Formulation of Magneto-Responsive Hydrogels from Dually Cross-Linked Polysaccharides: Synthesis, Tuning and Evaluation of Rheological Properties
Abstract
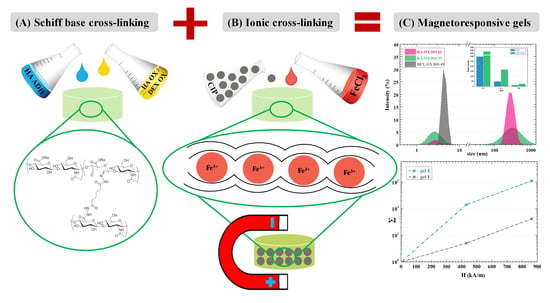
:1. Introduction
- The use of dynamic cross-linking is more favourable compared to non-reversible covalent cross-linking, as it provides pliable materials that can exhibit yield stress [53,63]. Such behaviour is advantageous in terms of materials processing, namely injectability and extrudability, but also promises a possibility to achieve significant MRE [26,64].
- Purely HA-based hydrogel matrices are rather scarce in the literature even though they may find use in tissue engineering, scaffold fabrication, and on-demand cell stimulation through an external impulse [22].
2. Results and Discussion
2.1. Polysaccharide Modification
2.2. Schiff Base Formation-Induced Gelation
2.3. Dual Cross-Linking of Hydrogels
2.4. Rheology
2.4.1. Schiff-Base Cross-Linked Hydrogels
2.4.2. Dually Cross-Linked Hydrogels
2.5. Cytotoxicity
2.6. Swelling
2.7. Mr Hydrogel Preparation
CIPs Characterization
2.8. CIP-Filled Hydrogel Magnetic Properties
2.9. Magnetorheology
2.9.1. Schiff Base Cross-Linked MR Hydrogels
2.9.2. Dually Cross-Linked Hydrogels
2.10. Porosity and Inner Morphology
3. Materials and Methods
3.1. Chemicals
3.2. Modification of HA
3.2.1. EDC-Mediated Reaction
3.2.2. DMTMM-Mediated Reaction
3.3. Oxidation of HA and Dextran
3.4. Schiff Base Linkage Formation
3.5. Dual Cross-Linking of Hydrogels
3.6. Determining DO
3.7. Nuclear Magnetic Resonance
3.8. Molecular Weight Determination
3.9. Dynamic Light-Scattering Measurement
3.10. Particle Size Analysis
3.11. Magnetometry
3.12. Rheology and Magnetorheology
3.13. Morphological Analysis
3.14. Swelling
3.15. Cytotoxicity Testing
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ADH | adipic acid dihydrazide |
| CIP | carbonyl iron particle |
| DEX-OX | oxidized dextran |
| DMSO | dimethyl sulfoxide |
| DMTMM | 4-(4,6-Dimethoxy-1,3,5-triazin-2-yl)-4-methylmorpholinium chloride |
| DO | degree of oxidation |
| DS | degree of substitution |
| ECM | extracellular matrix |
| EDC | 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide hydrochloride |
| GAG | glycosaminoglycan |
| HA | hyaluronan |
| HA-ADH | adipic acid dihydrazide-modified hyaluronan |
| HA-OX | oxidized hyaluronan |
| HoBt | 1-hydroxybenzotriazole hydrate |
| MR | magnetorheological |
| MRE | magnetorheological effect |
| MTT | 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium Bromide |
| PBS | phosphate buffered saline |
| RI | refractive index |
| SPION | superparamagnetic iron-oxide particle |
| UPW | ultrapure water |
References
- Vasile, C.; Pamfil, D.; Stoleru, E.; Baican, M. New Developments in Medical Applications of Hybrid Hydrogels Containing Natural Polymers. Molecules 2020, 25, 1539. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Wang, Z.; Han, F.; Jin, G.; Xu, L.; Xu, H.; Su, H.; Wang, H.; Le, Y.; Fu, Y.; et al. Mechano-regulation of vascular network formation without branches in 3D bioprinted cell-laden hydrogel constructs. Biotechnol. Bioeng. 2021, 118, 3787–3798. [Google Scholar] [CrossRef] [PubMed]
