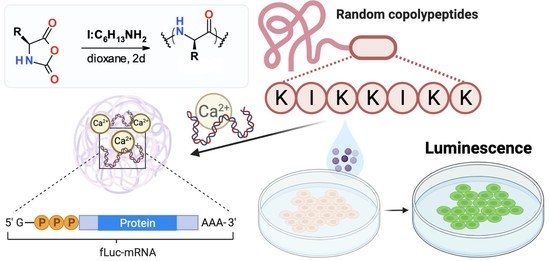
1. Introduction
Intracellular delivery of different genetic constructions in order to acquire the positive medical effect of such treatment represents emerging problem of modern biomedical science [
1]. It is of common knowledge, that many approaches for treatment of different severe diseases require control over protein expression. The first type of genetic drugs was plasmid DNA (pDNA), which allowed inducing the cellular production of necessary protein [
2]. However, pDNA effective action implies penetration to nucleus, which is quite challenging from delivery point of view. In this regard, application of messenger RNA (mRNA), which acts in cytoplasm, is more perspective [
3]. Moreover, mRNA does not integrate to the genome of the cells, which benefits from potential carcinogenicity point of view [
4].
In recent decades, the clinical role of mRNA-based therapeutics is constantly growing, mainly due to its great potential as vaccine [
5]. mRNA vaccines played a crucial role in struggling COVID-19 pandemic [
6]. However, mRNA vaccination is not only perspective for prevention of severe viral infections, but also possess great potential for immunotherapy of tumors [
4,
7,
8]. The latter approach requires the transfection of antigen-presenting cells with mRNA, which encodes tumor-associated antigen [
9] or chimeric antigen receptors (CARs) [
10].
Despite some obvious clinical success, the development of mRNA delivery systems represents an urgent research problem. Although viral vectors are highly effective, they still have a number of drawbacks, such as potential to trigger immunogenic responses and transgene mis-insertion risks [
4,
11,
12].
There are three major types of nanoplatforms applied for non-viral intracellular delivery of genetic constructions: lipoplexes, lipid nanoparticles and polyplexes [
13]. First ones are composed of positively charged lipids, which form self-organized spherical vesicles due to presence of distinct hydrophilic and hydrophobic parts within their molecules, and could encapsulate nucleic acids (NA) into their inner aqueous phase [
14]. Lipoplexes represent quite efficient class of transfection agents, which efficiently penetrate cellular interior and escape from endosomes [
15,
16,
17]. Despite good efficacy in vitro, lipoplexes possess some limitations for in vivo applications, such as uncertain stability in serum [
18] and toxicity [
19].
These features of lipoplexes have turned the interest to lipid nanoparticles (LNPs) as NA delivery vehicles [
20,
21]. The morphology of LNPs differs from traditional liposome bilayer, and characterized by inverted micelle formed by cationic/ionizable lipids around the encapsulated NA molecules [
22]. LNPs are more stable and quite versatile systems, which could serve as efficient NA delivery nanoplatforms in different applications [
23]. However, further development of LNPs involves the possibility of combining LNPs with polycations in order to increase their intracellular penetration, endosomal escape and transfection efficacy [
21].
Polycations represent an important type of non-viral vectors for delivery of genetic constructions [
24,
25]. During interaction of polycations with NA the condensation of NA into so called polyplexes (or polyion complexes) occurs [
26]. This process is quite simple and reproducible, which makes it very attractive for future clinical applications. The obtained polyplexes are nanoparticles with high density leading for easy cell internalization and enhanced protection from enzymatic degradation [
25]. The study of polycations application for stabilization of NA both in vitro and in vivo, as well as for promotion of nucleic acids intracellular penetration is vast and emerging. However, clinical application of polycations is still limited, mainly due to the low efficacy of such vectors. In order to give polycations a chance to serve as effective NA delivery vectors the study of different structural peculiarities of such polycations on the efficacy of NA binding, stabilization and transfection is of great importance.
