Antenatal Glucocorticoid Administration Promotes Cardiac Structure and Energy Metabolism Maturation in Preterm Fetuses
Abstract
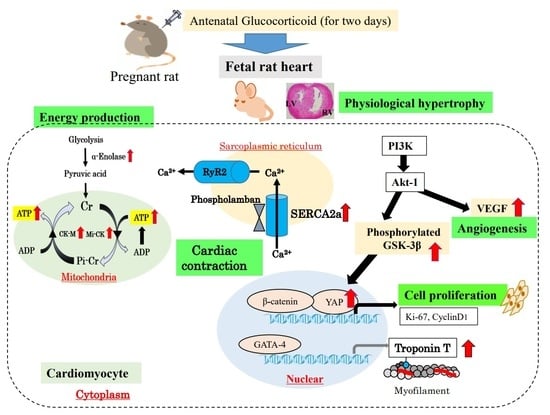
:1. Introduction
2. Cardiac Physiological Hypertrophy by Cardiomyocyte Proliferation with Antenatal GC Administration
3. Adenosine Triphosphate (ATP) Production in Glycolysis and Mitochondria Function
4. SR Calcium Transport ATPase 2a (SERCA2a) and Phospholamban in the Premature Fetal Rat Heart with Antenatal GC Administration
5. Cardiac Effects in Clinical Antenatal GC Therapy—From Bedside to Bench
6. Limitations
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Purisch, S.E.; Gyamfi-Bannerman, C. Epidemiology of preterm birth. Semin. Perinatol. 2017, 41, 387–391. [Google Scholar] [PubMed]
- Platt, M.J. Outcomes in preterm infants. Public Health 2014, 128, 399–403. [Google Scholar] [PubMed]
- Berger, H.; Melamed, N.; Davis, B.M.; Hasan, H.; Mawjee, K.; Barrett, J.; McDonald, S.D.; Geary, M.; Ray, J.G. Impact of diabetes, obesity and hypertension on preterm birth: Population-based study. PLoS ONE 2020, 25, e0228743. [Google Scholar]
- Simeoni, U.; Armengaud, J.B.; Siddeek, B.; Tolsa, J.F. Perinatal origins of adult disease. Neonatology 2018, 113, 393–399. [Google Scholar] [PubMed]
- Vrselja, A.; Pillow, J.J.; Black, M.J. Effect of preterm birth on cardiac and cardiomyocyte growth and the consequences of antenatal and postnatal glucocorticoid treatment. J. Clin. Med. 2021, 10, 3896. [Google Scholar] [PubMed]
- Schuermans, A.; Lewandowski, A.J. Understanding the preterm human heart: What do we know so far? Anat. Rec. 2022, 309, 2099–2112. [Google Scholar] [CrossRef]
- Lahti, J.; Lahti, M.; Pesonen, A.K.; Heinonen, K.; Kajantie, E.; Forsén, T.; Wahlbeck, K.; Osmond, C.; Barker, D.J.; Eriksson, J.G.; et al. Prenatal and childhood growth, and hospitalization for alcohol use disorders in adulthood: The Helsinki birth cohort study. PLoS ONE 2014, 29, e87404. [Google Scholar]
- McMullen, S.; Mostyn, A. Animal models for the study of the developmental origins of health and disease. Proc. Nutr. Soc. 2009, 68, 306–320. [Google Scholar]
- Williams, L.; Seki, Y.; Vuguin, P.M.; Charron, M.J. Animal models of in utero exposure to a high fat diet: A review. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2014, 1842, 507–519. [Google Scholar]
- Lurbe, E.; Ingelfinger, J. Developmental and early life origins of cardiometabolic risk factors: Novel findings and implications. Hypertens. 2021, 77, 308–318. [Google Scholar]
- Velazquez, M.A.; Fleming, T.P.; Watkins, A.J. Periconceptional environment and the developmental origins of disease. J. Endocrinol. 2019, 242, T33–T49. [Google Scholar] [PubMed]
- Baird, J.; Jacob, C.; Barker, M.; Fall, C.H.; Hanson, M.; Harvey, N.C.; Inskip, H.M.; Kumaran, K.; Cooper, C. Developmental Origins of Health and Disease: A Lifecourse approach to the prevention of non-communicable diseases. Healthcare 2017, 8, 14. [Google Scholar]