- Cui, P.; Qin, P.P.L.; Wang, X.; Chen, X.; Deng, Y.; Zhang, X. Nanoengineered hydrogels as 3D biomimetic extracellular matrix with injectable and sustained delivery capability for cartilage regeneration. Bioact. Mater. 2022, 19, 487–498. [Google Scholar] [CrossRef] [PubMed]
- Dangi, D.; Mattoo, M.; Kumar, V.; Sharma, P. Synthesis and characterization of galactomannan polymer hydrogel and sustained drug delivery. Carbohydr. Polym. Technol. Appl. 2022, 4, 100230. [Google Scholar] [CrossRef]
- Razali, N.A.M.; Lin, W.C. Accelerating the excisional wound closure by using the patterned microstructural nanofibrous mats/gentamicin-loaded hydrogel composite scaffold. Mater. Today Bio 2022, 16, 100347. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Wang, L.; Shen, Z.; Ren, J.; Chen, G.; Li, Q.; Zhou, Z. Super-Stretchable and Self-Healing hydrogel with a Three-Dimensional silver nanowires network structure for wearable sensor and electromagnetic interference shielding. Chem. Eng. J. 2022, 446, 137136. [Google Scholar] [CrossRef]
- Yin, X.; Wu, J.; Zhao, H.; Zhou, L.; He, T.; Fan, Y.; Chen, L.; Wang, K.; He, Y. A microgel-structured cellulose nanofibril coating with robust antifouling performance for highly efficient oil/water and immiscible organic solvent separation. Colloids Surfaces A Physicochem. Eng. Asp. 2022, 647, 128875. [Google Scholar] [CrossRef]
- Ren, J.; Li, R.; Wang, X.; Li, M.; Yang, W. A superabsorbent hydrogel for removal of dyes from aqueous solution. J. Polym. Environ. 2022, 30, 3327–3339. [Google Scholar] [CrossRef]
- Lu, H.; Li, X.; Yang, H.; Wu, J.; Zhang, Y.; Huang, H. Preparation and properties of riboflavin-loaded sanxan microcapsules. Food Hydrocoll. 2022, 129, 107641. [Google Scholar] [CrossRef]
- Lopes, P.M.P.; Moldovan, D.; Moldovan, M.; Carpa, R.; Saroşi, C.; Păşcuţă, P.; Moldovan, A.M.; Fechete, R.; Popescu, V. New Composite Hydrogel Based on Whey and Gelatin Crosslinked with Copper Sulphate. Materials 2022, 15, 2611. [Google Scholar] [CrossRef]
- Cvek, M.; Zahoranova, A.; Mrlik, M.; Sramkova, P.; Minarik, A.; Sedlacik, M. Poly(2-oxazoline)-based magnetic hydrogels: Synthesis, performance and cytotoxicity. Colloids Surfaces B Biointerfaces 2020, 190, 110912. [Google Scholar] [CrossRef]
- Zhang, S.D.; Zhai, Y.C.; Zhang, Z.F. Study on Polyvinly-Alcohol(PVA)/ Iron Oxide Black(Fe3O4) and Polyvinly-Alcohol(PVA)/ Iron Oxide Red(Fe2O3) Magnetic Sensitive Hydrogel. Adv. Mater. Res. 2011, 287–290, 2032–2035. [Google Scholar] [CrossRef]
- Bardajee, G.R.; Hooshyar, Z. One-pot synthesis of biocompatible superparamagnetic iron oxide nanoparticles/hydrogel based on salep: Characterization and drug delivery. Carbohydr. Polym. 2014, 101, 741–751. [Google Scholar] [CrossRef]
- Alveroğlu, E.; Sözeri, H.; Kurtan, U.; Şenel, M.; Baykal, A. Magnetic and spectroscopic properties of Polyacrylamide-CoFe2O4 magnetic hydrogel. J. Mol. Struct. 2013, 1036, 386–391. [Google Scholar] [CrossRef]
- Morillas, J.; de Vicente, J. Magnetorheology: A review. Soft Matter 2020, 16, 9614–9642. [Google Scholar] [CrossRef] [PubMed]
- Levy, M.; Luciani, N.; Alloyeau, D.; Elgrabli, D.; Deveaux, V.; Pechoux, C.; Chat, S.; Wang, G.; Vats, N.; Gendron, F.; et al. Long term in vivo biotransformation of iron oxide nanoparticles. Biomaterials 2011, 32, 3988–3999. [Google Scholar] [CrossRef]