There are three different physicochemical characteristics, which affect NA binding and transfection: positive charge, hydrogen bonding and hydrophobic interactions. While the first one is responsible for binding of NA, the second one is responsible for compactization of interpolyelectrolyte complexes (IPECs) into dense nanoparticles [
27]. Hydrophobization of polycations usually benefits their ability for effective transfection and lowering toxicity [
28,
29,
30]. The reason for this is the reduction of charge density by introduction of hydrophobic units, formation of stable and compact nanostructures, which better penetrate the cells. Hydrophobic modification of polycations is also believed to be responsible for better interaction with cell membranes and endosomolytic properties. In addition, hydrophobic interactions within the polyplex could cause the destabilization of polyplex, leading to better intracellular release of loaded NA [
27,
31].
Among different polycations many studies have been devoted to the application of poly(L-lysine) (PLys). PLys could be easily obtained by ring-opening polymerization of corresponding N-carboxyanhydride, and resulting polymer contains primary ε-amino groups in the side chains of each monomer unit, which provide great positive charge density. This allows an efficient binding of DNA or RNA molecules at physiological pHs. Other advantageous features of PLys are its biodegradability and efficient intracellular penetration. However, there are several drawbacks accompanying PLys application. These are toxicity for molecules with molecular weight (MW) above 30,000 and poor efficacy of transfection, which could be caused by too strong binding of NA with polycation.
Different research groups were focused on solving PLys poor transfection efficacy by chemical modification of PLys. Hydrophobization of PLys was performed in several studies and allowed to reach better results on transfection. Such hydrophobization could be performed by polymeranalogous reaction of prepared polymer, or by addition of hydrophobic monomer during polymer synthesis [
32,
33]. As example of the former approach, low and high MW (4000 and 25,000) PLys were modified by several endogenous lipids to substitute 10% of ε-NH2 groups of the polymer [
34]. The transfection efficacy correlated with the degree of substitution, and overall, the lipid-modified high MW PLys was found to be about 20–25% more effective than unmodified PLys. Hydrophobized PLys modified during polymerization stage are generally represented by block copolymers. Self-assembled PEG-b-PLys-b-PLeu [
35] micelles and PLys-b-PLeu with different length of Leu blocks [
36] were obtained and studied as gene delivery vehicles. It was shown by the authors of mentioned studies that increasing of hydrophobic block length benefits the transfection efficacy.
Despite some number of studies devoted to the application of amphiphilic Lys-containing block-copolymers, there is an unexplored possibility of synthesis and application of random copolymers of lysine with other hydrophobic amino acids, such as leucine (Leu) or isoleucine (Ile), in NA delivery. Random copolymers of poly(amino acids) were described earlier [
33,
37] and showed different properties as compared to block-copolymers. One of the peculiarities of random copolymers is their flexibility, which could greatly affect NA binding and transfection. PLys is known to form α-helix [
38], while Ile sequences in proteins are known to participate in the formation of β-sheets [
39,
40] (
Figure 1). These structures are more or less rigid, which should affect the formation of polyplexes and intracellular release of entrapped genetic construction. Copolymerization of Lys and Ile into random copolymer should lead to flexible polymer chain with reduced positive charge as compared to pure PLys. At the same time, the presence of Ile should benefit penetration through cytoplasmic membrane and escape from early endosomes via hydrophobic interactions with phospholipids. Furthermore, decreasing of the charge density of polycation by introduction of hydrophobic units into should facilitate the release of NA from IPECs (
Figure 1).
In this study, we have obtained random copolymers of lysine and isoleucine (P(Lys-co-Ile)) and assessed their properties in polyplexes with mRNA and pDNA. In addition, the study of their cytotoxicity and transfection efficacy were performed and discussed.
