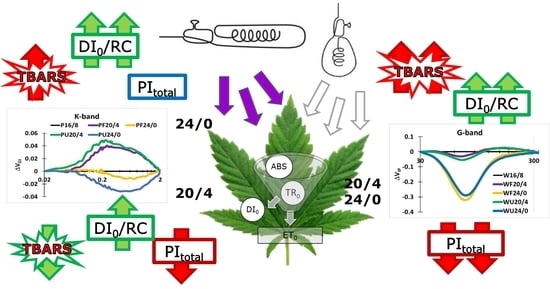
Effect of Prolonged Photoperiod on Light-Dependent Photosynthetic Reactions in Cannabis
Abstract
:1. Introduction
2. Results and Discussion
2.1. Fast Chlorophyll a Fluorescence Transients
2.2. Analysis of JIP-Test Parameters
2.3. Performance Index (PItotal) and Partitioning of Total Driving Forces
2.4. TBARS Content as a Measure of Oxidative Stress
2.5. Evaluation of FT-IR Spectra
3. Materials and Methods
3.1. Plant Material and Experimental Approach
3.2. Chlorophyll a Fluorescence Measurements
3.3. Determination of the Lipid Peroxidation Level
3.4. Fourier Transform Infrared Spectroscopy (FT-IR) Measurements
3.5. Data Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Moscariello, C.; Matassa, S.; Esposito, G.; Papirio, S. From residue to resource: The multifaceted environmental and bioeconomy potential of industrial hemp (Cannabis sativa L.). Resour. Conserv. Recycl. 2021, 175, 105864. [Google Scholar] [CrossRef]
- Fike, J. Industrial hemp: Renewed opportunities for an ancient crop. Crit. Rev. Plant Sci. 2016, 35, 406–424. [Google Scholar] [CrossRef]
- Industrial Hemp Market by Type (Hemp Seed, Hemp Seed Oil, Hemp Fiber, and CBD Hemp Oil), Application (Food, Beverages, Personal Care Products, Textiles, and Pharmaceuticals), Source (Organic and Conventional), and Region—Global Forecast to 2025. Available online: https://www.marketsandmarkets.com/Market-Reports/industrial-hemp-market-84188417.html (accessed on 27 July 2022).
- Giupponi, L.; Leoni, V.; Carrer, M.; Ceciliani, G.; Sala, S.; Panseri, S.; Pavlovic, R.; Giorgi, A. Overview on Italian hemp production chain, related productive and commercial activities and legislative framework. Ital. J. Agron. 2020, 15, 194–205. [Google Scholar] [CrossRef]
- Rupasinghe, H.V.; Davis, A.; Kumar, S.K.; Murray, B.; Zheljazkov, V.D. Industrial hemp (Cannabis sativa subsp. sativa) as an emerging source for value-added functional food ingredients and nutraceuticals. Molecules 2020, 25, 4078. [Google Scholar] [CrossRef] [PubMed]
- Jin, D.; Jin, S.; Chen, J. Cannabis indoor growing conditions, management practices, and post-harvest treatment: A review. Am. J. Plant Sci. 2019, 10, 925. [Google Scholar] [CrossRef]
- Proietti, S.; Moscatello, S.; Riccio, F.; Downey, P.; Battistelli, A. Continuous lighting promotes plant growth, light conversion efficiency, and nutritional quality of Eruca vesicaria (L.) Cav. in controlled environment with minor effects due to light quality. Front. Plant Sci. 2021, 12, 730119. [Google Scholar] [CrossRef] [PubMed]
- Osnato, M.; Cota, I.; Nebhnani, P.; Cereijo, U.; Pelaz, S. Photoperiod control of plant growth: Flowering time genes beyond flowering. Front. Plant Sci. 2021, 12, 805635. [Google Scholar] [CrossRef] [PubMed]
- Roeber, V.M.; Schmülling, T.; Cortleven, A. The photoperiod: Handling and causing stress in plants. Front. Plant Sci. 2021, 12, 781988. [Google Scholar] [CrossRef]
- Bhatta, M.; Sandro, P.; Smith, M.R.; Delaney, O.; Voss-Fels, K.P.; Gutierrez, L.; Hickey, L.T. Need for speed: Manipulating plant growth to accelerate breeding cycles. Curr. Opin. Plant Biol. 2021, 60, 101986. [Google Scholar] [CrossRef]
- Serrano-Bueno, G.; Romero-Campero, F.J.; Lucas-Reina, E.; Romero, J.M.; Valverde, F. Evolution of photoperiod sensing in plants and algae. Curr. Opin. Plant Biol. 2017, 37, 10–17. [Google Scholar] [CrossRef]
- Adams, S.; Langton, F. Photoperiod and plant growth: A review. J. Hortic. Sci. Biotechnol. 2005, 80, 2–10. [Google Scholar] [CrossRef]