- Mandy, M.; Nyirenda, M. Developmental origins of health and disease: The relevance to developing nations. Int. Health 2018, 10, 66–70. [Google Scholar]
- Guo, Y.; Pu, W.T. Cardiomyocyte maturation: New phase in development. Circ. Res. 2020, 126, 1086–1106. [Google Scholar]
- Karbassi, E.; Fenix, A.; Marchiano, S.; Muraoka, N.; Nakamura, K.; Yang, X.; Murry, C.E. Cardiomyocyte maturation: Advances in knowledge and implications for regenerative medicine. Nat. Rev. Cardiol. 2020, 17, 341–359. [Google Scholar]
- Langley-Evans, S.C. Nutritional programming of disease: Unravelling the mechanism. J. Anat. 2009, 215, 36–51. [Google Scholar]
- Parikh, S.S.; Blackwell, D.J.; Gomez-Hurtado, N.; Frisk, M.; Wang, L.; Kim, K.; Dahl, C.P.; Fiane, A.; Tønnessen, T.; Kryshtal, D.O.; et al. Thyroid and glucocorticoid hormones promote functional T-tubule development in human-induced pluripotent stem cell-derived cardiomyocytes. Circ. Res. 2017, 121, 323–1330. [Google Scholar]
- Fowden, A.L.; Mundy, L.; Silver, M. Developmental regulation of glucogenesis in the sheep fetus during late gestation. J. Physiol. 1998, 508, 937–947. [Google Scholar] [PubMed]
- Rog-Zielinska, E.A.; Richardson, R.V.; Denvir, M.A.; Chapman, K.E. Glucocorticoids and foetal heart maturation; implications for prematurity and foetal programming. J. Mol. Endocrinol. 2014, 52, R125–R135. [Google Scholar]
- American College of Obstetricians and Gynecologists’ Committee on Practice Bulletins—Obstetrics Collaborators. Practice Bulletin No. 171: Management of preterm labor. Obstet. Gynecol. 2016, 128, e155–e164. [Google Scholar]
- Roberts, D.; Brown, J.; Medley, N.; Dalziel, S.R. Antenatal corticosteroids for accelerating fetal lung maturation for women at risk of preterm birth. Cochrane Database Syst. Rev. 2017, 21, CD004454. [Google Scholar]
- Liggins, G.C.; Howie, R.N. A controlled trial of antepartum glucocorticoid treatment for prevention of the respiratory distress syndrome in premature infants. Pediatrics 1972, 50, 515–525. [Google Scholar] [PubMed]
- Stein, H.M.; Oyama, K.; Martinez, A.; Chappell, B.A.; Buh, E.; Blount, L.; Padbury, J.F. Effects of corticosteroids in preterm sheep on adaptation and sympathoadrenal mechanisms at birth. Am. J. Physiol. Endocrinol. Metab. 1993, 264, E763–E769. [Google Scholar]
- Kim, M.Y.; Eiby, Y.A.; Lumbers, E.R.; Wright, L.L.; Gibson, K.J.; Barnett, A.C.; Lingwood, B.E. Effects of glucocorticoid exposure on growth and structural maturation of the heart of the preterm piglet. PLoS ONE 2014, 27, e93407. [Google Scholar]
- Millage, A.R.; Latuga, M.S.; Aschner, J.L. Effect of perinatal glucocorticoids on vascular health and disease. Pediatr. Res. 2017, 81, 4–10. [Google Scholar]
- Agnew, E.J.; Ivy, J.R.; Stock, S.J.; Chapman, K.E. Glucocorticoids, antenatal corticosteroid therapy and fetal heart maturation. J. Mol. Endocrinol. 2018, 61, R61–R73. [Google Scholar]
