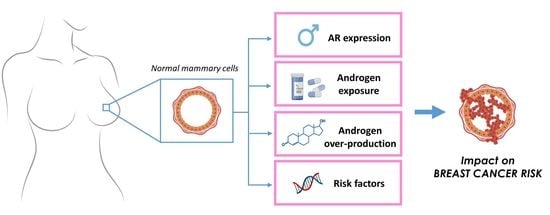
The Other Side of the Coin: May Androgens Have a Role in Breast Cancer Risk?
Abstract
:1. Introduction
2. AR Expression and Action in Normal Mammary Gland
3. Androgen Signalling in Normal Breast Epithelium and Cancer Susceptibility
4. Exogenous Androgen Exposure and Breast Cancer Incidence
5. Androgen Over-Production and Breast Cancer Risk
6. Androgens/AR and Breast Cancer Risk Factors
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Harbeck, N.; Penault-Llorca, F.; Cortes, J.; Gnant, M.; Houssami, N.; Poortmans, P.; Ruddy, K.; Tsang, J.; Cardoso, F. Breast cancer. Nat. Rev. Dis. Primers 2019, 5, 66. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.S.; Zhao, Z.; Yang, Z.N.; Xu, F.; Lu, H.J.; Zhu, Z.Y.; Shi, W.; Jiang, J.; Yao, P.P.; Zhu, H.P. Risk Factors and Preventions of Breast Cancer. Int. J. Biol. Sci. 2017, 13, 1387–1397. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Howell, A.; Anderson, A.S.; Clarke, R.B.; Duffy, S.W.; Evans, D.G.; Garcia-Closas, M.; Gescher, A.J.; Key, T.J.; Saxton, J.M.; Harvie, M.N. Risk determination and prevention of breast cancer. Breast Cancer Res. 2014, 16, 446. [Google Scholar] [CrossRef] [PubMed]
- Winters, S.; Martin, C.; Murphy, D.; Shokar, N.K. Breast Cancer Epidemiology, Prevention, and Screening. Prog. Mol. Biol. Transl. Sci. 2017, 151, 1–32. [Google Scholar] [CrossRef] [PubMed]
- Huh, S.J.; Oh, H.; Peterson, M.A.; Almendro, V.; Hu, R.; Bowden, M.; Lis, R.L.; Cotter, M.B.; Loda, M.; Barry, W.T.; et al. The Proliferative Activity of Mammary Epithelial Cells in Normal Tissue Predicts Breast Cancer Risk in Premenopausal Women. Cancer Res. 2016, 76, 1926–1934. [Google Scholar] [CrossRef] [Green Version]
- Brisken, C.; O’Malley, B. Hormone action in the mammary gland. Cold Spring Harb. Perspect. Biol. 2010, 2, a003178. [Google Scholar] [CrossRef] [PubMed]
- Bleach, R.; McIlroy, M. The Divergent Function of Androgen Receptor in Breast Cancer; Analysis of Steroid Mediators and Tumor Intracrinology. Front. Endocrinol. 2018, 9, 594. [Google Scholar] [CrossRef]
- Goldenberg, I.S. Testosterone Propionate Therapy in Breast Cancer. JAMA 1964, 188, 1069–1072. [Google Scholar] [CrossRef]
- Kennedy, B.J. Fluoxymesterone therapy in advanced breast cancer. N. Engl. J. Med. 1958, 259, 673–675. [Google Scholar] [CrossRef]
- Garay, J.P.; Park, B.H. Androgen receptor as a targeted therapy for breast cancer. Am. J. Cancer Res. 2012, 2, 434–445. [Google Scholar]
- Cole, M.P.; Jones, C.T.; Todd, I.D. A new anti-oestrogenic agent in late breast cancer. An early clinical appraisal of ICI46474. Br. J. Cancer 1971, 25, 270–275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Council on Drugs; Subcommittee on Breast and Genital Cancer; Committee on Research; American Medical Association (AMA). Androgens and estrogens in the treatment of disseminated mammary carcinoma—Retrospective study of 944 patients. JAMA 1960, 172, 1271–1283. [Google Scholar]
- Tsang, J.Y.; Ni, Y.B.; Chan, S.K.; Shao, M.M.; Law, B.K.; Tan, P.H.; Tse, G.M. Androgen receptor expression shows distinctive significance in ER positive and negative breast cancers. Ann. Surg. Oncol. 2014, 21, 2218–2228. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Koo, J.; Park, H.S.; Kim, J.H.; Choi, S.Y.; Lee, J.H.; Park, B.W.; Lee, K.S. Expression of androgen receptors in primary breast cancer. Ann. Oncol. 2010, 21, 488–492. [Google Scholar] [CrossRef] [PubMed]
- Ricciardelli, C.; Bianco-Miotto, T.; Jindal, S.; Butler, L.M.; Leung, S.; McNeil, C.M.; O’Toole, S.A.; Ebrahimie, E.; Millar, E.K.A.; Sakko, A.J.; et al. The Magnitude of Androgen Receptor Positivity in Breast Cancer Is Critical for Reliable Prediction of Disease Outcome. Clin. Cancer Res. 2018, 24, 2328–2341. [Google Scholar] [CrossRef] [Green Version]
- Jiang, H.S.; Kuang, X.Y.; Sun, W.L.; Xu, Y.; Zheng, Y.Z.; Liu, Y.R.; Lang, G.T.; Qiao, F.; Hu, X.; Shao, Z.M. Androgen receptor expression predicts different clinical outcomes for breast cancer patients stratified by hormone receptor status. Oncotarget 2016, 7, 41285–41293. [Google Scholar] [CrossRef] [Green Version]