- Jingli, G.; Haifei, X.; Yehua, H.; Wei, D.; Wei, H.; Chunyu, W.; Ning, G.; Haiyan, X.; Jimin, C. The internalization pathway, metabolic fate and biological effect of superparamagnetic iron oxide nanoparticles in the macrophage-like RAW264.7 cell. Sci. China Life Sci. Vol. 2011, 54, 793–805. [Google Scholar] [CrossRef]
- Mrlík, M.; Ilčíková, M.; Cvek, M.; Pavlínek, V.; Zahoranová, A.; Kroneková, Z.; Kasak, P. Carbonyl iron coated with a sulfobetaine moiety as a biocompatible system and the magnetorheological performance of its silicone oil suspensions. RSC Adv. 2016, 6, 32823–32830. [Google Scholar] [CrossRef]
- Cvek, M.; Mrlík, M.; Ilčíková, M.; Mosnáček, J.; Babayan, V.; Kuceková, Z.; Humpolíček, P.; Pavlínek, V. The chemical stability and cytotoxicity of carbonyl iron particles grafted with poly(glycidyl methacrylate) and the magnetorheological activity of their suspensions. RSC Adv. 2015, 5, 72816–72824. [Google Scholar] [CrossRef]
- Bossis, G.; Volkova, O.; Lacis, S.; Meunier, A. Magnetorheology: Fluids, Structures and Rheology. In Ferrofluids. Lecture Notes in Physics; Springer: Berlin, Germany, 2002. [Google Scholar] [CrossRef]
- Ruiz-López, J.A.; Hidalgo-Alvarez, R.; de Vicente, J. Towards a universal master curve in magnetorheology. Smart Mater. Struct. 2017, 26, 054001. [Google Scholar] [CrossRef]
- Gila-Vilchez, C.; Bonhome-Espinosa, A.B.; Kuzhir, P.; Zubarev, A.; Duran, J.D.G.; Lopez-Lopez, M.T. Rheology of magnetic alginate hydrogels. J. Rheol. 2018, 62, 1083–1096. [Google Scholar] [CrossRef]
- Bin, L.; Xu, C.; Wang, S.D.X. Alignment of magnetic particles in hydrogel matrix: A novel anisotropic magnetic hydrogels for soft robotics. J. Intell. Mater. Syst. Struct. 2021, 32, 1432–1440. [Google Scholar] [CrossRef]
- Galindo-Gonzalez, C.; Gantz, S.; Ourry, L.; Mammeri, F.; Ammar-Merah, S.; Ponton, A. Elaboration and Rheological Investigation of Magnetic Sensitive Nanocomposite Biopolymer Networks. Macromolecules 2014, 47, 3136–3144. [Google Scholar] [CrossRef]
- Borin, D.; Stepanov, G.; Musikhin, A.; Zubarev, A.; Bakhtiiarov, A.; Storozhenko, P. Magnetorheological Effect of Magnetoactive Elastomer with a Permalloy Filler. Polymers 2020, 12, 2371. [Google Scholar] [CrossRef]
- Rich, J.P.; McKinley, G.H.; Doyle, P.S. Arrested Chain Growth During Magnetic Directed Particle Assembly in Yield Stress Matrix Fluids. Langmuir 2012, 28, 3683–3689. [Google Scholar] [CrossRef]
- Liu, J.; Flores, G.A.; Sheng, R. In-vitro investigation of blood embolization in cancer treatment using magnetorheological fluids. J. Magn. Magn. Mater. 2001, 225, 209–217. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, D.; Chen, Y.; Li, Z. A Comparative Study of Ferrofluid Seal and Magnetorheological Fluid Seal. IEEE Trans. Magn. 2018, 54, 4601207. [Google Scholar] [CrossRef]
- Nardecchia, S.; Chocarro-Wrona, C.; Sánchez-Moreno, P.; Zambrano-Marín, J.R.; Marchal, J.A.; de Vicente, J. Living magnetorheological composites: From the synthesis to the in vitro characterization. Smart Mater. Struct. 2021, 30, 065015. [Google Scholar] [CrossRef]
- Fang, Y.; Yang, X.; Lin, Y.; Shi, J.; Prominski, A.; Clayton, C.; Ostroff, E.; Tian, B. Dissecting Biological and Synthetic Soft–Hard Interfaces for Tissue-Like Systems. Chem. Rev. 2022, 122, 5233–5276. [Google Scholar] [CrossRef]
- de Moraes Porto, I.C.C. Polymer Biocompatibility. In Polymerization; InTech: London, UK, 2012. [Google Scholar] [CrossRef]