- Juanita, K.J.; Andrew, J.W.; Abigail, L.F.; Dino, A.G. Glucocorticoid maturation of fetal cardiovascular function. Trends. Mol. Med. 2020, 26, 170–184. [Google Scholar]
- Hanson, M.A.; Gluckman, P.D. Early developmental conditioning of later health and disease: Physiology or pathophysiology? Physiol. Rev. 2014, 94, 1027–1076. [Google Scholar]
- Ivy, J.R.; Carter, R.N.; Zhao, J.F.; Buckley, C.; Urquijo, H.; Rog-Zielinska, E.A.; Panting, E.; Hrabalkova, L.; Nicholson, C.; Agnew, E.J.; et al. Glucocorticoids regulate mitochondrial fatty acid oxidation in fetal cardiomyocytes. J. Physiol. 2021, 599, 4901–4924. [Google Scholar]
- Tsiarli, M.A.; Rudine, A.; Kendall, N.; Pratt, M.O.; Krall, R.; Thiels, E.; DeFranco, D.B.; Monaghan, A.P. Antenatal dexamethasone exposure differentially affects distinct cortical neural progenitor cells and triggers long-term changes in murine cerebral architecture and behavior. Transl. Psychiatry 2017, 7, e1153. [Google Scholar]
- Noorlander, C.W.; De Graan, P.N.; Middeldorp, J.; Van Beers, J.J.; Visser, G.H. Ontogeny of hippocampal corticosteroid receptors: Effects of antenatal glucocorticoids in human and mouse. J. Comp. Neurol. 2006, 499, 924–932. [Google Scholar] [CrossRef] [PubMed]
- Augusto, F.S.; Matthew, W.K.; Judith, R.B.; Paranthaman, S.K.; Haruo, U.; Masatoshi, S.; Lucy, F.; Watanabe, S.; Sarah, S.; Boris, W.K.; et al. Low-dose betamethasone-acetate for fetal lung maturation in preterm sheep. Am. J. Obstet. Gynecol. 2018, 218, 132. [Google Scholar]
- Samtani, M.N.; Pyszczynski, N.A.; Dubois, D.C.; Almon, R.R.; Jusko, W.J. Modeling glucocorticoidmediated fetal lung maturation: I. Temporal patterns of corticosteroids in rat pregnancy. J. Pharmacol. Exp. Ther. 2006, 317, 117–126. [Google Scholar] [CrossRef] [Green Version]
- Samtani, M.N.; Pyszczynski, N.A.; Dubois, D.C.; Almon, R.R.; Jusko, W.J. Modeling glucocorticoidmediated fetal lung maturation: II. Temporal patterns of gene expression in fetal rat lung. J. Pharmacol. Exp. Ther. 2006, 317, 127–138. [Google Scholar] [CrossRef] [PubMed]
- Takagi, Y.; Takeba, Y.; Sakurai, K.; Mizuno, M.; Tsuzuki, Y.; Osada, Y.; Asoh, K.; Akashi, Y.; Matsumoto, N. Implication of cyclic AMP/cAMP-responsive element binding protein pathway contributes both surfactant protein B production and cell proliferation in the preterm infant lung with antenatal glucocorticoid administration. J. St. Marianna Univ. 2014, 5, 95–105. [Google Scholar]
- Porrello, E.R.; Mahmoud, A.I.; Simpson, E.; Hill, J.A.; Richardson, J.A.; Olson, E.N.; Sadek, H.A. Transient regenerative potential of the neonatal mouse heart. Science 2011, 331, 1078–1080. [Google Scholar] [CrossRef]
- Tsuzuki, Y.; Takeba, Y.; Kumai, T.; Matsumoto, N.; Aso, K.; Murano, K.; Mizuno, M.; Kobayashi, S. Identification and expression of cardiac contractry protein troponin T by antenatal glucocorticoid therapy in fetal and infant rats. Pediatr. Cardiol. Card. Surg. 2008, 4, 35–43. [Google Scholar]