- Hickey, T.E.; Selth, L.A.; Chia, K.M.; Laven-Law, G.; Milioli, H.H.; Roden, D.; Jindal, S.; Hui, M.; Finlay-Schultz, J.; Ebrahimie, E.; et al. The androgen receptor is a tumor suppressor in estrogen receptor-positive breast cancer. Nat. Med. 2021, 27, 310–320. [Google Scholar] [CrossRef]
- Ando, S.; De Amicis, F.; Rago, V.; Carpino, A.; Maggiolini, M.; Panno, M.L.; Lanzino, M. Breast cancer: From estrogen to androgen receptor. Mol. Cell Endocrinol. 2002, 193, 121–128. [Google Scholar] [CrossRef]
- Lanzino, M.; Sisci, D.; Morelli, C.; Garofalo, C.; Catalano, S.; Casaburi, I.; Capparelli, C.; Giordano, C.; Giordano, F.; Maggiolini, M.; et al. Inhibition of cyclin D1 expression by androgen receptor in breast cancer cells—Identification of a novel androgen response element. Nucleic Acids Res. 2010, 38, 5351–5365. [Google Scholar] [CrossRef] [PubMed]
- Lanzino, M.; Maris, P.; Sirianni, R.; Barone, I.; Casaburi, I.; Chimento, A.; Giordano, C.; Morelli, C.; Sisci, D.; Rizza, P.; et al. DAX-1, as an androgen-target gene, inhibits aromatase expression: A novel mechanism blocking estrogen-dependent breast cancer cell proliferation. Cell Death Dis. 2013, 4, e724. [Google Scholar] [CrossRef] [Green Version]
- De Amicis, F.; Chiodo, C.; Morelli, C.; Casaburi, I.; Marsico, S.; Bruno, R.; Sisci, D.; Ando, S.; Lanzino, M. AIB1 sequestration by androgen receptor inhibits estrogen-dependent cyclin D1 expression in breast cancer cells. BMC Cancer 2019, 19, 1038. [Google Scholar] [CrossRef] [Green Version]
- Cops, E.J.; Bianco-Miotto, T.; Moore, N.L.; Clarke, C.L.; Birrell, S.N.; Butler, L.M.; Tilley, W.D. Antiproliferative actions of the synthetic androgen, mibolerone, in breast cancer cells are mediated by both androgen and progesterone receptors. J. Steroid Biochem. Mol. Biol. 2008, 110, 236–243. [Google Scholar] [CrossRef]
- Liao, D.J.; Dickson, R.B. Roles of androgens in the development, growth, and carcinogenesis of the mammary gland. J. Steroid Biochem. Mol. Biol. 2002, 80, 175–189. [Google Scholar] [CrossRef]
- Peters, A.A.; Buchanan, G.; Ricciardelli, C.; Bianco-Miotto, T.; Centenera, M.M.; Harris, J.M.; Jindal, S.; Segara, D.; Jia, L.; Moore, N.L.; et al. Androgen receptor inhibits estrogen receptor-alpha activity and is prognostic in breast cancer. Cancer Res. 2009, 69, 6131–6140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Overmoyer, B.; Sanz-Altamira, P.; Taylor, R.P.; Hancock, M.L.; Dalton, J.T.; Johnston, M.A.; Steiner, M.S. Enobosarm: A targeted therapy for metastatic, androgen receptor positive, breast cancer. J. Clin. Oncol. 2014, 32, 568. [Google Scholar] [CrossRef]
- Hu, R.; Dawood, S.; Holmes, M.D.; Collins, L.C.; Schnitt, S.J.; Cole, K.; Marotti, J.D.; Hankinson, S.E.; Colditz, G.A.; Tamimi, R.M. Androgen receptor expression and breast cancer survival in postmenopausal women. Clin. Cancer Res. 2011, 17, 1867–1874. [Google Scholar] [CrossRef] [Green Version]
- Sutton, L.M.; Cao, D.; Sarode, V.; Molberg, K.H.; Torgbe, K.; Haley, B.; Peng, Y. Decreased androgen receptor expression is associated with distant metastases in patients with androgen receptor-expressing triple-negative breast carcinoma. Am. J. Clin. Pathol. 2012, 138, 511–516. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Petrossian, K.; Huang, M.J.; Saeki, K.; Kanaya, N.; Chang, G.; Somlo, G.; Chen, S. Functional characterization of androgen receptor in two patient-derived xenograft models of triple negative breast cancer. J. Steroid Biochem. Mol. Biol. 2021, 206, 105791. [Google Scholar] [CrossRef]
- Wang, Y.; Romigh, T.; He, X.; Tan, M.H.; Orloff, M.S.; Silverman, R.H.; Heston, W.D.; Eng, C. Differential regulation of PTEN expression by androgen receptor in prostate and breast cancers. Oncogene 2011, 30, 4327–4338. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; He, X.; Yu, Q.; Eng, C. Androgen receptor-induced tumor suppressor, KLLN, inhibits breast cancer growth and transcriptionally activates p53/p73-mediated apoptosis in breast carcinomas. Hum. Mol. Genet. 2013, 22, 2263–2272. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hickey, T.E.; Robinson, J.L.; Carroll, J.S.; Tilley, W.D. Minireview: The androgen receptor in breast tissues: Growth inhibitor, tumor suppressor, oncogene? Mol. Endocrinol. 2012, 26, 1252–1267. [Google Scholar] [CrossRef] [PubMed]
