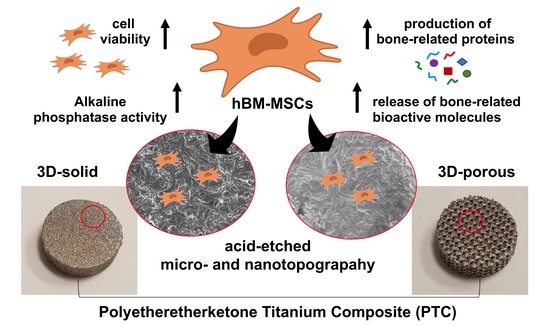
Early Osteogenic Marker Expression in hMSCs Cultured onto Acid Etching-Derived Micro- and Nanotopography 3D-Printed Titanium Surfaces
Abstract
:1. Introduction
2. Results and Discussion
2.1. Surface Characterization
2.1.1. Microscale Surface Morphology Characterization
2.1.2. Microscale Roughness Measurement
2.2. hBM-MSCs Response to the Different Surface Morphologies
2.2.1. Cell Viability and Morphology
2.2.2. Osteogenic Markers
2.3. Texture Analysis of SEM Images
3. Materials and Methods
3.1. Implant Preparation and Characterization
3.1.1. Implant Manufacturing
3.1.2. Investigation of Implant Morphology Surface
3.1.3. Investigation of Implant Roughness Surface
3.2. Biological Investigation
3.2.1. hBM-MSCs Culture and Seeding
3.2.2. Cell Viability
3.2.3. DNA Content Quantification
3.2.4. Secreted Protein Quantification
3.2.5. ECM Proteins Extraction and ELISA Assay
3.2.6. ALP Activity
3.2.7. Scanning Electron Microscopy of Cell-Cultured Implants
3.2.8. Immunofluorescence
3.3. Texture Analysis of SEM Images
3.4. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Maradit Kremers, H.; Larson, D.R.; Crowson, C.S.; Kremers, W.K.; Washington, R.E.; Steiner, C.A.; Jiranek, W.A.; Berry, D.J. Prevalence of Total Hip and Knee Replacement in the United States. J. Bone Jt. Surg. Am. 2015, 97, 1386–1397. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ong, K.L.; Yun, B.M.; White, J.B. New Biomaterials for Orthopedic Implants. ORR 2015, 7, 107–130. [Google Scholar] [CrossRef] [Green Version]
- Mobbs, R.J.; Chung, M.; Rao, P.J. Bone Graft Substitutes for Anterior Lumbar Interbody Fusion. Orthop. Surg. 2013, 5, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Kowalczewski, C.J.; Saul, J.M. Biomaterials for the Delivery of Growth Factors and Other Therapeutic Agents in Tissue Engineering Approaches to Bone Regeneration. Front. Pharmacol. 2018, 9, 513. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, L.; Yang, G.; Johnson, B.N.; Jia, X. Three-Dimensional (3D) Printed Scaffold and Material Selection for Bone Repair. Acta Biomater. 2019, 84, 16–33. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Wang, Q.; Liu, G. A Review of 3D Printed Bone Implants. Micromachines 2022, 13, 528. [Google Scholar] [CrossRef]
- Teo, A.; Mishra, A.; Park, I.; Kim, Y.-J.; Park, W.-T.; Yoon, Y. Polymeric Biomaterials for Medical Implants & Devices. ACS Biomater. Sci. Eng. 2016, 2, 454–472. [Google Scholar] [CrossRef]
- Wei, S.; Ma, J.-X.; Xu, L.; Gu, X.-S.; Ma, X.-L. Biodegradable Materials for Bone Defect Repair. Mil. Med. Res. 2020, 7, 54. [Google Scholar] [CrossRef]
- Ou, S.; Chen, C.; Hosseinkhani, H.; Yu, C.; Shen, Y.; Ou, K. Surface Properties of Nano–Structural Silicon–Doped Carbon Films for Biomedical Applications. Int. J. Nanotechnol. 2013, 10, 945–958. [Google Scholar] [CrossRef]