- Sakurai, K.; Osada, Y.; Takeba, Y.; Mizuno, M.; Tsuzuki, Y.; Ohta, Y.; Ootaki, M.; Iiri, T.; Aso, K.; Yamamoto, H.; et al. Exposure of immature rat heart to antenatal glucocorticoid results in cardiac proliferation. Pediatr. Int. 2019, 61, 31–42. [Google Scholar] [CrossRef]
- Nakamura, Y.; Takeba, Y.; Kobayashi, T.; Ootaki, M.; Ohta, Y.; Kida, K.; Sakurai, K.; Gen, K.; Watanabe, M.; Iiri, T.; et al. Yap contributes to cardiomyocyte proliferation in the fetal rat heart epicardium with antenatal glucocorticoid administration. J. St. Marian. Univ. 2020, 11, 109–122. [Google Scholar] [CrossRef]
- DeBosch, B.; Treskov, I.; Lupu, T.S.; Weinheimer, C.; Kovacs, A.; Courtois, M.; Muslin, A.J. Akt1 is required for physiological cardiac growth. Circulation 2006, 113, 2097–2104. [Google Scholar] [CrossRef]
- Failor, K.; Desyatnikov, Y.; Finger, L.A.; Firestone, G.L. Glucocorticoid-induced degradation of glycogen synthase kinase-3 protein is triggered by serum- and glucocorticoid- induced protein kinase and Akt signaling and controls β-catenin dynamics and tight junction formation in mammary epithelial tumor cells. Mol. Endocrinol. 2007, 21, 2403–2415. [Google Scholar] [PubMed]
- Pal, R.; Khanna, A. Role of smad-and wnt-dependent pathways in embryonic cardiac development. Stem Cells Dev. 2006, 15, 29–39. [Google Scholar] [CrossRef] [PubMed]
- Buikema, J.W.; Zwetsloot, P.P.; Doevendans, P.A.; Sluijter, J.P.; Domian, I.J. Expanding mouse ventricular cardiomyocytes through GSK-3 inhibition. Curr. Protoc. Cell Biol. 2013, 61, 23.9.1–23.9.10. [Google Scholar] [PubMed]
- Walsh, K.; Shiojima, I. Cardiac growth and angiogenesis coordinated by intertissue interactions. J. Clin. Investig. 2007, 117, 3176–3179. [Google Scholar] [CrossRef]
- van Berlo, J.H.; Elrod, J.W.; Aronow, B.J.; Pu, W.T.; Molkentin, J.D. Serine 105 phosphorylation of transcription factor GATA4 is necessary for stress-induced cardiac hypertrophy in vivo. Proc. Natl. Acad. Sci. USA 2011, 108, 12331–12336. [Google Scholar]
- Stechschulte, L.A.; Wuesche, L.; Marino, J.S.; Hill, J.W.; Eng, C.; Hinds, T.D., Jr. Glucocorticoid receptor β stimulates Akt1 growth pathway by attenuation of PTEN. J. Biol. Chem. 2014, 289, 17885–17894. [Google Scholar]
- Todd, H.; Min, Z.; Jun, W.; Margarita, B.C.; Ela, K.; Randy, L.J.; James, F.M. Hippo pathway inhibits Wnt signaling to restrain cardiomyocyte proliferation and heart size. Science 2011, 332, 458–461. [Google Scholar]
- Reini, S.A.; Wood, C.E.; Keller-Wood, M. The ontogeny of genes related to ovine fetal cardiac growth. Gene Expr. Patterns 2009, 9, 122–128. [Google Scholar]
- Foglia, M.J.; Poss, K.D. Building and re-building he heart by cardiomyocyte proliferation. Development 2016, 143, 729–740. [Google Scholar]
- Singh, A.; Ramesh, S.; Cibi, D.M.; Yun, L.S.; Li, J.; Li, L.; Manderfield, L.J.; Olson, E.N.; Epstein, J.A.; Singh, M.K. Hippo signaling mediators Yap and Taz are required in the epicardium for coronary vasculature development. Cell Rep. 2016, 15, 1384–1393. [Google Scholar]