- Tibbitt, M.W.; Anseth, K.S. Hydrogels as extracellular matrix mimics for 3D cell culture. Biotechnol. Bioeng. 2009, 103, 655–663. [Google Scholar] [CrossRef]
- Siglreitmeier, M.; Wu, B.; Kollmann, T.; Neubauer, M.; Nagy, G.; Schwahn, D.; Pipich, V.; Faivre, D.; Zahn, D.; Fery, A.; et al. Multifunctional layered magnetic composites. Beilstein J. Nanotechnol. 2015, 6, 134–148. [Google Scholar] [CrossRef] [PubMed]
- de Marco, C.; Alcântara, C.C.J.; Kim, S.; Briatico, F.; Kadioglu, A.; de Bernardis, G.; Chen, X.; Marano, C.; Nelson, B.J.; Pané, S. Indirect 3D and 4D Printing of Soft Robotic Microstructures. Adv. Mater. Technol. 2019, 4, 1900332. [Google Scholar] [CrossRef]
- Tognato, R.; Armiento, A.R.; Bonfrate, V.; Levato, R.; Malda, J.; Alini, M.; Eglin, D.; Giancane, G.; Serra, T. A Stimuli-Responsive Nanocomposite for 3D Anisotropic Cell-Guidance and Magnetic Soft Robotics. Adv. Funct. Mater. 2019, 29, 1804647. [Google Scholar] [CrossRef]
- Löwik, D.W.P.M.; Shklyarevskiy, I.O.; Ruizendaal, L.; Christianen, P.C.M.; Maan, J.C.; van Hest, J.C.M. A Highly Ordered Material from Magnetically Aligned Peptide Amphiphile Nanofiber Assemblies. Adv. Mater. 2007, 19, 1191–1195. [Google Scholar] [CrossRef]
- Lopez-Lopez, M.T.; Rodriguez, I.A.; Rodriguez-Arco, L.; Carriel, V.; Bonhome-Espinosa, A.B.; Campos, F.; Zubarev, A.; Duran, J.D.G. Synthesis, characterization and in vivo evaluation of biocompatible ferrogels. J. Magn. Magn. Mater. 2017, 431, 110–114. [Google Scholar] [CrossRef]
- Akama, S.; Ikeda, J.; Kawai, M.; Mitsumata, T. A Feature in Magnetorheological Effect for Polysaccharide Magnetic Hydrogels. Chem. Lett. 2018, 47, 1240–1242. [Google Scholar] [CrossRef]
- Abrougui, M.M.; Lopez-Lopez, M.T.; Duran, J.D.G. Mechanical properties of magnetic gels containing rod-like composite particles. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2019, 377. [Google Scholar] [CrossRef]
- Abrougui, M.M.; Srasra, E.; Lopez-Lopez, M.T.; Duran, J.D.G. Rheology of magnetic colloids containing clusters of particle platelets and polymer nanofibres. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2020, 378. [Google Scholar] [CrossRef] [Green Version]
- Zhao, X.; Kim, J.; Cezar, C.A.; Huebsch, N.; Lee, K.; Bouhadir, K.; Mooney, D.J. Active scaffolds for on-demand drug and cell delivery. Proc. Natl. Acad. Sci. USA 2011, 108, 67–72. [Google Scholar] [CrossRef]
- Popa, E.; Santo, V.; Rodrigues, M.; Gomes, M. Magnetically-Responsive Hydrogels for Modulation of Chondrogenic Commitment of Human Adipose-Derived Stem Cells. Polymers 2016, 8, 28. [Google Scholar] [CrossRef]
- Ikeda, J.; Takahashi, D.; Watanabe, M.; Kawai, M.; Mitsumata, T. Particle Size in Secondary Particle and Magnetic Response for Carrageenan Magnetic Hydrogels. Gels 2019, 5, 39. [Google Scholar] [CrossRef] [PubMed]
- Amorim, S.; Reis, C.A.; Reis, R.L.; Pires, R.A. Extracellular Matrix Mimics Using Hyaluronan-Based Biomaterials. Trends Biotechnol. 2021, 39, 90–104. [Google Scholar] [CrossRef] [PubMed]
- Jongprasitkul, H.; Turunen, S.; Parihar, V.S.; Annurakshita, S.; Kellomäki, M. Photocross-linkable Methacrylated Polypeptides and Polysaccharides for Casting, Injecting, and 3D Fabrication. Biomacromolecules 2021, 22, 481–493. [Google Scholar] [CrossRef] [PubMed]