- Fioretti, F.M.; Sita-Lumsden, A.; Bevan, C.L.; Brooke, G.N. Revising the role of the androgen receptor in breast cancer. J. Mol. Endocrinol. 2014, 52, R257–R265. [Google Scholar] [CrossRef] [PubMed]
- Zhu, A.; Li, Y.; Song, W.; Xu, Y.; Yang, F.; Zhang, W.; Yin, Y.; Guan, X. Antiproliferative Effect of Androgen Receptor Inhibition in Mesenchymal Stem-Like Triple-Negative Breast Cancer. Cell Physiol. Biochem. 2016, 38, 1003–1014. [Google Scholar] [CrossRef]
- Giovannelli, P.; Di Donato, M.; Auricchio, F.; Castoria, G.; Migliaccio, A. Androgens Induce Invasiveness of Triple Negative Breast Cancer Cells through AR/Src/PI3-K Complex Assembly. Sci. Rep. 2019, 9, 4490. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahram, M.; Bawadi, R.; Abdullah, M.S.; Alsafadi, D.B.; Abaza, H.; Abdallah, S.; Mustafa, E. Involvement of beta-catenin in Androgen-induced Mesenchymal Transition of Breast MDA-MB-453 Cancer Cells. Endocr. Res. 2021, 46, 114–128. [Google Scholar] [CrossRef] [PubMed]
- Chia, K.; O’Brien, M.; Brown, M.; Lim, E. Targeting the androgen receptor in breast cancer. Curr. Oncol. Rep. 2015, 17, 4. [Google Scholar] [CrossRef]
- Cochrane, D.R.; Bernales, S.; Jacobsen, B.M.; Cittelly, D.M.; Howe, E.N.; D’Amato, N.C.; Spoelstra, N.S.; Edgerton, S.M.; Jean, A.; Guerrero, J.; et al. Role of the androgen receptor in breast cancer and preclinical analysis of enzalutamide. Breast Cancer Res. 2014, 16, R7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ni, M.; Chen, Y.; Lim, E.; Wimberly, H.; Bailey, S.T.; Imai, Y.; Rimm, D.L.; Liu, X.S.; Brown, M. Targeting androgen receptor in estrogen receptor-negative breast cancer. Cancer Cell 2011, 20, 119–131. [Google Scholar] [CrossRef] [Green Version]
- Barton, V.N.; D’Amato, N.C.; Gordon, M.A.; Lind, H.T.; Spoelstra, N.S.; Babbs, B.L.; Heinz, R.E.; Elias, A.; Jedlicka, P.; Jacobsen, B.M.; et al. Multiple molecular subtypes of triple-negative breast cancer critically rely on androgen receptor and respond to enzalutamide in vivo. Mol. Cancer 2015, 14, 769–778. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Michmerhuizen, A.R.; Spratt, D.E.; Pierce, L.J.; Speers, C.W. Are we there yet? Understanding androgen receptor signaling in breast cancer. NPJ Breast Cancer 2020, 6, 47. [Google Scholar] [CrossRef]
- Tarulli, G.A.; Laven-Law, G.; Shehata, M.; Walters, K.A.; Denis, I.M.; Rahman, M.M.; Handelsman, D.J.; Dean, N.R.; Tilley, W.D.; Hickey, T.E. Androgen Receptor Signalling Promotes a Luminal Phenotype in Mammary Epithelial Cells. J. Mammary Gland Biol. Neoplasia 2019, 24, 99–108. [Google Scholar] [CrossRef]
- Nguyen, Q.H.; Pervolarakis, N.; Blake, K.; Ma, D.; Davis, R.T.; James, N.; Phung, A.T.; Willey, E.; Kumar, R.; Jabart, E.; et al. Profiling human breast epithelial cells using single cell RNA sequencing identifies cell diversity. Nat. Commun. 2018, 9, 2028. [Google Scholar] [CrossRef]
- Clarke, R.B.; Howell, A.; Potten, C.S.; Anderson, E. Dissociation between steroid receptor expression and cell proliferation in the human breast. Cancer Res. 1997, 57, 4987–4991. [Google Scholar]
- Wang, F.; Dohogne, Z.; Yang, J.; Liu, Y.; Soibam, B. Predictors of breast cancer cell types and their prognostic power in breast cancer patients. BMC Genom. 2018, 19, 137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tarulli, G.A.; Butler, L.M.; Tilley, W.D.; Hickey, T.E. Bringing androgens up a NOTCH in breast cancer. Endocr. Relat. Cancer 2014, 21, T183–T202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bach, K.; Pensa, S.; Grzelak, M.; Hadfield, J.; Adams, D.J.; Marioni, J.C.; Khaled, W.T. Differentiation dynamics of mammary epithelial cells revealed by single-cell RNA sequencing. Nat. Commun. 2017, 8, 2128. [Google Scholar] [CrossRef] [Green Version]
- Sflomos, G.; Dormoy, V.; Metsalu, T.; Jeitziner, R.; Battista, L.; Scabia, V.; Raffoul, W.; Delaloye, J.F.; Treboux, A.; Fiche, M.; et al. A Preclinical Model for ERalpha-Positive Breast Cancer Points to the Epithelial Microenvironment as Determinant of Luminal Phenotype and Hormone Response. Cancer Cell 2016, 29, 407–422. [Google Scholar] [CrossRef] [Green Version]