- Han, H.-C.; Lo, H.-C.; Wu, C.-Y.; Chen, K.-H.; Chen, L.-C.; Ou, K.-L.; Hosseinkhani, H. Nano-Textured Fluidic Biochip as Biological Filter for Selective Survival of Neuronal Cells. J. Biomed. Mater. Res. A 2015, 103, 2015–2023. [Google Scholar] [CrossRef]
- Muthiah, N.; Yolcu, Y.U.; Alan, N.; Agarwal, N.; Hamilton, D.K.; Ozpinar, A. Evolution of Polyetheretherketone (PEEK) and Titanium Interbody Devices for Spinal Procedures: A Comprehensive Review of the Literature. Eur. Spine J. 2022, 1–10. [Google Scholar] [CrossRef] [PubMed]
- McGilvray, K.C.; Waldorff, E.I.; Easley, J.; Seim, H.B.; Zhang, N.; Linovitz, R.J.; Ryaby, J.T.; Puttlitz, C.M. Evaluation of a Polyetheretherketone (PEEK) Titanium Composite Interbody Spacer in an Ovine Lumbar Interbody Fusion Model: Biomechanical, Microcomputed Tomographic, and Histologic Analyses. Spine J. 2017, 17, 1907–1916. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.; Xu, S.; Zhou, S.; Xu, W.; Leary, M.; Choong, P.; Qian, M.; Brandt, M.; Xie, Y.M. Topological Design and Additive Manufacturing of Porous Metals for Bone Scaffolds and Orthopaedic Implants: A Review. Biomaterials 2016, 83, 127–141. [Google Scholar] [CrossRef] [PubMed]
- Sidambe, A.T. Biocompatibility of Advanced Manufactured Titanium Implants—A Review. Materials 2014, 7, 8168–8188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vercellino, M.; Ceccarelli, G.; Cristofaro, F.; Balli, M.; Bertoglio, F.; Bruni, G.; Benedetti, L.; Avanzini, M.A.; Imbriani, M.; Visai, L. Nanostructured TiO2 Surfaces Promote Human Bone Marrow Mesenchymal Stem Cells Differentiation to Osteoblasts. Nanomaterials 2016, 6, 124. [Google Scholar] [CrossRef] [Green Version]
- Lincks, J.; Boyan, B.D.; Blanchard, C.R.; Lohmann, C.H.; Liu, Y.; Cochran, D.L.; Dean, D.D.; Schwartz, Z. Response of MG63 Osteoblast-like Cells to Titanium and Titanium Alloy Is Dependent on Surface Roughness and Composition. Biomaterials 1998, 19, 2219–2232. [Google Scholar] [CrossRef]
- Arpornmaeklong, P.; Pripatnanont, P.; Chookiatsiri, C.; Tangtrakulwanich, B. Effects of Titanium Surface Microtopography and Simvastatin on Growth and Osteogenic Differentiation of Human Mesenchymal Stem Cells in Estrogen-Deprived Cell Culture. Int. J. Oral Maxillofac. Implant. 2017, 32, e35–e46. [Google Scholar] [CrossRef] [Green Version]
- Quinn, J.; McFadden, R.; Chan, C.-W.; Carson, L. Titanium for Orthopedic Applications: An Overview of Surface Modification to Improve Biocompatibility and Prevent Bacterial Biofilm Formation. iScience 2020, 23, 101745. [Google Scholar] [CrossRef]
- Giner, L.; Mercadé, M.; Torrent, S.; Punset, M.; Pérez, R.A.; Delgado, L.M.; Gil, F.J. Double Acid Etching Treatment of Dental Implants for Enhanced Biological Properties. J. Appl. Biomater. Funct. Mater. 2018, 16, 83–89. [Google Scholar] [CrossRef] [Green Version]
- Schliephake, H.; Scharnweber, D. Chemical and Biological Functionalization of Titanium for Dental Implants. J. Mater. Chem. 2008, 18, 2404–2414. [Google Scholar] [CrossRef]