- Teong, B.; Wu, S.C.; Chang, C.M.; Chen, J.W.; Chen, H.T.; Chen, C.H.; Chang, J.K.; Ho, M.L. The stiffness of a crosslinked hyaluronan hydrogel affects its chondro-induction activity on hADSCs. J. Biomed. Mater. Res. Part B Appl. Biomater. 2018, 106, 808–816. [Google Scholar] [CrossRef] [PubMed]
- Bobula, T.; Buffa, R.; Hermannová, M.; Kohutová, L.; Procházková, P.; Vágnerová, H.; Čepa, M.; Wolfová, L.; Židek, O.; Velebný, V. A novel photopolymerizable derivative of hyaluronan for designed hydrogel formation. Carbohydr. Polym. 2017, 161, 277–285. [Google Scholar] [CrossRef]
- Staubli, F.; Stoddart, M.J.; D’Este, M.; Schwab, A. Pre-culture of human mesenchymal stromal cells in spheroids facilitates chondrogenesis at a low total cell count upon embedding in biomaterials to generate cartilage microtissues. Acta Biomater. 2022, 143, 253–265. [Google Scholar] [CrossRef]
- Santhanam, S.; Liang, J.; Baid, R.; Ravi, N. Investigating thiol-modification on hyaluronan via carbodiimide chemistry using response surface methodology. J. Biomed. Mater. Res. Part A 2015, 103, 2300–2308. [Google Scholar] [CrossRef]
- Köwitsch, A.; Niepel, M.S.; Michanetzis, G.P.A.; Missirlis, Y.F.; Groth, T. Effect of Immobilized Thiolated Glycosaminoglycans on Fibronectin Adsorption and Behavior of Fibroblasts. Macromol. Biosci. 2016, 16, 381–394. [Google Scholar] [CrossRef]
- Barthold, J.E.; McCreery, K.P.; Martinez, J.; Bellerjeau, C.; Ding, Y.; Bryant, S.J.; Whiting, G.L.; Neu, C.P. Particulate ECM biomaterial ink is 3D printed and naturally crosslinked to form structurally-layered and lubricated cartilage tissue mimics. Biofabrication 2022, 14, 025021. [Google Scholar] [CrossRef]
- Buffa, R.; Odstrčilová, L.; Šedová, P.; Basarabová, I.; Novotný, J.; Velebný, V. Conjugates of modified hyaluronic acid with amino compounds for biomedical applications. Carbohydr. Polym. 2018, 189, 273–279. [Google Scholar] [CrossRef]
- Uman, S.; Dhand, A.; Burdick, J.A. Recent advances in shear-thinning and self-healing hydrogels for biomedical applications. J. Appl. Polym. Sci. 2020, 137, 48668. [Google Scholar] [CrossRef]
- Wang, L.L.; Highley, C.B.; Yeh, Y.C.; Galarraga, J.H.; Uman, S.; Burdick, J.A. Three-dimensional extrusion bioprinting of single- and double-network hydrogels containing dynamic covalent crosslinks. J. Biomed. Mater. Res. Part A 2018, 106, 865–875. [Google Scholar] [CrossRef] [PubMed]
- Shi, W.; Huang, J.; Fang, R.; Mingjie, M. Imparting Functionality to the Hydrogel by Magnetic-Field-Induced Nano-assembly and Macro-response. Appl. Mater. Interfaces 2020, 12, 5177–5194. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Zeng, Y.; Zhao, Y.; Yang, B.; Ossipov, D.; Tai, C.W.; Dai, J.W.; Xu, C.G. Biocompatible Injectable Magnetic Hydrogel Formed by Dynamic Coordination Network. Appl. Mater. Interfaces 2019, 11, 46233–46240. [Google Scholar] [CrossRef]
- Zhang, Y.; Sun, Y.; Yang, X.; Hilbornand, J.; Heerschapand, A.; Ossipov, D.A. Injectable in situ forming hybrid iron oxide-hyaluronic acid hydrogel for magnetic resonance imaging and drug delivery. Macromol. Biosci. 2014, 14, 1249–1259. [Google Scholar] [CrossRef]
- Tay, A.; Sohrabi, A.; Poole, K.; Seidlits, S.; Carlo, D.D. A 3D magnetic hyaluronic acid hydrogel for magnetomechanical neuromodulation of primary dorsal root ganglion neurons. Adv. Mater. 2018, 30, 1800927. [Google Scholar] [CrossRef]