- Blakemore, J.; Naftolin, F. Aromatase: Contributions to Physiology and Disease in Women and Men. Physiology 2016, 31, 258–269. [Google Scholar] [CrossRef] [Green Version]
- Lanigan, F.; O’Connor, D.; Martin, F.; Gallagher, W.M. Molecular links between mammary gland development and breast cancer. Cell. Mol. Life Sci. 2007, 64, 3159–3184. [Google Scholar] [CrossRef]
- Gao, Y.R.; Walters, K.A.; Desai, R.; Zhou, H.; Handelsman, D.J.; Simanainen, U. Androgen receptor inactivation resulted in acceleration in pubertal mammary gland growth, upregulation of ERalpha expression, and Wnt/beta-catenin signaling in female mice. Endocrinology 2014, 155, 4951–4963. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yeh, S.; Hu, Y.C.; Wang, P.H.; Xie, C.; Xu, Q.; Tsai, M.Y.; Dong, Z.; Wang, R.S.; Lee, T.H.; Chang, C. Abnormal mammary gland development and growth retardation in female mice and MCF7 breast cancer cells lacking androgen receptor. J. Exp. Med. 2003, 198, 1899–1908. [Google Scholar] [CrossRef]
- Bulun, S.E.; Fang, Z.J.; Gurates, B.; Tamura, M.; Yilmaz, B.; Amin, S.; Yang, S.J. Arornatase in health and disease. Endocrinologist 2003, 13, 269–276. [Google Scholar] [CrossRef]
- Melo, K.F.S.; Mendonca, B.B.; Billerbeck, A.E.C.; Costa, E.M.F.; Inacio, M.; Silva, F.A.Q.; Leal, A.M.O.; Latronico, A.C.; Arnhold, I.J.P. Clinical, hormonal, behavioral, and genetic characteristics of androgen insensitivity syndrome in a Brazilian cohort: Five novel mutations in the androgen receptor gene. J. Clin. Endocr. Metab. 2003, 88, 3241–3250. [Google Scholar] [CrossRef] [Green Version]
- Karagiannis, A.; Harsoulis, F. Gonadal dysfunction in systemic diseases. Eur. J. Endocrinol. 2005, 152, 501–513. [Google Scholar] [CrossRef]
- Sansone, A.; Romanelli, F.; Sansone, M.; Lenzi, A.; Di Luigi, L. Gynecomastia and hormones. Endocrine 2017, 55, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Secreto, G.; Girombelli, A.; Krogh, V. Androgen excess in breast cancer development: Implications for prevention and treatment. Endocr. Relat. Cancer 2019, 26, R81–R94. [Google Scholar] [CrossRef]
- Zhou, J.; Ng, S.; Adesanya-Famuiya, O.; Anderson, K.; Bondy, C.A. Testosterone inhibits estrogen-induced mammary epithelial proliferation and suppresses estrogen receptor expression. FASEB J. 2000, 14, 1725–1730. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clarke, R.B.; Anderson, E.; Howell, A. Steroid receptors in human breast cancer. Trends Endocrinol. Metab. 2004, 15, 316–323. [Google Scholar] [CrossRef]
- Schippinger, W.; Regitnig, P.; Dandachi, N.; Wernecke, K.D.; Bauernhofer, T.; Samonigg, H.; Moinfar, F. Evaluation of the prognostic significance of androgen receptor expression in metastatic breast cancer. Virchows Arch. 2006, 449, 24–30. [Google Scholar] [CrossRef]
- Kraby, M.R.; Valla, M.; Opdahl, S.; Haugen, O.A.; Sawicka, J.E.; Engstrom, M.J.; Bofin, A.M. The prognostic value of androgen receptors in breast cancer subtypes. Breast Cancer Res. Treat. 2018, 172, 283–296. [Google Scholar] [CrossRef] [PubMed]
- Chamberlain, N.L.; Driver, E.D.; Miesfeld, R.L. The length and location of CAG trinucleotide repeats in the androgen receptor N-terminal domain affect transactivation function. Nucleic Acids Res. 1994, 22, 3181–3186. [Google Scholar] [CrossRef] [Green Version]
- Esteban, E.; Rodon, N.; Via, M.; Gonzalez-Perez, E.; Santamaria, J.; Dugoujon, J.M.; Chennawi, F.E.; Melhaoui, M.; Cherkaoui, M.; Vona, G.; et al. Androgen receptor CAG and GGC polymorphisms in Mediterraneans: Repeat dynamics and population relationships. J. Hum. Genet. 2006, 51, 129–136. [Google Scholar] [CrossRef] [Green Version]
- Hao, Y.; Montiel, R.; Li, B.; Huang, E.; Zeng, L.; Huang, Y. Association between androgen receptor gene CAG repeat polymorphism and breast cancer risk: A meta-analysis. Breast Cancer Res. Treat. 2010, 124, 815–820. [Google Scholar] [CrossRef]
- La Spada, A.R.; Wilson, E.M.; Lubahn, D.B.; Harding, A.E.; Fischbeck, K.H. Androgen receptor gene mutations in X-linked spinal and bulbar muscular atrophy. Nature 1991, 352, 77–79. [Google Scholar] [CrossRef]