- Islam, M.J.; Ryu, B.R.; Azad, M.O.K.; Rahman, M.H.; Cheong, E.J.; Lim, J.-D.; Lim, Y.-S. Cannabinoids accumulation in hemp (Cannabis sativa L.) plants under LED light spectra and their discrete role as a stress marker. Biology 2021, 10, 710. [Google Scholar] [CrossRef]
- Gendron, J.M.; Leung, C.C.; Liu, W. Energy as a seasonal signal for growth and reproduction. Curr. Opin. Plant Biol. 2021, 63, 102092. [Google Scholar] [CrossRef] [PubMed]
- Griebel, T.; Zeier, J. Light regulation and daytime dependency of inducible plant defenses in Arabidopsis: Phytochrome signaling controls systemic acquired resistance rather than local defense. Plant Physiol. 2008, 147, 790–801. [Google Scholar] [CrossRef] [PubMed]
- Haque, M.S.; Kjaer, K.H.; Rosenqvist, E.; Ottosen, C.-O. Continuous light increases growth, daily carbon gain, antioxidants, and alters carbohydrate metabolism in a cultivated and a wild tomato species. Front. Plant Sci. 2015, 6, 522. [Google Scholar] [CrossRef] [PubMed]
- Velez-Ramirez, A.I.; van Ieperen, W.; Vreugdenhil, D.; Millenaar, F.F. Plants under continuous light. Trends Plant Sci. 2011, 16, 310–318. [Google Scholar] [CrossRef]
- Ksas, B.; Becuwe, N.; Chevalier, A.; Havaux, M. Plant tolerance to excess light energy and photooxidative damage relies on plastoquinone biosynthesis. Sci. Rep. 2015, 5, srep10919. [Google Scholar] [CrossRef]
- Krieger-Liszkay, A.; Trebst, A. Tocopherol is the scavenger of singlet oxygen produced by the triplet states of chlorophyll in the PSII reaction centre. J. Exp. Bot. 2006, 57, 1677–1684. [Google Scholar] [CrossRef]
- Tsaliki, E.; Kalivas, A.; Jankauskiene, Z.; Irakli, M.; Cook, C.; Grigoriadis, I.; Panoras, I.; Vasilakoglou, I.; Dhima, K. Fibre and seed productivity of industrial hemp (Cannabis sativa L.) varieties under mediterranean conditions. Agronomy 2021, 11, 171. [Google Scholar] [CrossRef]
- Hall, J.; Bhattarai, S.P.; Midmore, D.J. The effects of photoperiod on phenological development and yields of industrial hemp. J. Nat. Fibers 2014, 11, 87–106. [Google Scholar] [CrossRef]
- Potter, D.J. A review of the cultivation and processing of cannabis (Cannabis sativa L.) for production of prescription medicines in the UK. Drug Test Anal. 2014, 6, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Morrison, V.; Llewellyn, D.; Zheng, Y. Cannabis yield, potency, and leaf photosynthesis respond differently to increasing light levels in an indoor environment. Front. Plant Sci. 2021, 12, 456. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Anderson, S.L.; Brym, Z.T.; Pearson, B.J. Photoperiodic flowering response of essential oil, grain, and fiber hemp (Cannabis sativa L.) cultivars. Front. Plant Sci. 2021, 12, 694153. [Google Scholar] [CrossRef]
- Cheng, X.; Wang, R.; Liu, X.; Zhou, L.; Dong, M.; Rehman, M.; Fahad, S.; Liu, L.; Deng, G. Effects of light spectra on morphology, gaseous exchange, and antioxidant capacity of industrial hemp. Front. Plant Sci. 2022, 13, 937436. [Google Scholar] [CrossRef] [PubMed]
- Lalge, A.; Cerny, P.; Trojan, V.; Vyhnanek, T. The effects of red, blue and white light on the growth and development of Cannabis sativa L. Mendel Net. 2017, 8, 646–651. [Google Scholar]
- Reichel, P.; Munz, S.; Hartung, J.; Präger, A.; Kotiranta, S.; Burgel, L.; Schober, T.; Graeff-Hönninger, S. Impact of three different light spectra on the yield, morphology and growth trajectory of three different Cannabis sativa L. strains. Plants 2021, 10, 1866. [Google Scholar] [CrossRef]
- Kalaji, H.M.; Oukarroum, A.; Alexandrov, V.; Kouzmanova, M.; Brestic, M.; Zivcak, M.; Samborska, I.A.; Cetner, M.D.; Allakhverdiev, S.I.; Goltsev, V. Identification of nutrient deficiency in maize and tomato plants by in vivo chlorophyll a fluorescence measurements. Plant Physiol. Biochem. 2014, 81, 16–25. [Google Scholar] [CrossRef]