3. Materials and Methods
3.1. Materials
ε-Z-L-lysine (Lys(Z)), L-isoleucine (Ile), α-pinene, triphosgene, trifluoroacetic acid (TFA), n-butylamine, trifluoromethanesulfonic acid (TFMSA), dimethyl sulfoxide-d6 (DMSO-d6, 99.8%), heparin sodium salt (Mw 8000–12,000), Ribonuclease A (RNase) were obtained from Sigma–Aldrich (Darmstadt, Germany) and used without additional purification. All organic solvents, i.e., N,N-dimethylformamide (DMF), dimethyl sulfoxide (DMSO) 1,4-dioxane, petroleum ether, ethyl acetate and some others were purchased from Vecton Ltd. (St. Petersburg, Russia), and purified before use according to standard protocols. For purification of synthesized polymers, Spectra/Pore® dialysis bags (MWCO:1000 and 10,000, Rancho Dominguez, CA, USA) were used. Amicon Ultra filter tubes with 10,000 and 50,000 MWCO (0.5 mL) were purchased from Merck (Darmstadt, Germany). Cy3-NHS fluorescent dye was purchased from Lumiprobe (Moscow, Russia). Ethidium bromide (EtBr) and agarose were purchased from Molecular Probes (Eugene, OR, USA).
SEC column calibration was performed with the use of poly(methyl methacrylate) (PMMA) standards (Mw 17,000–250,000; Đ ≤ 1.14) purchased from Supelco (Bellefonte, PA, USA). Spin columns (molecular weight cut-off (MWCO) 3000; VivaScience, Sartorius Group, Göttingen, Germany) and Eppendorf tubes with filter (30,000 MWCO, Amicon Ultra 0.5 mL, Merck, Darmstadt, Germany) were used for dialysis, IPECs purification, and separation.
Cy3-labeled 23-base pairs duplex of oligothymidine and oligoadenine (oligo-dT-dA) was purchased from Biobeagle (St. Petersburg, Russia). Firefly luciferase encoding mRNA was prepared as described below. Plasmids encoding luciferase (pCLuc4) and GFP (pEGFP-C2) were a kind gifts of Dr. Marika Ruponen (School of Pharmacy, University of Eastern Finland, Kuopio, Finland). GFP encoding messenger RNA (EGFP-mRNA) was obtained from IBA (Göttingen, Germany). Lipofectamine 2000 Transfection agent (Invitrogen, Carlsbad, CA, USA).
Branched poly(ethylene imine) (bPEI 25k; Mw = 25,000 and Mn = 10,000 according to GPC; Sigma-Aldrich, St Louis, MO, USA) was used as control in transfection studies.
The plastic for cell cultivation was obtained from Sigma-Aldrich (Darmstadt, Germany) and Sarstedt AG and Co (Nümbrecht, Germany). Transfection analysis was performed with Gaussia Luciferase kit (Thermo Fisher Scientific, Roskilde, Denmark).
All other materials are described further upon their appearance in the text.
3.2. Instruments
The magnetic stirrer MR Hei-Mix S (Heidolph, Schwabach, Germany), Schlenk reaction tubes with rubber septum (Aldrich, Munich, Germany) and rotary evaporator Hei-VAP Precision ML/G3B (Schwabach, Heidolph, Germany) were used for polymer synthesis. 1H NMR spectroscopic data were recorded with equipment of Magnetic Resonance Research Centre of St. Petersburg State University: Bruker Avance spectrometer (400.13 MHz for 1H and 100.61 MHz for 13C) in DMSO-d6 or in D2O. Shimadzu LC-20 Prominence system supplied with refractometric RID 10-A detector (Kyoto, Japan) and 7.5 × 300 mm Agilent PLgel MIXED-D column (Chrom Tech, Apple Valley, MN, USA) were applied for size exclusion chromatography (SEC) analysis.
An ultrasound homogenizer (Sonopuls HD2070, Bandelin, Berlin, Germany), Vortex (Thermo Fisher Scientific, Waltham, MA, USA) and TS-100C thermo-shaker BioSan (Riga, Latvia) were applied for polyplexes formation. The Vivaspin column ultra-filtration and particle separation were performed with Sigma 2–16 KL centrifuge (Sigma, Darmstadt, Germany).