- Davies, J.E.; Mendes, V.C.; Ko, J.C.H.; Ajami, E. Topographic Scale-Range Synergy at the Functional Bone/Implant Interface. Biomaterials 2014, 35, 25–35. [Google Scholar] [CrossRef]
- Mishra, S.; Chowdhary, R. PEEK Materials as an Alternative to Titanium in Dental Implants: A Systematic Review. Clin. Implant. Dent. Relat. Res. 2019, 21, 208–222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jarman-Smith, M. Evolving Uses for Implantable PEEK and PEEK Based Compounds. Med. Device Technol. 2008, 19, 12–15. [Google Scholar] [PubMed]
- Verma, S.; Sharma, N.; Kango, S.; Sharma, S. Developments of PEEK (Polyetheretherketone) as a Biomedical Material: A Focused Review. Eur. Polym. J. 2021, 147, 110295. [Google Scholar] [CrossRef]
- Flejszar, M.; Chmielarz, P. Surface Modifications of Poly(Ether Ether Ketone) via Polymerization Methods-Current Status and Future Prospects. Materials 2020, 13, 999. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Teton, Z.E.; Cheaney, B.; Obayashi, J.T.; Than, K.D. PEEK Interbody Devices for Multilevel Anterior Cervical Discectomy and Fusion: Association with More than 6-Fold Higher Rates of Pseudarthrosis Compared to Structural Allograft. J. Neurosurg. Spine 2020, 32, 696–702. [Google Scholar] [CrossRef]
- Rutkovskiy, A.; Stensløkken, K.-O.; Vaage, I.J. Osteoblast Differentiation at a Glance. Med. Sci. Monit Basic Res. 2016, 22, 95–106. [Google Scholar] [CrossRef] [Green Version]
- Zhou, L.; Quan, R.; Yang, J.; Xu, H. Healing of Bone Defects by Induced Pluripotent Stem Cell-Derived Bone Marrow Mesenchymal Stem Cells Seeded on Hydroxyapatite-Zirconia. Ann. Transl. Med. 2021, 9, 1723. [Google Scholar] [CrossRef]
- Gonzalez-Vilchis, R.A.; Piedra-Ramirez, A.; Patiño-Morales, C.C.; Sanchez-Gomez, C.; Beltran-Vargas, N.E. Sources, Characteristics, and Therapeutic Applications of Mesenchymal Cells in Tissue Engineering. Tissue Eng. Regen. Med. 2022. [Google Scholar] [CrossRef]
- Li, J.; Liu, Y.; Zhang, Y.; Yao, B.; Enhejirigala; Li, Z.; Song, W.; Wang, Y.; Duan, X.; Yuan, X.; et al. Biophysical and Biochemical Cues of Biomaterials Guide Mesenchymal Stem Cell Behaviors. Front. Cell Dev. Biol. 2021, 9, 640388. [Google Scholar] [CrossRef]
- de Peppo, G.M.; Marolt, D. Modulating the Biochemical and Biophysical Culture Environment to Enhance Osteogenic Differentiation and Maturation of Human Pluripotent Stem Cell-Derived Mesenchymal Progenitors. Stem Cell Res. Ther. 2013, 4, 106. [Google Scholar] [CrossRef] [Green Version]
- Naqvi, S.; McNamara, L. Stem Cell Mechanobiology and the Role of Biomaterials in Governing Mechanotransduction and Matrix Production for Tissue Regeneration. Front. Bioeng. Biotechnol. 2020, 8, 597661. [Google Scholar] [CrossRef] [PubMed]
- McMurray, R.J.; Dalby, M.J.; Tsimbouri, P.M. Using Biomaterials to Study Stem Cell Mechanotransduction, Growth and Differentiation. J. Tissue Eng. Regen. Med. 2015, 9, 528–539. [Google Scholar] [CrossRef] [PubMed]