- Barbucci, R.; Giani, G.; Fedi, S.; Bottari, S.; Casolaro, M. Biohydrogels with magnetic nanoparticles as crosslinker: Characteristics and potential use for controlled antitumor drug-delivery. Acta Biomater. 2012, 8, 4244–4252. [Google Scholar] [CrossRef]
- Tran, K.A.; Kraus, E.; Clark, A.T.; Bennett, A.; Pogoda, K.; Cheng, X.; Cebers, A.; Janmey, P.; Galie, P.A. Dynamic Tuning of Viscoelastic Hydrogels with Carbonyl Iron Microparticles Reveals the Rapid Response of Cells to Three-Dimensional Substrate Mechanics. ACS Appl. Mater. Interfaces 2021, 13, 20947–20959. [Google Scholar] [CrossRef]
- Koand, E.S.; Kimand, C.; Choi, Y.; Lee, K.Y. 3D printing of self-healing ferrogel prepared from glycol chitosan, oxidized hyaluronate, and iron oxide nanoparticles. Carbohydr. Polym. 2020, 245, 116496. [Google Scholar] [CrossRef]
- Choi, Y.; Kim, C.; Kim, H.S.; Moon, C.; Lee, K.Y. 3D Printing of dynamic tissue scaffold by combining self-healing hydrogel and self-healing ferrogel. Colloids Surfaces B Biointerfaces 2021, 208, 112108. [Google Scholar] [CrossRef]
- Mo, C.; Xiang, L.; Chen, Y. Advances in Injectable and Self-healing Polysaccharide Hydrogel Based on the Schiff Base Reaction. Macromol. Rapid Commun. 2021, 42, 2100025. [Google Scholar] [CrossRef]
- Townsend, J.M.; Beck, C.E.; Gehrke, S.H.; Berkland, C.J.; Detamore, M.S. Flow behavior prior to crosslinking: The need for precursor rheology for placement of hydrogels in medical applications and for 3D bioprinting. Prog. Polym. Sci. 2019, 91, 126–140. [Google Scholar] [CrossRef] [PubMed]
- Zuo, X.; Tang, H.; Zhu, X.; Zhang, D.; Gao, W. Injectable magnetic hydrogels for self-regulating magnetic hyperthermia and drug release. Mod. Phys. Lett. B 2021, 35. [Google Scholar] [CrossRef]
- Jahanban-Esfahlan, R.; Derakhshankhah, H.; Haghshenas, B.; Massoumi, B.; Abbasian, M.; Jaymand, M. A bio-inspired magnetic natural hydrogel containing gelatin and alginate as a drug delivery system for cancer chemotherapy. Int. J. Biol. Macromol. 2020, 156, 438–445. [Google Scholar] [CrossRef] [PubMed]
- Bulpitt, P.; Aeschlimann, D. New strategy for chemical modification of hyaluronic acid: Preparation of functionalized derivatives and their use in the formation of novel biocompatible hydrogels. J. Biomed. Mater. Res. 1999, 47, 152–169. [Google Scholar] [CrossRef]
- Maia, J.; Carvalho, R.A.; Coelho, J.F.J.; Simões, P.N.; Gil, M.H. Insight on the periodate oxidation of dextran and its structural vicissitudes. Polymer 2011, 52, 258–265. [Google Scholar] [CrossRef]
- Nonsuwan, P.; Matsugami, A.; Hayashi, F.; Hyon, S.H.; Matsumura, K. Controlling the degradation of an oxidized dextran-based hydrogel independent of the mechanical properties. Carbohydr. Polym. 2019, 204, 131–141. [Google Scholar] [CrossRef]
- Mendichi, R.; Soltes, L.; Schieroni, A.G. Evaluation of Radius of Gyration and Intrinsic Viscosity Molar Mass Dependence and Stiffness of Hyaluronan. Biomacromolecules 2004, 4, 1805–1810. [Google Scholar] [CrossRef]
- Hersloef, A.; Sundeloef, L.O.; Edsman, K. Interaction between polyelectrolyte and surfactant of opposite charge: Hydrodynamic effects in the sodium hyaluronate/tetradecyltrimethylammonium bromide/sodium chloride/water system. J. Phys. Chem. 1992, 96, 2345–2348. [Google Scholar] [CrossRef]