- Spurdle, A.B.; Antoniou, A.C.; Duffy, D.L.; Pandeya, N.; Kelemen, L.; Chen, X.; Peock, S.; Cook, M.R.; Smith, P.L.; Purdie, D.M.; et al. The androgen receptor CAG repeat polymorphism and modification of breast cancer risk in BRCA1 and BRCA2 mutation carriers. Breast Cancer Res. 2005, 7, R176–R183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weng, H.; Li, S.; Huang, J.-Y.; He, Z.-Q.; Meng, X.-Y.; Cao, Y.; Fang, C.; Zeng, X.-T. Androgen receptor gene polymorphisms and risk of prostate cancer: A meta-analysis. Sci. Rep. 2017, 7, 40554. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haiman, C.A.; Brown, M.; Hankinson, S.E.; Spiegelman, D.; Colditz, G.A.; Willett, W.C.; Kantoff, P.W.; Hunter, D.J. The androgen receptor CAG repeat polymorphism and risk of breast cancer in the Nurses’ Health Study. Cancer Res. 2002, 62, 1045–1049. [Google Scholar]
- Gonzalez, A.; Javier Dorta, F.; Rodriguez, G.; Brito, B.; Rodriguez, M.A.; Cabrera, A.; Diaz-Chico, J.C.; Reyes, R.; Aguirre-Jaime, A.; Nicolas Diaz-Chico, B. Increased risk of breast cancer in women bearing a combination of large CAG and GGN repeats in the exon 1 of the androgen receptor gene. Eur. J. Cancer 2007, 43, 2373–2380. [Google Scholar] [CrossRef] [PubMed]
- Mehdipour, P.; Pirouzpanah, S.; Kheirollahi, M.; Atri, M. Androgen receptor gene CAG repeat polymorphism and breast cancer risk in Iranian women: A case-control study. Breast J. 2011, 17, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Mao, Q.; Qiu, M.; Dong, G.; Xia, W.; Zhang, S.; Xu, Y.; Wang, J.; Rong, Y.; Xu, L.; Jiang, F. CAG repeat polymorphisms in the androgen receptor and breast cancer risk in women: A meta-analysis of 17 studies. OncoTargets Ther. 2015, 8, 2111–2120. [Google Scholar] [CrossRef] [Green Version]
- Santagata, S.; Thakkar, A.; Ergonul, A.; Wang, B.; Woo, T.; Hu, R.; Harrell, J.C.; McNamara, G.; Schwede, M.; Culhane, A.C.; et al. Taxonomy of breast cancer based on normal cell phenotype predicts outcome. J. Clin. Investig. 2014, 124, 859–870. [Google Scholar] [CrossRef] [PubMed]
- Santisteban, M.; Reynolds, C.; Barr Fritcher, E.G.; Frost, M.H.; Vierkant, R.A.; Anderson, S.S.; Degnim, A.C.; Visscher, D.W.; Pankratz, V.S.; Hartmann, L.C. Ki67: A time-varying biomarker of risk of breast cancer in atypical hyperplasia. Breast Cancer Res. Treat. 2010, 121, 431–437. [Google Scholar] [CrossRef] [PubMed]
- Kensler, K.H.; Beca, F.; Baker, G.M.; Heng, Y.J.; Beck, A.H.; Schnitt, S.J.; Hazra, A.; Rosner, B.A.; Eliassen, A.H.; Hankinson, S.E.; et al. Androgen receptor expression in normal breast tissue and subsequent breast cancer risk. NPJ Breast Cancer 2018, 4, 33. [Google Scholar] [CrossRef]
- Lanzino, M.; De Amicis, F.; McPhaul, M.J.; Marsico, S.; Panno, M.L.; Ando, S. Endogenous coactivator ARA70 interacts with estrogen receptor alpha (ERalpha) and modulates the functional ERalpha/androgen receptor interplay in MCF-7 cells. J. Biol. Chem. 2005, 280, 20421–20430. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Need, E.F.; Selth, L.A.; Harris, T.J.; Birrell, S.N.; Tilley, W.D.; Buchanan, G. Research resource: Interplay between the genomic and transcriptional networks of androgen receptor and estrogen receptor alpha in luminal breast cancer cells. Mol. Endocrinol. 2012, 26, 1941–1952. [Google Scholar] [CrossRef] [Green Version]
- Yu, Z.; He, S.; Wang, D.; Patel, H.K.; Miller, C.P.; Brown, J.L.; Hattersley, G.; Saeh, J.C. Selective Androgen Receptor Modulator RAD140 Inhibits the Growth of Androgen/Estrogen Receptor-Positive Breast Cancer Models with a Distinct Mechanism of Action. Clin. Cancer Res. 2017, 23, 7608–7620. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nappi, R.E.; Martini, E.; Terreno, E.; Albani, F.; Santamaria, V.; Tonani, S.; Chiovato, L.; Polatti, F. Management of hypoactive sexual desire disorder in women: Current and emerging therapies. Int. J. Women’s Health 2010, 2, 167–175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vegunta, S.; Kling, J.M.; Kapoor, E. Androgen Therapy in Women. J. Women’s Health 2020, 29, 57–64. [Google Scholar] [CrossRef]