- Zhou, Q.; Li, L.; Zhao, B.; Guan, K.L. The hippo pathway in heart development, regeneration, and diseases. Circ. Res. 2015, 116, 1431–1447. [Google Scholar] [CrossRef] [PubMed]
- Xin, M.; Kim, Y.; Sutherland, L.B.; Qi, X.; McAnally, J.; Schwartz, R.J.; Richardson, J.A.; Bassel-Duby, R.; Olson, E.N. Regulation of insulin-like growth factor signaling by yap governs cardiomyocyte proliferation and embryonic heart size. Sci. Signal. 2011, 4, ra70. [Google Scholar] [CrossRef]
- Maillet, M.; van Berlo, J.H.; Molkentin, J.D. Molecular basis of physiological heart growth: Fundamental concepts and new players. Nat. Rev. Mol. Cell Biol. 2012, 14, 38–48. [Google Scholar] [CrossRef] [PubMed]
- Reini, S.A.; Dutta, G.; Wood, C.E.; Keller-Wood, M. Cardiac corticosteroid receptors mediate the enlargement of the ovine fetal heart induced by chronic increases in maternal cortisol. J. Endocrinol. 2008, 198, 419–427. [Google Scholar] [CrossRef]
- Lopaschuk, G.D.; Collins-Nakai, R.L.; Itoi, T. Developmental changes in energy substrate use by the heart. Cardiovasc. Res. 1992, 26, 1172–1180. [Google Scholar] [CrossRef]
- Sano, H.I.; Toki, T.; Naito, Y.; Tomita, M. Developmental changes in the balance of glycolytic ATP production and oxidative phosphorylation in ventricular cells: A simulation study. J. Theor. Biol. 2017, 419, 269–277. [Google Scholar] [CrossRef]
- Deloulme, J.C.; Helies, A.; Ledig, M.; Lucas, M.; Sensenbrenner, M. A comparative study of the distribution of alpha- and gamma-enolase subunits in cultured rat neural cells and fibroblasts. Int. J. Dev. Neurosci. 1997, 15, 183–194. [Google Scholar] [CrossRef]
- Kashiwaya, Y.; Sato, K.; Tsuchiya, N.; Thomas, S.; Fell, D.A.; Veech, R.L.; Passonneau, J.V. Control of glucose utilization in working perfused rat heart. J. Biol. Chem. 1994, 269, 25502–25514. [Google Scholar] [CrossRef]
- Tsuzuki, Y.; Takeba, Y.; Kumai, T.; Matsumoto, N.; Mizuno, M.; Murano, K.; Asoh, K.; Takagi, M.; Yamamoto, H.; Kobayashi, S. Antenatal glucocorticoid therapy increase cardiac α-enolase levels in fetus and neonate rats. Life Sci. 2009, 85, 609–616. [Google Scholar] [CrossRef]
- Dowell, R.T. Phosphorylcreatine shuttle enzymes during perinatal heart development. Biochem. Med. Metab. Biol. 1987, 37, 374–384. [Google Scholar] [CrossRef]
- Wyss, M.; Kaddurah-Daouk, R. Creatine and creatinine metabolism. Physiol. Rev. 2000, 80, 1107–1213. [Google Scholar] [CrossRef] [PubMed]
- Ventura-Clapier, R.; Garnier, A.; Veksler, V. Energy metabolism in heart failure. J. Physiol. 2004, 555, 1–13. [Google Scholar] [CrossRef]
- Saupe, K.W.; Spindler, M.; Tian, R.; Ingwall, J.S. Impaired cardiac energetics in mice lacking muscle-specific isoenzymes of creatine kinase. Circ. Res. 1998, 82, 898–907. [Google Scholar] [CrossRef] [PubMed]