- Langenbach, F.; Handschel, J. Effects of Dexamethasone, Ascorbic Acid and β-Glycerophosphate on the Osteogenic Differentiation of Stem Cells in Vitro. Stem Cell Res. Ther. 2013, 4, 117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lutolf, M.P.; Gilbert, P.M.; Blau, H.M. Designing Materials to Direct Stem-Cell Fate. Nature 2009, 462, 433–441. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martin, J.Y.; Schwartz, Z.; Hummert, T.W.; Schraub, D.M.; Simpson, J.; Lankford, J.; Dean, D.D.; Cochran, D.L.; Boyan, B.D. Effect of Titanium Surface Roughness on Proliferation, Differentiation, and Protein Synthesis of Human Osteoblast-like Cells (MG63). J. Biomed. Mater. Res. 1995, 29, 389–401. [Google Scholar] [CrossRef]
- Webster, T.J.; Ejiofor, J.U. Increased Osteoblast Adhesion on Nanophase Metals: Ti, Ti6Al4V, and CoCrMo. Biomaterials 2004, 25, 4731–4739. [Google Scholar] [CrossRef]
- Marconi, G.D.; Fonticoli, L.; Della Rocca, Y.; Oliva, S.; Rajan, T.S.; Trubiani, O.; Murmura, G.; Diomede, F.; Pizzicannella, J. Enhanced Extracellular Matrix Deposition on Titanium Implant Surfaces: Cellular and Molecular Evidences. Biomedicines 2021, 9, 1710. [Google Scholar] [CrossRef]
- Khang, D.; Choi, J.; Im, Y.-M.; Kim, Y.-J.; Jang, J.-H.; Kang, S.S.; Nam, T.-H.; Song, J.; Park, J.-W. Role of Subnano-, Nano- and Submicron-Surface Features on Osteoblast Differentiation of Bone Marrow Mesenchymal Stem Cells. Biomaterials 2012, 33, 5997–6007. [Google Scholar] [CrossRef]
- Thibault, R.A.; Scott Baggett, L.; Mikos, A.G.; Kasper, F.K. Osteogenic Differentiation of Mesenchymal Stem Cells on Pregenerated Extracellular Matrix Scaffolds in the Absence of Osteogenic Cell Culture Supplements. Tissue Eng. Part A 2010, 16, 431–440. [Google Scholar] [CrossRef]
- Cooper, L.F.; Zhou, Y.; Takebe, J.; Guo, J.; Abron, A.; Holmén, A.; Ellingsen, J.E. Fluoride Modification Effects on Osteoblast Behavior and Bone Formation at TiO2 Grit-Blasted c.p. Titanium Endosseous Implants. Biomaterials 2006, 27, 926–936. [Google Scholar] [CrossRef] [PubMed]
- Scarfì, S. Use of Bone Morphogenetic Proteins in Mesenchymal Stem Cell Stimulation of Cartilage and Bone Repair. World J. Stem Cells 2016, 8, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Furumatsu, T.; Shen, Z.N.; Kawai, A.; Nishida, K.; Manabe, H.; Oohashi, T.; Inoue, H.; Ninomiya, Y. Vascular Endothelial Growth Factor Principally Acts as the Main Angiogenic Factor in the Early Stage of Human Osteoblastogenesis. J. Biochem. 2003, 133, 633–639. [Google Scholar] [CrossRef] [PubMed]
- Mayer, H.; Bertram, H.; Lindenmaier, W.; Korff, T.; Weber, H.; Weich, H. Vascular Endothelial Growth Factor (VEGF-A) Expression in Human Mesenchymal Stem Cells: Autocrine and Paracrine Role on Osteoblastic and Endothelial Differentiation. J. Cell. Biochem. 2005, 95, 827–839. [Google Scholar] [CrossRef] [PubMed]