- Glaser, R.L.; York, A.E.; Dimitrakakis, C. Incidence of invasive breast cancer in women treated with testosterone implants: A prospective 10-year cohort study. BMC Cancer 2019, 19, 1271. [Google Scholar] [CrossRef] [PubMed]
- Donovitz, G.; Cotten, M. Breast Cancer Incidence Reduction in Women Treated with Subcutaneous Testosterone: Testosterone Therapy and Breast Cancer Incidence Study. Eur. J. Breast Health 2021, 17, 150–156. [Google Scholar] [CrossRef] [PubMed]
- Gera, R.; Tayeh, S.; Chehade, H.E.; Mokbel, K. Does Transdermal Testosterone Increase the Risk of Developing Breast Cancer? A Systematic Review. Anticancer Res. 2018, 38, 6615–6620. [Google Scholar] [CrossRef] [Green Version]
- Hofling, M.; Hirschberg, A.L.; Skoog, L.; Tani, E.; Hagerstrom, T.; von Schoultz, B. Testosterone inhibits estrogen/progestogen-induced breast cell proliferation in postmenopausal women. Menopause 2007, 14, 183–190. [Google Scholar] [CrossRef]
- Eigeliene, N.; Elo, T.; Linhala, M.; Hurme, S.; Erkkola, R.; Harkonen, P. Androgens inhibit the stimulatory action of 17beta-estradiol on normal human breast tissue in explant cultures. J. Clin. Endocrinol. Metab. 2012, 97, E1116–E1127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ochnik, A.M.; Moore, N.L.; Jankovic-Karasoulos, T.; Bianco-Miotto, T.; Ryan, N.K.; Thomas, M.R.; Birrell, S.N.; Butler, L.M.; Tilley, W.D.; Hickey, T.E. Antiandrogenic actions of medroxyprogesterone acetate on epithelial cells within normal human breast tissues cultured ex vivo. Menopause 2014, 21, 79–88. [Google Scholar] [CrossRef]
- Shamseddin, M.; De Martino, F.; Constantin, C.; Scabia, V.; Lancelot, A.S.; Laszlo, C.; Ayyannan, A.; Battista, L.; Raffoul, W.; Gailloud-Matthieu, M.C.; et al. Contraceptive progestins with androgenic properties stimulate breast epithelial cell proliferation. EMBO Mol. Med. 2021, 13, e14314. [Google Scholar] [CrossRef] [PubMed]
- Tamimi, R.M.; Hankinson, S.E.; Chen, W.Y.; Rosner, B.; Colditz, G.A. Combined estrogen and testosterone use and risk of breast cancer in postmenopausal women. Arch. Intern. Med. 2006, 166, 1483–1489. [Google Scholar] [CrossRef] [Green Version]
- De Blok, C.J.M.; Wiepjes, C.M.; Nota, N.M.; van Engelen, K.; Adank, M.A.; Dreijerink, K.M.A.; Barbe, E.; Konings, I.; den Heijer, M. Breast cancer risk in transgender people receiving hormone treatment: Nationwide cohort study in the Netherlands. BMJ 2019, 365, l1652. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Irwig, M.S. Testosterone therapy for transgender men. Lancet Diabetes Endocrinol. 2017, 5, 301–311. [Google Scholar] [CrossRef]
- Fundytus, A.; Saad, N.; Logie, N.; Roldan Urgoiti, G. Breast cancer in transgender female-to-male individuals: A case report of androgen receptor-positive breast cancer. Breast J. 2020, 26, 1007–1012. [Google Scholar] [CrossRef]
- Light, M.; McFarlane, T.; Ives, A.; Shah, B.; Lim, E.; Grossmann, M.; Zajac, J.D.; Cheung, A.S. Testosterone therapy considerations in oestrogen, progesterone and androgen receptor-positive breast cancer in a transgender man. Clin. Endocrinol. 2020, 93, 355–357. [Google Scholar] [CrossRef]
- Nikolic, D.V.; Djordjevic, M.L.; Granic, M.; Nikolic, A.T.; Stanimirovic, V.V.; Zdravkovic, D.; Jelic, S. Importance of revealing a rare case of breast cancer in a female to male transsexual after bilateral mastectomy. World J. Surg. Oncol. 2012, 10, 280. [Google Scholar] [CrossRef] [Green Version]
- Tanini, S.; Fisher, A.D.; Meattini, I.; Bianchi, S.; Ristori, J.; Maggi, M.; Lo Russo, G. Testosterone and Breast Cancer in Transmen: Case Reports, Review of the Literature, and Clinical Observation. Clin. Breast Cancer 2019, 19, e271–e275. [Google Scholar] [CrossRef] [PubMed]
- Elhassan, Y.S.; Idkowiak, J.; Smith, K.; Asia, M.; Gleeson, H.; Webster, R.; Arlt, W.; O’Reilly, M.W. Causes, Patterns, and Severity of Androgen Excess in 1205 Consecutively Recruited Women. J. Clin. Endocrinol. Metab. 2018, 103, 1214–1223. [Google Scholar] [CrossRef] [PubMed]
- Secreto, G.; Zumoff, B. Role of androgen excess in the development of estrogen receptor-positive and estrogen receptor-negative breast cancer. Anticancer Res. 2012, 32, 3223–3228. [Google Scholar] [PubMed]