- Montano, M.M.; Lim, R.W. Glucocorticoid effects on the skeletal muscle differentiation program: Analysis of clonal proliferation, morphological differentiation and the expression of muscle-specific and regulatory genes. Endocr. Res. 1997, 23, 37–57. [Google Scholar] [CrossRef] [PubMed]
- Yoshiko, Y.; Hirao, K.; Maeda, N. Dexamethasone regulates the actions of endogenous insulin-like growth factor-II during myogenic differentiation. Life Sci. 1998, 63, 77–85. [Google Scholar] [CrossRef]
- Mizuno, M.; Takeba, Y.; Matsumoto, N.; Tsuzuki, Y.; Asoh, K.; Takagi, M.; Kobayashi, S.; Yamamoto, H. Antenatal glucocorticoid therapy accelerates ATP production with creatine kinase increase in the growth-enhanced fetal rat heart. Circ. J. 2010, 74, 171–180. [Google Scholar] [CrossRef]
- Jones, L.R.; Cala, S.E. Biochemical evidence for functional heterogeneity of cardiac sarcoplasmic reticulum vesicles. J. Biol. Chem. 1981, 25, 11809–11818. [Google Scholar] [CrossRef]
- Kawamura, Y.; Ishiwata, T.; Takizawa, M.; Ishida, H.; Asano, Y.; Nonoyama, S. Fetal and neonatal development of Ca2+ transients and functional sarcoplasmic reticulum in beating mouse hearts. Circ. J. 2010, 74, 1442–1450. [Google Scholar] [CrossRef]
- Nakanishi, T.; Okuda, H.; Kamata, K.; Abe, K.; Sekiguchi, M.; Takao, A. Development of myocardial contractile system in the fetal rabbit. Pediatr. Res. 1987, 22, 201–207. [Google Scholar] [CrossRef]
- Barry, W.H.; Bridge, J.H. Intracellular calcium homeostasis in cardiac myocytes. Circulation 1993, 87, 1806–1815. [Google Scholar] [CrossRef]
- Monte, D.F.; Hajjar, J.R. Targeting calcium cycling proteins in heart failure through gene transfer. J. Physiol. 2003, 541, 49–61. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Okuda, S.; Yano, M. Excitation-contraction coupling and intracellular calcium cycling in failing hearts. Clin. Calcium. 2013, 23, 471–480. [Google Scholar] [PubMed]
- Sakurai, K.; Takeba, Y.; Mizuno, M.; Asoh, K.; Tsuzuki, Y.; Matsumoto, N.; Yamamoto, H. Antenatal glucocorticoid administration enhances sarcoplasmic reticulum calcium transport ATPase 2a and phospholamban expression in the immature fetal rat heart. J. St. Marian. Univ. 2013, 4, 69–80. [Google Scholar]
- Crowley, P. WITHDRAWN: Prophylactic corticosteroids for preterm birth. Cochrane Database Syst. Rev. 2000, 18, CD000065. [Google Scholar]
- Evans, D.L. Cardiovascular adaptations to exercise and training. Vet. Clin. N. Am. Equine Pract. 1985, 11, 513–531. [Google Scholar] [CrossRef]
- Padbury, J.F.; Polk, D.H.; Ervin, M.G.; Berry, L.M.; Ikegami, M.; Jobe, A.H. Postnatal Cardiovascular and Metabolic Responses to a Single Intramuscular Dose of Betamethasone in Fetal Sheep Born Prematurely by Cesarean Section. Pediatr. Res. 1995, 38, 709–715. [Google Scholar] [CrossRef]
- Martin, J.L.; Stefan, E.; Thomas, E. What is the role of β-adrenergic signaling in heart failure? Circ. Res. 2003, 14, 896–906. [Google Scholar]