- Kok, C.M.; Rudin, A. Relationship between the hydrodynamic radius and the radius of gyration of a polymer in solution. Die Makromol. Chemie Rapid Commun. 1981, 2, 655–659. [Google Scholar] [CrossRef]
- Zhou, H.X.; Szabo, A. Theory and simulation of the time-dependent rate coefficients of diffusion-influenced reactions. Biophys. J. 1996, 71, 2440–2457. [Google Scholar] [CrossRef]
- Zellermann, A.M.; Bergmann, D.; Mayer, C. Cation induced conformation changes in hyaluronate solution. Eur. Polym. J. 2013, 49, 70–79. [Google Scholar] [CrossRef]
- Xu, C.; Hung, C.; Cao, Y.; Liu, H.H. Tunable Crosslinking, Reversible Phase Transition, and 3D Printing of Hyaluronic Acid Hydrogels via Dynamic Coordination of Innate Carboxyl Groups and Metallic Ions. ACS Appl. Bio Mater. 2021, 4, 2408–2428. [Google Scholar] [CrossRef] [PubMed]
- Radhakrishnan, J.; Subramanian, A.; Krishnan, U.M.; Sethuraman, S. Injectable and 3D Bioprinted Polysaccharide Hydrogels: From Cartilage to Osteochondral Tissue Engineering. Biomacromolecules 2017, 18, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.; Lue, A.; Zhang, L. Effects of Crosslinking Methods on Structure and Properties of Cellulose/PVA Hydrogels. Macromol. Chem. Phys. 2008, 209, 1266–1273. [Google Scholar] [CrossRef]
- Radulescu, D.M.; Neacsu, I.A.; Grumezescu, A.M.; Andronescu, E. New Insights of Scaffolds Based on Hydrogels in Tissue Engineering. Polymers 2022, 14, 799. [Google Scholar] [CrossRef]
- Hölzl, K.; Lin, S.; Tytgat, L.; Vlierberghe, S.V.; Gu, L.; Ovsianikov, A. Bioink properties before, during and after 3D bioprinting. Biofabrication 2016, 8, 032002. [Google Scholar] [CrossRef]
- Wang, B.; Moura, A.G.; Chen, J.; Erturk, A.; Hu, Y. Characterization of hydrogel structural damping. Extrem. Mech. 2020, 40, 100841. [Google Scholar] [CrossRef]
- LoPachin, R.M.; Gavin, T. Molecular Mechanisms of Aldehyde Toxicity: A Chemical Perspective. Chem. Res. Toxicol. 2014, 27, 1081–1091. [Google Scholar] [CrossRef]
- Gřundělová, L.; Gregorova, A.; Mráček, A.; Vícha, R.; Smolka, P.; Minařík, A. Viscoelastic and mechanical properties of hyaluronan films and hydrogels modified by carbodiimide. Carbohydr. Polym. 2015, 119, 142–148. [Google Scholar] [CrossRef]
- Tumanski, S. Chapter Magnetic Materials. In Handbook of Magnetic Measurements; CRC Press: Boca Raton, FL, USA, 2011. [Google Scholar] [CrossRef]
- Genovese, D.B. Shear rheology of hard-sphere, dispersed, and aggregated suspensions, and filler-matrix composites. Adv. Colloid Interface Sci. 2012, 171, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Gila-Vilchez, C.; Duran, J.D.G.; Gonzalez-Caballero, F.; Zubarev, A.; Lopez-Lopez, M.T. Magnetorheology of alginate ferrogels. Smart Mater. Struct. 2019, 28, 035018. [Google Scholar] [CrossRef]
- Sözeri, H.; Alveroğlu, E.; Kurtan, U.; Şenel, M.; Baykal, A. Magnetic hydrogel with high coercivity. Mater. Res. Bull. 2013, 48, 2751–2757. [Google Scholar] [CrossRef]
- Cox, J.S.G.; Kennedy, G.R.; King, J.; Marshall, P.R.; Rutherford, D. Structure of and Iron-Dextran Complex. J. Pharm. Sci. 1972, 24, 513–517. [Google Scholar] [CrossRef] [PubMed]