- Houghton, L.C.; Knight, J.A.; Wei, Y.; Romeo, R.D.; Goldberg, M.; Andrulis, I.L.; Bradbury, A.R.; Buys, S.S.; Daly, M.B.; John, E.M.; et al. Association of Prepubertal and Adolescent Androgen Concentrations with Timing of Breast Development and Family History of Breast Cancer. JAMA Netw. Open 2019, 2, e190083. [Google Scholar] [CrossRef] [Green Version]
- Secreto, G.; Sieri, S.; Agnoli, C.; Grioni, S.; Muti, P.; Zumoff, B.; Sant, M.; Meneghini, E.; Krogh, V. A novel approach to breast cancer prevention: Reducing excessive ovarian androgen production in elderly women. Breast Cancer Res. Treat. 2016, 158, 553–561. [Google Scholar] [CrossRef] [PubMed]
- Endogenous, H.; Breast Cancer Collaborative, G.; Key, T.J.; Appleby, P.N.; Reeves, G.K.; Travis, R.C.; Alberg, A.J.; Barricarte, A.; Berrino, F.; Krogh, V.; et al. Sex hormones and risk of breast cancer in premenopausal women: A collaborative reanalysis of individual participant data from seven prospective studies. Lancet Oncol. 2013, 14, 1009–1019. [Google Scholar] [CrossRef] [Green Version]
- Zeleniuch-Jacquotte, A.; Afanasyeva, Y.; Kaaks, R.; Rinaldi, S.; Scarmo, S.; Liu, M.; Arslan, A.A.; Toniolo, P.; Shore, R.E.; Koenig, K.L. Premenopausal serum androgens and breast cancer risk: A nested case-control study. Breast Cancer Res. 2012, 14, R32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaaks, R.; Tikk, K.; Sookthai, D.; Schock, H.; Johnson, T.; Tjonneland, A.; Olsen, A.; Overvad, K.; Clavel-Chapelon, F.; Dossus, L.; et al. Premenopausal serum sex hormone levels in relation to breast cancer risk, overall and by hormone receptor status—Results from the EPIC cohort. Int. J. Cancer 2014, 134, 1947–1957. [Google Scholar] [CrossRef] [Green Version]
- Dorgan, J.F.; Longcope, C.; Stephenson, H.E., Jr.; Falk, R.T.; Miller, R.; Franz, C.; Kahle, L.; Campbell, W.S.; Tangrea, J.A.; Schatzkin, A. Relation of prediagnostic serum estrogen and androgen levels to breast cancer risk. Cancer Epidemiol. Biomark. Prev. 1996, 5, 533–539. [Google Scholar]
- Zhang, X.; Tworoger, S.S.; Eliassen, A.H.; Hankinson, S.E. Postmenopausal plasma sex hormone levels and breast cancer risk over 20 years of follow-up. Breast Cancer Res. Treat. 2013, 137, 883–892. [Google Scholar] [CrossRef] [Green Version]
- Dimitrakakis, C.; Zhou, J.; Bondy, C.A. Androgens and mammary growth and neoplasia. Fertil. Steril. 2002, 77 (Suppl. S4), S26–S33. [Google Scholar] [CrossRef]
- Lee, O.; Heinz, R.E.; Ivancic, D.; Muzzio, M.; Chatterton, R.T.; Zalles, C.M.; Keeney, K.; Phan, B.; Liu, D.; Scholtens, D.; et al. Breast Hormone Concentrations in Random Fine-Needle Aspirates of Healthy Women Associate with Cytological Atypia and Gene Methylation. Cancer Prev Res. 2018, 11, 557–568. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chatterton, R.T.; Heinz, R.E.; Fought, A.J.; Ivancic, D.; Shappell, C.; Allu, S.; Gapstur, S.; Scholtens, D.M.; Gann, P.H.; Khan, S.A. Nipple Aspirate Fluid Hormone Concentrations and Breast Cancer Risk. Horm. Cancer 2016, 7, 127–136. [Google Scholar] [CrossRef] [Green Version]
- Evans, D.G.; Howell, S.J.; Howell, A. Personalized prevention in high risk individuals: Managing hormones and beyond. Breast 2018, 39, 139–147. [Google Scholar] [CrossRef] [PubMed]
- McCormack, V.A.; dos Santos Silva, I. Breast density and parenchymal patterns as markers of breast cancer risk: A meta-analysis. Cancer Epidemiol. Biomark. Prev. 2006, 15, 1159–1169. [Google Scholar] [CrossRef] [Green Version]
- Martin, L.J.; Boyd, N.F. Mammographic density. Potential mechanisms of breast cancer risk associated with mammographic density: Hypotheses based on epidemiological evidence. Breast Cancer Res. 2008, 10, 201. [Google Scholar] [CrossRef] [PubMed]
- Gabrielson, M.; Azam, S.; Hardell, E.; Holm, M.; Ubhayasekera, K.A.; Eriksson, M.; Backlund, M.; Bergquist, J.; Czene, K.; Hall, P. Hormonal determinants of mammographic density and density change. Breast Cancer Res. 2020, 22, 95. [Google Scholar] [CrossRef]
- Bertrand, K.A.; Eliassen, A.H.; Hankinson, S.E.; Rosner, B.A.; Tamimi, R.M. Circulating Hormones and Mammographic Density in Premenopausal Women. Horm. Cancer 2018, 9, 117–127. [Google Scholar] [CrossRef] [PubMed]