- Kalaji, H.M.; Bąba, W.; Gediga, K.; Goltsev, V.; Samborska, I.A.; Cetner, M.D.; Dimitrova, S.; Piszcz, U.; Bielecki, K.; Karmowska, K. Chlorophyll fluorescence as a tool for nutrient status identification in rapeseed plants. Photosynth. Res. 2018, 136, 329–343. [Google Scholar] [CrossRef]
- Małkowski, E.; Sitko, K.; Szopiński, M.; Gieroń, Ż.; Pogrzeba, M.; Kalaji, H.M.; Zieleźnik-Rusinowska, P. Hormesis in plants: The role of oxidative stress, auxins and photosynthesis in corn treated with Cd or Pb. Int. J. Mol. Sci. 2020, 21, 2099. [Google Scholar] [CrossRef]
- Dąbrowski, P.; Baczewska, A.H.; Pawluśkiewicz, B.; Paunov, M.; Alexantrov, V.; Goltsev, V.; Kalaji, M.H. Prompt chlorophyll a fluorescence as a rapid tool for diagnostic changes in PSII structure inhibited by salt stress in Perennial ryegrass. J. Photochem. Photobiol. B Biol. 2016, 157, 22–31. [Google Scholar] [CrossRef]
- Linić, I.; Mlinarić, S.; Brkljačić, L.; Pavlović, I.; Smolko, A.; Salopek-Sondi, B. Ferulic acid and Salicylic acid foliar treatments reduce short-term salt stress in Chinese cabbage by increasing phenolic compounds accumulation and photosynthetic performance. Plants 2021, 10, 2346. [Google Scholar] [CrossRef] [PubMed]
- Dąbrowski, P.; Baczewska-Dąbrowska, A.H.; Kalaji, H.M.; Goltsev, V.; Paunov, M.; Rapacz, M.; Wójcik-Jagła, M.; Pawluśkiewicz, B.; Bąba, W.; Brestic, M. Exploration of chlorophyll a fluorescence and plant gas exchange parameters as indicators of drought tolerance in perennial ryegrass. Sensors 2019, 19, 2736. [Google Scholar] [CrossRef] [PubMed]
- Mlinarić, S.; Cesar, V.; Lepeduš, H. Antioxidative response and photosynthetic regulatory mechanisms in common fig leaves after short-term chilling stress. Ann. Appl. Biol. 2021, 178, 315–327. [Google Scholar] [CrossRef]
- Tóth, S.Z.; Schansker, G.; Strasser, R.J. A non-invasive assay of the plastoquinone pool redox state based on the OJIP-transient. Photosynth. Res. 2007, 93, 193–203. [Google Scholar] [CrossRef]
- Gomes, M.T.G.; da Luz, A.C.; dos Santos, M.R.; Batitucci, M.d.C.P.; Silva, D.M.; Falqueto, A.R. Drought tolerance of passion fruit plants assessed by the OJIP chlorophyll a fluorescence transient. Sci. Hortic. 2012, 142, 49–56. [Google Scholar] [CrossRef]
- Strasser, R.J.; Tsimilli-Michael, M.; Dangre, D.; Rai, M. Biophysical phenomics reveals functional building blocks of plants systems biology: A case study for the evaluation of the impact of mycorrhization with Piriformospora indica. In Advanced Techniques in Soil Microbiology; Varma, A., Oelmüller, R., Eds.; Springer Verlag: Berlin/Heidelberg, Germany, 2007; pp. 319–341. [Google Scholar]
- Yusuf, M.A.; Kumar, D.; Rajwanshi, R.; Strasser, R.J.; Tsimilli-Michael, M.; Govindjee; Sarin, N.B. Overexpression of g-tocopherol methyl transferase gene in transgenic Brassica juncea plants alleviates abiotic stress: Physiological and chlorophyll a fluorescence measurements. Biochim. Biophys. Acta 2010, 1797, 1428–1438. [Google Scholar] [CrossRef] [PubMed]
- Mlinarić, S.; Begović, L.; Tripić, N.; Piškor, A.; Cesar, V. Evaluation of light-dependent photosynthetic reactions in Reynoutria japonica Houtt. leaves grown at different light conditions. Front. Plant Sci. 2021, 12, 612702. [Google Scholar] [CrossRef]
- Oukarroum, A.; El Madidi, S.; Schansker, G.; Strasser, R.J. Probing the responses of barley cultivars (Hordeum vulgare L.) by chlorophyll a fluorescence OLKJIP under drought stress and re-watering. Environ. Exp. Bot. 2007, 60, 438–446. [Google Scholar] [CrossRef]