A dynamic and electrophoretic light-scattering (DLS and ELS) instrument Zetasizer Nano ZS (Malvern, Enigma Business Park, United Kingdom) equipped with a He–Ne laser beam at λ = 633 nm was used for measurements of particle hydrodynamic radius, particle size distribution and surface charge. Nucleic acids were quantified spectrophotometrically with application of Nanodrop 2000c spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA). The photometrical detection of aldehyde groups was performed with UV–Vis-1800 Shimadzu (Kyoto, Japan). Microplate Spectrophotometer-Fluorometer Fluoroskan Ascent reader (Thermo Fisher Scientific, Waltham, MA, USA) was used for quantitative analysis. Nanoparticles morphology was analyzed in Centre for Molecular and Cell Technologies of St. Petersburg State University by using transmission electron microscope Zeiss Libra 200FE (Carl Zeiss, Oberkochen, Germany).
Agarose gel electrophoresis was performed with BlueMarine 200 system (Serva Electrophoresis GmbH, Heidelberg, Germany) and analyzed with SkyLight Table ECX-F20 (Vilber, Collégien, France).
PCR amplification was performed with equipment purchased in Biometra Thistle Scientific (Glasgow, UK).
The cell uptake efficiency was determined using CELENA S Digital Imaging System (Logos Biosystems, GE Healthcare, South Korea). Transfection efficiency was visualized by the Cytell Cell Imaging instrument (GE Healthcare, Washington, Issaquah, USA) and quantified with Microplate Spectrophotometer-Fluorometer Fluoroskan Ascent reader (Thermo Fisher Scientific, Waltham, MA, USA).
All other instruments are described further upon their appearance in the text.
3.3. Cells Culturing
Human retinal pigment epithelial (ARPE-19) and corneal epithelial (HCE) cells were obtained from American Type Culture Collection (ATCC, Manassas, VA, USA). Medium materials for cell culture were obtained as follows. ARPE-19 growth medium contained Dulbecco’s modified eagle medium (DMEM, Gibco Laboratories, Gaithersburg, MD, USA), fetal bovine serum (FBS, 10 vol%, Thermo Fischer Scientific, Roskilde, Denmark), L-glutamine (1 vol%), penicillin (1 vol%), streptomycin (1 vol%). 500 mL of HCE growth medium contained 402.5 mL of DMEM F12 (Gibco Laboratories, Gaithersburg, MD, USA), 75 mL of FBS (Thermo Fischer Scientific, Roskilde, Denmark), penicillin (1 vol%), streptomycin (1 vol%), L-Glutamine (5 mL), epidermal growth factor (EGF, 0.005 mg), insulin (2.5 mg), DMSO (2.5 mL), cholera toxin (0.05 mg), 1 M HEPES (7.5 mL, Thermo Fischer Scientific, Roskilde, Denmark).
3.4. Methods
3.4.1. Synthesis and Characterization of Cationic Polypeptides
Polypeptides were synthesized by ring-opening polymerization (ROP) of α-amino acid N-carboxyanhydrides (NCA) as random structures (
Figure 2). NCA monomers of Lys(Z) and Ile were prepared via the reaction of α-amino acids with triphosgene in anhydrous dioxane under argon atmosphere with addition of α-pinene [
33]. Synthesized NCAs were purified by recrystallization from anhydrous ethyl acetate/n-hexane. Yields: Lys(Z) NCA—85%, Ile NCA—69%. The structure and purity of NCAs were confirmed by 1H NMR at 25 °C in CDCl3 (400 MHz Avance instrument, Bruker, Karlsruhe, Germany). Lys(Z) NCA: δ 7.43–7.28 (m, 5H), 6.97 (s, 1H), 5.12 (s, 2H), 4.97 (s, 1H), 4.32–4.23 (t, J = 5.2, 1H) (s, 1H), 3.29–3.14 (m, 2H), 2.03–1.90 (m, 1H), 1.90–1.75 (m, 1H), 1.73–1.28 (m, 4H); Ile NCA: 0.836 (t, 3H), 0.871 (d, 3H), 1.236 (dq, 2H), 1.941 (qtd,1H), 4.28 (d, 1H).
NCAs were polymerized to produce P(Lys(Z)-co-Ile) using n-hexylamine as initiator and the following molar ratio of monomers: [Lys(Z) NCA]/[Ile NCA] = 80/20. The NCAs to initiator molar ratios varied as 10, 20 and 50. Polymerization was initiated by dropwise addition of n-hexylamine to 4 wt% solution of NCAs in anhydrous 1,4-dioxane. The mixture was stirred under inert atmosphere at room temperature during 48 h. The product was precipitated with 50 volume excess of diethyl ether. The precipitate was dissolved in DMF and dialyzed against water for 36 h (MWCO 1000).