- Lee, J.A.; Sohn, J.A.; Oh, S.; Choi, B.M. Perinatal risk factors of symptomatic preterm patent ductus arteriosus and secondary ligation. Pediatr. Neonatol. 2020, 61, 439–446. [Google Scholar] [CrossRef]
- Kim, H.N.; Cho, G.J.; Ahn, K.Y.; Lee, U.S.; Kim, H.K.; Cho, H.J.; Kim, G.H.; Kim, W.; Jeong, H.M.; Park, C.J.; et al. A case of Torsade de Pointes associated with hypopituitarism due to hemorrhagic fever with renal syndrome. J. Korean Med. Sci. 2001, 16, 355–358. [Google Scholar] [CrossRef]
- Kanamori, K.; Yamashita, R.; Tsutsui, K.; Hara, M.; Murakawa, Y. Long QT syndrome associated with adrenal insufficiency in a patient with isolated adrenocorticotropic hormone deficiency. Intern. Med. 2014, 53, 2329–2331. [Google Scholar] [CrossRef]
- Kang, G.D.; Kim, E.S.; Park, S.M.; Kim, J.E.; Lee, H.J.; Park, G.D.; Han, R.K.; Oh, J.D. Acquired long QT syndrome manifesting with Torsades de Pointes in a patient with panhypopituitarism due to radiotherapy. Korean Circ. J. 2013, 43, 340–342. [Google Scholar] [CrossRef] [PubMed]
- Yasser, Y.E.; Ann, E.B. Antenatal corticosteroid therapy for fetal maturation. Obstet. Gynecol. 2017, 130, e102–e109. [Google Scholar]
- Hsu, C.N.; Tain, Y.L. Animal models for DOHaD research: Focus on hypertension of developmental origins. Biomedicines 2021, 9, 623. [Google Scholar] [PubMed]
- Wolf, G. Adult type 2 diabetes induced by intrauterine growth retardation. Nutr. Rev. 2003, 61, 176–179. [Google Scholar]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sakurai, K.; Takeba, Y.; Osada, Y.; Mizuno, M.; Tsuzuki, Y.; Aso, K.; Kida, K.; Ohta, Y.; Ootaki, M.; Iiri, T.; et al. Antenatal Glucocorticoid Administration Promotes Cardiac Structure and Energy Metabolism Maturation in Preterm Fetuses. Int. J. Mol. Sci. 2022, 23, 10186. https://doi.org/10.3390/ijms231710186
Sakurai K, Takeba Y, Osada Y, Mizuno M, Tsuzuki Y, Aso K, Kida K, Ohta Y, Ootaki M, Iiri T, et al. Antenatal Glucocorticoid Administration Promotes Cardiac Structure and Energy Metabolism Maturation in Preterm Fetuses. International Journal of Molecular Sciences. 2022; 23(17):10186. https://doi.org/10.3390/ijms231710186
Chicago/Turabian StyleSakurai, Kenzo, Yuko Takeba, Yosuke Osada, Masanori Mizuno, Yoshimitsu Tsuzuki, Kentaro Aso, Keisuke Kida, Yuki Ohta, Masanori Ootaki, Taroh Iiri, and et al. 2022. "Antenatal Glucocorticoid Administration Promotes Cardiac Structure and Energy Metabolism Maturation in Preterm Fetuses" International Journal of Molecular Sciences 23, no. 17: 10186. https://doi.org/10.3390/ijms231710186
APA StyleSakurai, K., Takeba, Y., Osada, Y., Mizuno, M., Tsuzuki, Y., Aso, K., Kida, K., Ohta, Y., Ootaki, M., Iiri, T., Hokuto, I., Shimizu, N., & Matsumoto, N. (2022). Antenatal Glucocorticoid Administration Promotes Cardiac Structure and Energy Metabolism Maturation in Preterm Fetuses. International Journal of Molecular Sciences, 23(17), 10186. https://doi.org/10.3390/ijms231710186