- Lei, L.; Wang, S.; Wu, H.; Ju, W.; Peng, J.; Qahtan, A.S.A.; Chen, C.; Lu, Y.; Peng, J.; Zhang, X.; et al. Optimization of Release Pattern of FGF-2 and BMP-2 for Osteogenic Differentiation of Low-Population Density HMSCs. J. Biomed. Mater. Res. Part A 2015, 103, 252–261. [Google Scholar] [CrossRef]
- Lin, X.; Patil, S.; Gao, Y.-G.; Qian, A. The Bone Extracellular Matrix in Bone Formation and Regeneration. Front. Pharm. 2020, 11, 757. [Google Scholar] [CrossRef]
- Unal, M.; Creecy, A.; Nyman, J.S. The Role of Matrix Composition in the Mechanical Behavior of Bone. Curr. Osteoporos. Rep. 2018, 16, 205–215. [Google Scholar] [CrossRef]
- Aubin, J.E. Regulation of Osteoblast Formation and Function. Rev. Endocr. Metab. Disord. 2001, 2, 81–94. [Google Scholar] [CrossRef]
- Stacey, M.W.; Grubbs, J.; Asmar, A.; Pryor, J.; Elsayed-Ali, H.; Cao, W.; Beskok, A.; Dutta, D.; Darby, D.A.; Fecteau, A.; et al. Decorin Expression, Straw-like Structure, and Differentiation of Human Costal Cartilage. Connect. Tissue Res. 2012, 53, 415–421. [Google Scholar] [CrossRef] [Green Version]
- Tsao, Y.-T.; Huang, Y.-J.; Wu, H.-H.; Liu, Y.-A.; Liu, Y.-S.; Lee, O.K. Osteocalcin Mediates Biomineralization during Osteogenic Maturation in Human Mesenchymal Stromal Cells. Int. J. Mol. Sci. 2017, 18, 159. [Google Scholar] [CrossRef]
- Knepper-Nicolai, B.; Reinstorf, A.; Hofinger, I.; Flade, K.; Wenz, R.; Pompe, W. Influence of Osteocalcin and Collagen I on the Mechanical and Biological Properties of Biocement D. Biomol. Eng. 2002, 19, 227–231. [Google Scholar] [CrossRef]
- Martinez, M.A.F.; Balderrama, Í.d.F.; Karam, P.S.B.H.; de Oliveira, R.C.; de Oliveira, F.A.; Grandini, C.R.; Vicente, F.B.; Stavropoulos, A.; Zangrando, M.S.R.; Sant’Ana, A.C.P. Surface Roughness of Titanium Disks Influences the Adhesion, Proliferation and Differentiation of Osteogenic Properties Derived from Human. Int. J. Implant. Dent. 2020, 6, 46. [Google Scholar] [CrossRef] [PubMed]
- Alves, N.M.; Pashkuleva, I.; Reis, R.L.; Mano, J.F. Controlling Cell Behavior through the Design of Polymer Surfaces. Small 2010, 6, 2208–2220. [Google Scholar] [CrossRef] [Green Version]
- Majhy, B.; Priyadarshini, P.; Sen, A.K. Effect of Surface Energy and Roughness on Cell Adhesion and Growth—Facile Surface Modification for Enhanced Cell Culture. RSC Adv. 2021, 11, 15467–15476. [Google Scholar] [CrossRef] [PubMed]
- Decuzzi, P.; Ferrari, M. Modulating Cellular Adhesion through Nanotopography. Biomaterials 2010, 31, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Gentile, F.; Tirinato, L.; Battista, E.; Causa, F.; Liberale, C.; di Fabrizio, E.M.; Decuzzi, P. Cells Preferentially Grow on Rough Substrates. Biomaterials 2010, 31, 7205–7212. [Google Scholar] [CrossRef]
- Gentile, F.; La Rocca, R.; Marinaro, G.; Nicastri, A.; Toma, A.; Paonessa, F.; Cojoc, G.; Liberale, C.; Benfenati, F.; di Fabrizio, E.; et al. Differential Cell Adhesion on Mesoporous Silicon Substrates. ACS Appl. Mater. Interfaces 2012, 4, 2903–2911. [Google Scholar] [CrossRef]