Molecular-weight characteristics (weight average and number average molecular weights, Mw and Mn, respectively, as well as dispersity, Đ) of polymers were evaluated by SEC with application of DMF with 0.1 M LiBr was used as a mobile phase. The analysis was performed at 40 °C under 1.0 mL/min of the mobile phase flow rate. SEC LC Solutions software (Shimadzu, Kyoto, Japan) was used for calculations of Mw, Mn and Đ regarding the calibration curve plotted for PMMA standards.
The amino acid compositions of obtained polypeptides were determined using 1H NMR at 25 °C in DMSO-d6 at 400 MHz. PLys, δ ppm: n-hexylamine—0.8 (CH3); lysine—4.1–4.3 (CH), 2.97 (NH-CH2), Z-protection—5.0 (O-CH2-C6H5), 7.1–7.4 (O-CH2-C6H5); P(Lys-co-Ile): δ ppm: n-hexylamine—0.9 (CH3), lysine—4.1–4.3 (CH), 3.0 (NH-CH2), Z-protection—5.0 (O-CH2-C6H5); 7.1–7.4 (O-CH2-C6H5); isoleucine—0.5–0.8 (CH3), 1.1–1.4 (CH2CH3), 1.5–1.7 (CH(CH3)(C2H5)), 3.4–3.6 ((CO)(NH)CHCH(CH3)(C2H5)).
The Z-protection groups were removed by 9% trifluoromethanesulfonic acid in trifluoroacetic acid (TFMSA/TFA, Sigma Aldrich) according to general procedure [
27]. The peptide products were dissolved in DMF, dialyzed against water for 36 h (MWCO 1000) and lyophilized under vacuum. The removal of Z-protection was detected with application of
1H NMR in D
2O by disappearance of aromatic signals at 7.1–7.4 ppm.
Composition of copolymers was determined via quantitative HPLC analysis of free amino acids obtained after total acidic hydrolysis of copolymers with the use of protocol developed earlier [
33]. An example of chromatogram as well as details on chromatographic conditions and calibration plots can be found in
Supplementary Materials (
Figure S1).
3.4.2. Preparation and Characterization of Polypeptide Particles
Polypeptide nanoparticles suspensions in 0.01 M PBS at pH 7.4 were obtained by 30 s sonication treatment of polycations using 10% power of ultrasonic homogenizer with application of ultrasound homogenizer (70 W, acoustic energy—0.21 kJ).
Average hydrodynamic diameter of particles (DH) and polydispersity index (PDI) were measured at 25 °C by DLS at scattering angle of 173°. DLS experiments were performed in 0.01 M PBS, pH 7.4, whereas ζ-potential determination by ELS was carried out in deionized water. Stability of peptide nanoparticles colloidal suspensions were examined by DLS in PBS pH 7.4 and Opti MEM (FBS-free) for 14 days.
The morphology was investigated by transmission electron microscopy (TEM). The samples were prepared by dropping 3 μL of particles’ suspension (0.3 mg/mL) on copper grid (300 mesh) covered with carbon and formvar, and further staining with 3 w/w% uranyl acetate solution for 1 min. The grids were washed gently with pure water and dried for 30 min before the measurement.
3.4.3. In Vitro Transcription of mRNA
The gen of interest (firefly luciferase) mRNA (fLuc mRNA) was cloned into a modified pSMART vector. The open reading frame of the gene was flanked by the Kozak sequence in the 5′UTR and the human a-globin sequence in the 3′UTR and poly A tail. The template for in vitro transcription was prepared by PCR (Biometra Thistle Scientific, Glasgow, UK) amplification of the gene. The required amount of plasmid was linearized by SpeI restriction enzyme.