- Lundstrom, E.; Carlstrom, K.; Naessen, S.; Soderqvist, G. Dehydroepiandrosterone and/or its metabolites: Possible androgen receptor antagonistic effects on digitized mammographic breast density in normal breast tissue of postmenopausal women. Horm. Mol. Biol. Clin. Investig. 2018, 25, 35. [Google Scholar] [CrossRef]
- Chen, F.; Knecht, K.; Birzin, E.; Fisher, J.; Wilkinson, H.; Mojena, M.; Moreno, C.T.; Schmidt, A.; Harada, S.; Freedman, L.P.; et al. Direct agonist/antagonist functions of dehydroepiandrosterone. Endocrinology 2005, 146, 4568–4576. [Google Scholar] [CrossRef] [Green Version]
- Rebbeck, T.R.; Kantoff, P.W.; Krithivas, K.; Neuhausen, S.; Blackwood, M.A.; Godwin, A.K.; Daly, M.B.; Narod, S.A.; Garber, J.E.; Lynch, H.T.; et al. Modification of BRCA1-associated breast cancer risk by the polymorphic androgen-receptor CAG repeat. Am. J. Hum. Genet. 1999, 64, 1371–1377. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, J.J.; Irvine, R.A.; Buchanan, G.; Koh, S.S.; Park, J.M.; Tilley, W.D.; Stallcup, M.R.; Press, M.F.; Coetzee, G.A. Breast cancer susceptibility gene 1 (BRCAI) is a coactivator of the androgen receptor. Cancer Res. 2000, 60, 5946–5949. [Google Scholar]

| Identifier | Title | Phase | Treatment | Activity | Disease |
|---|---|---|---|---|---|
| NCT02463032 | A Phase 2 Open Label, Multi-Center, Multinational, Randomized, Parallel Design Study Investigating The Efficacy and Safety Of GTx-024 On Metastatic or Locally Advanced ER+/AR+ Breast Cancer (BC) in Postmenopausal Women | 2 | GTx-024 | Selective-AR modulator | ER+ AR+ metastatic or locally advanced BC |
| NCT02007512 | A phase 2, randomized, double-blind, placebo-controlled, multicenter study of efficacy and safety of enzalutamide in combination with exemestane in patients with advanced breast cancer that is estrogen or progesterone receptor-positive and her2-normal | 2 | Enzalutamide/ Exemestane/ Placebo | AR inhibitor/ Aromatase inhibitor | ER+ or PR+ and HER2 normal advanced BC |
| NCT01889238 | A phase 2, single-arm, open-label, multicenter study of the clinical activity and safety of enzalutamide in patients with advanced, androgen receptor-positive, triple-negative breast cancer | 2 | Enzalutamide | AR inhibitor | AR+ TNBCadvanced BC |
| NCT03650894 | A Phase II Study of Nivolumab Combined With Bicalutamide and Ipilimumab in Metastatic HER2-negative Breast Cancer | 2 | Nivolumab/ Ipilimumab/ Bicalutamide | anti-PD-1/ anit-CTLA4/ AR inhibitor | HER 2- metastatic or unresectable BC |
| NCT02091960 | A Phase 2, Multicenter, Open-label Study to Assess the Efficacy and Safety of Enzalutamide With Trastuzumab in Subjects With HER2+ AR+ Metastatic or Locally Advanced Breast Cancer | 2 | Enzalutamide/ Trastuzumab | AR inhibitor/ HER2 inhibitor | AR+ HER2+ metastatic or locally advanced BC |
| NCT00725374 | An Exploratory, Double-blind, Randomized, Placebo-controlled Trial to Investigate the Tissue Specific Effects of 2.5 mg Tibolone on Breast Cancer in Postmenopausal Women, in Particular on Breast Tissue Proliferation | 3 | Tibolone/ Placebo | Selective-ER modulator | BC |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chiodo, C.; Morelli, C.; Cavaliere, F.; Sisci, D.; Lanzino, M. The Other Side of the Coin: May Androgens Have a Role in Breast Cancer Risk? Int. J. Mol. Sci. 2022, 23, 424. https://doi.org/10.3390/ijms23010424
Chiodo C, Morelli C, Cavaliere F, Sisci D, Lanzino M. The Other Side of the Coin: May Androgens Have a Role in Breast Cancer Risk? International Journal of Molecular Sciences. 2022; 23(1):424. https://doi.org/10.3390/ijms23010424
Chicago/Turabian StyleChiodo, Chiara, Catia Morelli, Fabiola Cavaliere, Diego Sisci, and Marilena Lanzino. 2022. "The Other Side of the Coin: May Androgens Have a Role in Breast Cancer Risk?" International Journal of Molecular Sciences 23, no. 1: 424. https://doi.org/10.3390/ijms23010424
APA StyleChiodo, C., Morelli, C., Cavaliere, F., Sisci, D., & Lanzino, M. (2022). The Other Side of the Coin: May Androgens Have a Role in Breast Cancer Risk? International Journal of Molecular Sciences, 23(1), 424. https://doi.org/10.3390/ijms23010424