- Bloise, N.; Fassina, L.; Focarete, M.L.; Lotti, N.; Visai, L. Haralick’s Texture Analysis to Predict Cellular Proliferation on Randomly Oriented Electrospun Nanomaterials. Nanosc. Adv. 2022, 4, 1330–1335. [Google Scholar] [CrossRef]
- Löfstedt, T.; Brynolfsson, P.; Asklund, T.; Nyholm, T.; Garpebring, A. Gray-Level Invariant Haralick Texture Features. PLoS ONE 2019, 14, e0212110. [Google Scholar] [CrossRef]
- Ulaby, F.T.; Kouyate, F.; Brisco, B.; Williams, T.H.L. Textural Infornation in SAR Images. IEEE Trans. Geosci. Remote Sens. 1986, GE-24, 235–245. [Google Scholar] [CrossRef]
- Raheja, J.; Kumar, S.; Chaudhary, A. Fabric Defect Detection Based on GLCM and Gabor Filter: A Comparison. Opt. Int. J. Light Electron. Opt. 2013, 124, 6469–6474. [Google Scholar] [CrossRef]
- Ou, X.; Pan, W.; Xiao, P. In Vivo Skin Capacitive Imaging Analysis by Using Grey Level Co-Occurrence Matrix (GLCM). Int. J. Pharm. 2014, 460, 28–32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mayerhoefer, M.E.; Schima, W.; Trattnig, S.; Pinker, K.; Berger-Kulemann, V.; Ba-Ssalamah, A. Texture-Based Classification of Focal Liver Lesions on MRI at 3.0 Tesla: A Feasibility Study in Cysts and Hemangiomas. J. Magn. Reson. Imaging 2010, 32, 352–359. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Giger, M.L.; Lan, L.; Bancroft Brown, J.; MacMahon, A.; Mussman, M.; Olopade, O.I.; Sennett, C. Computerized Analysis of Mammographic Parenchymal Patterns on a Large Clinical Dataset of Full-Field Digital Mammograms: Robustness Study with Two High-Risk Datasets. J. Digit. Imaging 2012, 25, 591–598. [Google Scholar] [CrossRef] [Green Version]
- Nie, K.; Chen, J.-H.; Yu, H.J.; Chu, Y.; Nalcioglu, O.; Su, M.-Y. Quantitative Analysis of Lesion Morphology and Texture Features for Diagnostic Prediction in Breast MRI. Acad. Radiol. 2008, 15, 1513–1525. [Google Scholar] [CrossRef] [Green Version]
- Brynolfsson, P.; Nilsson, D.; Henriksson, R.; Hauksson, J.; Karlsson, M.; Garpebring, A.; Birgander, R.; Trygg, J.; Nyholm, T.; Asklund, T. ADC Texture--an Imaging Biomarker for High-Grade Glioma? Med. Phys. 2014, 41, 101903. [Google Scholar] [CrossRef]
- Aerts, H.J.W.L.; Velazquez, E.R.; Leijenaar, R.T.H.; Parmar, C.; Grossmann, P.; Carvalho, S.; Cavalho, S.; Bussink, J.; Monshouwer, R.; Haibe-Kains, B.; et al. Decoding Tumour Phenotype by Noninvasive Imaging Using a Quantitative Radiomics Approach. Nat. Commun. 2014, 5, 4006. [Google Scholar] [CrossRef]
- Cho, H.; Park, H. Classification of Low-Grade and High-Grade Glioma Using Multi-Modal Image Radiomics Features. In Proceedings of the 2017 39th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Jeju, Korea, 11–15 July 2017. [Google Scholar] [CrossRef]