mRNA was synthesized with the T7 RNA polymerase [
51]. 500 μL of reaction contained: 50 ng/μL linearized plasmid (BIOCAD, Saint Petersburg, Russia), 2 mM Ribonucleotide Triphosphates (NEB, Ipswich, MA, USA), 1x Transcription buffer (40 mM TrisHCl pH 8, (Sigma Aldrich, St. Louis, MO, USA), 6 mM MgCl
2 (Sigma Aldrich, St. Louis, MO, USA), 2 mM spermidine (Panreac, Barcelona, Spain), 5 mM DTT (Panreac, Barcelona, Spain), 1 unit/μL RNaseIn (Solar Bio, Beijing, China), 0.2 μg/μL T7 RNA polymerase (BIOCAD, Saint Petersburg, Russia). The reaction was incubated at 37 °C for 4 h. The product was treated with 25 units of DNase I (Neb, Ipswich, MA, USA) and purified by LiCl (Sigma Aldrich, St. Louis, MO, USA) precipitation. The capping reaction was performed by Vaccinia Capping System [
52]. 1000 μL of reaction medium contained 1x capping buffer (50 mM Tris-HCl pH 8.5 mM KCl (Panreac, Barcelona, Spain), 1 mM MgCl
2, 1 mM DTT), 0.5 mM Guanosine Triphosphate (Neb, Ipswich, MA, USA), 0.1 mM S-Adenosyl methionine (Neb, Ipswich, MA, USA), 1U/μL RNAseIn, 7.5 ng/μL Vaccinia Capping Enzyme (BIOCAD, Saint Petersburg, Russia), 500 μg of denaturated RNA. The reaction medium was incubated at 37 °C for 1 h. The final Luc mRNA was purified with silica columns [
26] (Promega, Madison, WI, USA).
The 1 mg/mL stock solution of nuclease resistant Luc mRNA in 1 mM sodium citrate buffer was prepared and stored in the freezer. The stability of Luc mRNA was testified before application by 1% agarose denaturing gel electrophoresis (
Figure S7,
Supplementary Materials).
3.4.4. Condensation of mRNA with Calcium Chloride
mRNA was turned into condensed form by calcium chloride at 20:1 [nucleotide:Ca
2+] molar ratios. Briefly, 10 µg of mRNA was mixed with 0.16 µg of CaCl
2, the mixture volume was then adjusted to 100 µL using 1xTE buffer. Calcium chloride was overall used at a concentration of 1.4 × 10
−7 M established as safe concentration for intra- and extracellular environment [
41]. Hydrodynamic size and ζ-potential of prepared mRNA-Ca
2+ complexes were 87 nm (PDI 0.22) and −32.9 mV, respectively.
3.4.5. Encapsulation of mRNA
mRNA-Ca2+ complexes were then incorporated into positively charged polypeptides through polyelectrolyte complexation between phosphate groups and ε-amino groups of lysine with varying N/P ratio [nitrogen to phosphate] using three methods.
Method 1: the polymers (P(Lys-co-Ile), PLys) and NA solutions (1 mg/mL) were mixed at predetermined N/P ratios and vortexed at 1000 rpm, and 25 °C during 30 s. The formed suspensions were further incubated at 25 °C during 30 min for particles ageing.
Method 2: the dispersion of polymer particles in water or buffer solution (1 mg/mL) was prepared as in method 1, but then subjected to ultrasonication during 30 s and diluted to a desired concentration. After that, the condensed mRNA-Ca2+ complex was quickly added to the suspension under 1500 rpm vortex stirring during 2 min. The mixture was left for 30 min at room temperature for ageing.
Method 3: the polymers (P(Lys-co-Ile); PLys) were dissolved in a 70% ethanol-water mixture at concentration of 1 mg/mL. Particles were formed varying N/P ratio [nitrogen to phosphate]. For that, the appropriate volume of polymer solution was quickly mixed with mRNA-Ca2+ complexes (prepared in TE buffer) at 1500 rpm stirring, the reaction mixture was left for 30 min at room temperature and then washed with 0.01 M PBS pH 7.4 via ultracentrifugation (MWCO 10,000) to purify from ethanol.