- Bendix, P.M.; Koenderink, G.H.; Cuvelier, D.; Dogic, Z.; Koeleman, B.N.; Brieher, W.M.; Field, C.M.; Mahadevan, L.; Weitz, D.A. A Quantitative Analysis of Contractility in Active Cytoskeletal Protein Networks. Biophys. J. 2008, 94, 3126–3136. [Google Scholar] [CrossRef]
- Laskin, G.S.; Gordon, B.S. Changes to the Skeletal Muscle Gene Expression Signature in Response to Nutrient and/or Mechanical Stimuli. FASEB J. 2022, 36, R3761. [Google Scholar] [CrossRef]
- Singh, G.; Chanda, A. Mechanical properties of whole-body soft human tissues: A review. Biomed. Mater. 2021, 16. [Google Scholar] [CrossRef]
- D’Este, M.; Eglin, D.; Alini, M. A systematic analysis of DMTMM vs EDC/NHS for ligation of amines to Hyaluronan in water. Carbohydr. Polym. 2014, 108, 239–246. [Google Scholar] [CrossRef]
- Huiru, Z.; Heindel, N.D. Determination of Degree of Substitution of Formyl Groups in Polyaldehyde Dextran by the Hydroxylamine Hydrochloride Method. Pharm. Res. 1991, 8, 400–402. [Google Scholar] [CrossRef]















| Sample Name | HA-ADH (EDC) | HA-ADH (DMTMM) | HA-OX DO 35 | HA-OX DO 62 | DEX-OX DO 49 |
|---|---|---|---|---|---|
| gel A | ✓ | ✓ | |||
| gel B | ✓ | ✓ | |||
| gel C | ✓ | ✓ | |||
| gel D | ✓ | ✓ | |||
| gel E | ✓ | ✓ | |||
| gel F | ✓ | ✓ |
| Initial Molecular Weight (kDa) | Mass of HA (g) | Molar Amount of HA (mmol) | Weight Fraction of HA (wt.%) | Mass of NaIO4 (g) | Molar Amount of NaIO4 (mmol) | Time (hours) | |
|---|---|---|---|---|---|---|---|
| HA-OX A | 1500 | 1.5 | 3.8 | 1 | 0.88 | 4.1 | 10 |
| HA-OX B | 1180 | 1.5 | 3.8 | 1 | 0.88 | 4.1 | 10 |
| DEX-OX | 70 | 3 | 18.5 | 13 | 1.58 | 7.4 | 4 |
| Yield (g) | Yield (%) | DO | Final Molecular Weigth (kDa) | |
|---|---|---|---|---|
| HA-OX A | 0.55 | 36 | 62 | 7.6 |
| HA-OX B | 0.78 | 51 | 35 | 8.4 |
| DEX-OX | 2.66 | 89 | 49 | 8.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vítková, L.; Musilová, L.; Achbergerová, E.; Kolařík, R.; Mrlík, M.; Korpasová, K.; Mahelová, L.; Capáková, Z.; Mráček, A. Formulation of Magneto-Responsive Hydrogels from Dually Cross-Linked Polysaccharides: Synthesis, Tuning and Evaluation of Rheological Properties. Int. J. Mol. Sci. 2022, 23, 9633. https://doi.org/10.3390/ijms23179633
Vítková L, Musilová L, Achbergerová E, Kolařík R, Mrlík M, Korpasová K, Mahelová L, Capáková Z, Mráček A. Formulation of Magneto-Responsive Hydrogels from Dually Cross-Linked Polysaccharides: Synthesis, Tuning and Evaluation of Rheological Properties. International Journal of Molecular Sciences. 2022; 23(17):9633. https://doi.org/10.3390/ijms23179633
Chicago/Turabian StyleVítková, Lenka, Lenka Musilová, Eva Achbergerová, Roman Kolařík, Miroslav Mrlík, Kateřina Korpasová, Leona Mahelová, Zdenka Capáková, and Aleš Mráček. 2022. "Formulation of Magneto-Responsive Hydrogels from Dually Cross-Linked Polysaccharides: Synthesis, Tuning and Evaluation of Rheological Properties" International Journal of Molecular Sciences 23, no. 17: 9633. https://doi.org/10.3390/ijms23179633
APA StyleVítková, L., Musilová, L., Achbergerová, E., Kolařík, R., Mrlík, M., Korpasová, K., Mahelová, L., Capáková, Z., & Mráček, A. (2022). Formulation of Magneto-Responsive Hydrogels from Dually Cross-Linked Polysaccharides: Synthesis, Tuning and Evaluation of Rheological Properties. International Journal of Molecular Sciences, 23(17), 9633. https://doi.org/10.3390/ijms23179633