- Ceccarelli, G.; Bloise, N.; Mantelli, M.; Gastaldi, G.; Fassina, L.; De Angelis, M.G.C.; Ferrari, D.; Imbriani, M.; Visai, L. A Comparative Analysis of the in Vitro Effects of Pulsed Electromagnetic Field Treatment on Osteogenic Differentiation of Two Different Mesenchymal Cell Lineages. Biores. Open Access 2013, 2, 283–294. [Google Scholar] [CrossRef]
- Bloise, N.; Patrucco, A.; Bruni, G.; Montagna, G.; Caringella, R.; Fassina, L.; Tonin, C.; Visai, L. In Vitro Production of Calcified Bone Matrix onto Wool Keratin Scaffods via Osteogenic Factors and Electromagnetic Stimulus. Materials 2020, 13, 3052. [Google Scholar] [CrossRef]
- Saino, E.; Grandi, S.; Quartarone, E.; Maliardi, V.; Galli, D.; Bloise, N.; Fassina, L.; De Angelis, M.G.C.; Mustarelli, P.; Imbriani, M.; et al. In Vitro Calcified Matrix Deposition by Human Osteoblasts onto a Zinc-Containing Bioactive Glass. Eur. Cell Mater. 2011, 21, 59–72; discussion 72. [Google Scholar] [CrossRef]
- Haralick, R.M.; Shanmugam, K.; Dinstein, I. Textural Features for Image Classification. IEEE Trans. Syst. Man Cybern. 1973, SMC-3, 610–621. [Google Scholar] [CrossRef] [Green Version]








| Group | Material | Manufacturing | Post-Process | Porosity |
|---|---|---|---|---|
| MF | Ti-6Al-4V | machined | non-etched | solid |
| PF | Ti-6Al-4V | 3D-printed | non-etched | solid |
| PFA | Ti-6Al-4V | 3D-printed | etched | solid |
| P3 | Ti-6Al-4V | 3D-printed | non-etched | porous |
| P3A | Ti-6Al-4V | 3D-printed | etched | porous |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bloise, N.; Waldorff, E.I.; Montagna, G.; Bruni, G.; Fassina, L.; Fang, S.; Zhang, N.; Jiang, J.; Ryaby, J.T.; Visai, L. Early Osteogenic Marker Expression in hMSCs Cultured onto Acid Etching-Derived Micro- and Nanotopography 3D-Printed Titanium Surfaces. Int. J. Mol. Sci. 2022, 23, 7070. https://doi.org/10.3390/ijms23137070
Bloise N, Waldorff EI, Montagna G, Bruni G, Fassina L, Fang S, Zhang N, Jiang J, Ryaby JT, Visai L. Early Osteogenic Marker Expression in hMSCs Cultured onto Acid Etching-Derived Micro- and Nanotopography 3D-Printed Titanium Surfaces. International Journal of Molecular Sciences. 2022; 23(13):7070. https://doi.org/10.3390/ijms23137070
Chicago/Turabian StyleBloise, Nora, Erik I. Waldorff, Giulia Montagna, Giovanna Bruni, Lorenzo Fassina, Samuel Fang, Nianli Zhang, Jiechao Jiang, James T. Ryaby, and Livia Visai. 2022. "Early Osteogenic Marker Expression in hMSCs Cultured onto Acid Etching-Derived Micro- and Nanotopography 3D-Printed Titanium Surfaces" International Journal of Molecular Sciences 23, no. 13: 7070. https://doi.org/10.3390/ijms23137070
APA StyleBloise, N., Waldorff, E. I., Montagna, G., Bruni, G., Fassina, L., Fang, S., Zhang, N., Jiang, J., Ryaby, J. T., & Visai, L. (2022). Early Osteogenic Marker Expression in hMSCs Cultured onto Acid Etching-Derived Micro- and Nanotopography 3D-Printed Titanium Surfaces. International Journal of Molecular Sciences, 23(13), 7070. https://doi.org/10.3390/ijms23137070