The concentrations of mRNA were evaluated using RiboGreen fluorometric assay (Promega, Madison, WI, USA) [
44]. Ribogreen dye was diluted 200 times with a TE buffer in a dark plastic tube and stirred. For the calibration curve RNA standard solution (Ribosomal RNA standard, 16S and 23S rRNA from E. Coli) was diluted with a TE buffer to prepare final concentrations from 20 to 1000 ng/mL. 100 µL of each RNA concentration was mixed with 100 µL of Ribogreen solution in 96-well plates, the samples were kept at room temperature for 2–5 min and then analyzed using fluorimetry at an excitation wavelength of 480 nm and an emission wavelength 520 nm. mRNA sample was diluted with a TE buffer to a total mRNA concentration of 2 µg/mL. The concentration of mRNA samples was determined according to the same procedure and calculated via standard RNA calibration curve.
Effective binding of mRNA within polypeptide particles prevents staining of this NA with RiboGreen. Thus, only free mRNA was stained. This allowed direct determination of mRNA encapsulation efficacy without the need for separation of particles and the dye. For that, 100 µL of each particle suspension was mixed with 100 µL of Ribogreen solution in 96-well plates, the samples were analyzed as described above. Encapsulation efficiency was calculated using the following equation:
where m
1 is the initial input of mRNA, m
2 is the amount of non-bound mRNA determined by its fluorescence with application of RiboGreen Assay.
3.4.6. Agarose Gel Electrophoresis
To prepare the denaturing gel, 1 g of agarose was dissolved in 72 mL of water under heating, then 10 mL of 10X MOPS running buffer and 18 mL 37% formaldehyde (12.3 M) was added to the flask. The gel was poured using a well-comb and 1X MOPS was used as a running buffer. RNA samples were prepared as follows: 600 ng of free mRNA or particles, containing 600 ng RNA, were mixed with the equal volume of 1X Formaldehyde Loading Dye, containing ethidium bromide for visualization at a final concentration of 10 mg/mL. Samples were heated at 65–70 °C for 15 min and loaded to the gel. Electrophoresis was carried out at 6 V/cm until the bromophenol blue migrated at least ⅔ of the gel length and then the gels were imaged.
To study the enzymatic stabilization of mRNA by polyplexes RNase solution 3 µL (15 µg/mL) was added to the solution of pure mRNA 15 µL (150 ng) and 15 µL of polyplexes suspensions, containing the equivalent amount of mRNA. The reaction was stopped by addition of 2-fold wt/wt heparin towards RNase. To release mRNA from polyplexes before or after coincubation with RNase the 8-fold wt/wt excess of heparin towards polycation was added to polyplexes. Released mRNA was purified from heparin and polycation with application of Vivaspin columns (MWCO 100,000).
3.4.7. Cytotoxicity
The cytotoxicity of polyplexes with fLuc-mRNA prepared with varying N/P ratio from 2:1 to 20:1 was studied using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) reduction assay. Human retinal pigment epithelial (ARPE-19) and human corneal epithelial (HCE) cell lines were seeded into 96-well plates at a density of 20,000 and 23,000 cells/well. The cells were cultured in ARPE-19 and HCE cell culture media prepared according to the protocol (Experimental
Section 2.2). After 24 h, 10 µL of nanoparticles were added in 190 µL of Dulbecco’s Modified Eagle Medium (DMEM) F-12 with 10% fetal bovine serum (FBS) to each well in triplicate. The cells were exposed to the nanoparticles for 24 and 72 h. Thereafter, the medium was replaced with 10 μL MTT solution (5 mg/mL in DMEM F-12; filtered through 0.45 μm) and 100 μL of fresh DMEM F-12. The cells were incubated for 2 h at 37 °C and 5% CO2. Then, 100 μL of lysis buffer with sodium dodecyl sulfate and dimethylformamide was added to each well and the cells were incubated for an additional 3 h at 37 °C and 5% CO2. The absorbance of the wells was measured at 570 nm using a microplate reader (Viktor 2, Perkin Elmer, Waltham, MA, USA). Polypeptide without mRNA (PP) was used as control. The negative controls were DMEM F-12 (untreated cells) and 10% PBS (pH 7.4) in DMEM F-12 (solvent control). The mean value of solvent control (corrected for blank value) was set as 100% cell viability. The relative cell viability was calculated as following:
3.4.8. Cell Uptake
Cell uptake of polypeptide-mRNA nanoparticles into ARPE-19 cell line was assessed using Cy3 labeled polypeptides. The cells were incubated with nanoparticles in serum-free medium for 4 h, then the medium was removed and cells were washed with 1 M NaCl in order to wash out not penetrated particles. Then, 100 μL DMEM-F12 containing 2 × FBS and 2 × penicillin–streptomycin was added for another 20 h of incubation. After 24 h the cells were fixed using exposure of 200 μL 3.7% of formaldehyde–methanol per well for 15 min at 37 °C, washed three times with PBS. Cell membranes were permeabilized with 0.2% Triton X-100 in PBS for 15 min. Staining of the cell nuclei was performed using DAPI for 30 min according to a manufacturer protocol. The cells were washed with PBS and then with distilled water for three times. The cell uptake efficiency was determined by analyzing the fluorescence intensity of Cy3-polypeptide nanoparticles (λex = 532 nm, λem = 556 nm) using CELENA S Digital Imaging System (Logos Biosystems, GE Healthcare, Anyang, Kyonggi-do, South Korea).
3.4.9. Transfection of Cells with Plasmid DNA
To visualize the efficiency of nanocarriers developed to deliver and release nucleic acids, the transfection of ARPE-19 was performed using polypeptide nanoparticles with encapsulated 100 ng of plasmid DNA (pEGFP-C2) at N/P 4:1. Transfection efficiency was visualized by the Cytell Cell Imaging instrument (GE Healthcare, Washington, Issaquah, WA, USA).
Quantitative analysis on transfection efficiency of ARPE-19 and HCE was assessed using polypeptide particles, containing mRNA or pDNA coding luciferase genes.
3.4.10. Transfection of Cells with fLuc-mRNA
Cells (ARPE-19 and HCT) were seeded at a density of 50,000 cells per well in a 48-well plate. Next day, the cell culture medium was refreshed and 50 µL of nanoparticles, containing 1 µg of mRNA luc, with N/P ratio of 4:1 and 8:1 was added to each well. The cells were incubated with nanoparticles for 24 and 48 h separately. Substrate (One Glo Luciferase Assay System) was added directly to the cells in each well in a volume ratio of 1:1 (volume of cell liquid to substrate volume). Luminescence was measured after 10 min of incubation with substrate in a Black Corning plate with application of Microplate Spectrophotometer-Fluorometer Fluoroskan Ascent reader. The percentage of luminescence in the tested samples was normalized relative to the luminescence after transfection using bPEI-fLuc-mRNA, which was taken as 100%.
4. Conclusions
In this study, we have synthesized new polypeptides, namely, random copolymers of Lys and Ile. The best properties of polyplexes, such as size, ζ-potential and encapsulation efficacy were observed for polypeptides consisting of Lys and Ile in a ratio of 80/20 (mol%). Being amphiphilic polypeptides, P(Lys-co-Ile) could self-assemble into particles. Simple mixing of nucleic acids with such polymers in aqueous media results in spooling of nucleic acids over the polypeptide particles. In order to encapsulate nucleic acids into the inner part of polyplexes ultrasonication or phase inversion procedures could be applied. The latter one is more versatile, because it doesn’t require the ultrasound equipment, but only centrifuge, and results in spherical particles with smaller size.
The obtained P(Lys-co-Ile) polyplexes allowed effective protection of encapsulated mRNA towards RNAse. The toxicity of copolymers was significantly lower than that of just PLys, which is in good correlation with diminishing of charge density within obtained structures. The P(Lys-co-Ile) based polyplexes were found to effectively penetrate cells. These polymers also showed efficacy as vectors for transfection of cells with EGFP-mRNA. The efficacy was significantly higher than that observed for bPEI 25k. Altogether, this allows us to consider random amphiphilic copolymers as promising candidates for mRNA delivery applications, such as construction of antiviral and anticancer vaccines, and for therapeutic induction of missing proteins expression.