virus in pathogenesis of multiple sclerosis. Autoimmunity 2018, 51, 147–151. [Google Scholar] [CrossRef] [PubMed]
- Sospedra, M.; Martin, R. Molecular mimicry in multiple sclerosis. Autoimmunity 2006, 39, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Geginat, J.; Paroni, M.; Pagani, M.; Galimberti, D.; De Francesco, R.; Scarpini, E.; Abrignani, S. The Enigmatic Role of Viruses in Multiple Sclerosis: Molecular Mimicry or Disturbed Immune Surveillance? Trends Immunol. 2017, 38, 498–512. [Google Scholar] [CrossRef] [PubMed]
- Viglietta, V.; Baecher-Allan, C.; Weiner, H.L.; Hafler, D.A. Loss of functional suppression by CD4(+)CD25(+) regulatory T cells in patients with multiple sclerosis. J. Exp. Med. 2004, 199, 971–979. [Google Scholar] [CrossRef] [Green Version]
- Kumar, M.; Putzki, N.; Limmroth, V.; Remus, R.; Lindemann, M.; Knop, D.; Mueller, N.; Hardt, C.; Kreuzfelder, E.; Grosse-Wilde, H. CD4(+)CD25(+)FoxP3(+) T lymphocytes fail to suppress myelin basic protein-induced proliferation in patients with multiple sclerosis. J. Neuroimmunol. 2006, 180, 178–184. [Google Scholar] [CrossRef] [PubMed]
- Salou, M.; Nicol, B.; Garcia, A.; Laplaud, D.A. Involvement of CD8(+) T Cells in Multiple Sclerosis. Front. Immunol. 2015, 6, 604. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lassmann, H. Multiple Sclerosis Pathology. Cold Spring Harb. Perspect. Med. 2018, 8, a028936. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salou, M.; Garcia, A.; Michel, L.; Gainche-Salmon, A.; Loussouarn, D.; Nicol, B.; Guillot, F.; Hulin, P.; Nedellec, S.; Baron, D.; et al. Expanded CD8 T-cell sharing between periphery and CNS in multiple sclerosis. Ann. Clin. Transl. Neurol. 2015, 2, 609–622. [Google Scholar] [CrossRef]
- Bohmer, R.M.; Bandala-Sanchez, E.; Harrison, L.C. Forward Light Scatter Is a Simple Measure of T-Cell Activation and Proliferation but Is Not Universally Suited for Doublet Discrimination. Cytom. A 2011, 79, 646–652. [Google Scholar] [CrossRef] [PubMed]





| Study Groups | Age a | Gender (F/M) | Evolution (Years) a | Relapses/Year a | EDSS a | T/NT |
|---|---|---|---|---|---|---|
| MS (n = 29) | 30 ± 7.3 | 18/11 | 5.8 ± 5.8 | 1.6 ± 0.9 | 3.4 ± 2.1 | 15/14 |
| HC (n = 38) | 27.1 ± 4.8 | 23/15 | NA | NA | NA | NA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pérez-Saldívar, M.; Ordoñez, G.; Pineda, B.; Sotelo, J.; Martínez-Palomo, A.; Flores-Rivera, J.; Espinosa-Cantellano, M. T-Cell Response against Varicella Zoster Virus in Patients with Multiple Sclerosis during Relapse and Remission. Int. J. Mol. Sci. 2022, 23, 298. https://doi.org/10.3390/ijms23010298
Pérez-Saldívar M, Ordoñez G, Pineda B, Sotelo J, Martínez-Palomo A, Flores-Rivera J, Espinosa-Cantellano M. T-Cell Response against Varicella Zoster Virus in Patients with Multiple Sclerosis during Relapse and Remission. International Journal of Molecular Sciences. 2022; 23(1):298. https://doi.org/10.3390/ijms23010298
Chicago/Turabian StylePérez-Saldívar, Miriam, Graciela Ordoñez, Benjamín Pineda, Julio Sotelo, Adolfo Martínez-Palomo, José Flores-Rivera, and Martha Espinosa-Cantellano. 2022. "T-Cell Response against Varicella Zoster Virus in Patients with Multiple Sclerosis during Relapse and Remission" International Journal of Molecular Sciences 23, no. 1: 298. https://doi.org/10.3390/ijms23010298
APA StylePérez-Saldívar, M., Ordoñez, G., Pineda, B., Sotelo, J., Martínez-Palomo, A., Flores-Rivera, J., & Espinosa-Cantellano, M. (2022). T-Cell Response against Varicella Zoster Virus in Patients with Multiple Sclerosis during Relapse and Remission. International Journal of Molecular Sciences, 23(1), 298. https://doi.org/10.3390/ijms23010298