- Żurek, G.; Rybka, K.; Pogrzeba, M.; Krzyżak, J.; Prokopiuk, K. Chlorophyll a fluorescence in evaluation of the effect of heavy metal soil contamination on Perennial grasses. PLoS ONE 2014, 9, e91475. [Google Scholar] [CrossRef]
- Chen, S.; Yang, J.; Zhang, M.; Strasser, R.J.; Qiang, S. Classification and characteristics of heat tolerance in Ageratina adenophora populations using fast chlorophyll a fluorescence rise OJIP. Environ. Exp. Bot. 2016, 122, 126–140. [Google Scholar] [CrossRef]
- Chen, L.-S.; Cheng, L. Photosystem 2 is more tolerant to high temperature in apple (Malus domestica Borkh.) leaves than in fruit peel. Photosynthetica 2009, 47, 112–120. [Google Scholar] [CrossRef]
- Begović, L.; Mlinarić, S.; Antunović Dunić, J.; Katanić, Z.; Lončarić, Z.; Lepeduš, H.; Cesar, V. Response of Lemna minor L. to short-term cobalt exposure: The effect on photosynthetic electron transport chain and induction of oxidative damage. Aquat. Toxicol. 2016, 175, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Krüger, G.H.J.; De Villiers, M.F.; Strauss, A.J.; de Beer, M.; van Heerden, P.D.R.; Maldonado, R.; Strasser, R.J. Inhibition of photosystem II activities in soybean (Glycine max) genotypes differing in chilling sensitivity. South Afr. J. Bot. 2014, 95, 85–96. [Google Scholar] [CrossRef]
- Begović, L.; Galić, V.; Abičić, I.; Lončarić, Z.; Lalić, A.; Mlinarić, S. Implications of intra-seasonal climate variations on chlorophyll a fluorescence and biomass in winter barley breeding program. Photosynthetica 2020, 58, 995–1008. [Google Scholar] [CrossRef]
- Pavlović, I.; Mlinarić, S.; Tarkowská, D.; Oklestkova, J.; Novak, O.; Lepeduš, H.; Vujčić Bok, V.; Radić Brkanac, S.; Strnad, M.; Salopek Sondi, B. Early Brassica crops responses to salinity stress: A comparative analysis between Chinese cabbage, white cabbage and kale. Front. Plant Sci. 2019, 10, 450. [Google Scholar] [CrossRef]
- Tóth, S.Z.; Puthur, J.T.; Nagy, V.; Garab, G. Experimental evidence for ascorbate-dependent electron transport in leaves with inactive oxygen-evolving complexes. Plant Physiol. 2009, 149, 1568–1578. [Google Scholar] [CrossRef]
- Venkatesh, J.; Upadhyaya, C.P.; Yu, J.-W.; Hemavathi, A.; Kim, D.H.; Strasser, R.J.; Park, S.W. Chlorophyll a fluorescence transient analysis of transgenic potato overexpressing D-galacturonic acid reductase gene for salinity stress tolerance. Hortic. Environ. Biotechnol. 2012, 53, 320–328. [Google Scholar] [CrossRef]
- Kumar, D.; Singh, H.; Raj, S.; Soni, V. Chlorophyll a fluorescence kinetics of mung bean (Vigna radiata L.) grown under artificial continuous light. Biochem. Biophys. Rep. 2020, 24, 100813. [Google Scholar] [CrossRef]
- Zagorchev, L.; Atanasova, A.; Albanova, I.; Traianova, A.; Mladenov, P.; Kouzmanova, M.; Goltsev, V.; Kalaji, H.M.; Teofanova, D. Functional characterization of the photosynthetic machinery in Smicronix galls on the parasitic plant Cuscuta campestris by JIP-test. Cells 2021, 10, 1399. [Google Scholar] [CrossRef]
- Lazár, D. The polyphasic chlorophyll a fluorescence rise measured under high intensity of exciting light. Funct. Plant. Biol. 2006, 33, 9–30. [Google Scholar] [CrossRef]
- Dimitrova, S.; Paunov, M.; Pavlova, B.; Dankov, K.; Kouzmanova, M.; Velikova, V.; Tsonev, T.; Kalaji, H.; Goltsev, V. Photosynthetic efficiency of two Platanus orientalis L. ecotypes exposed to moderately high temperature JIP-test analysis. Photosynthetica 2020, 58, 12. [Google Scholar] [CrossRef] [Green Version]
- Zagorchev, L.; Traianova, A.; Teofanova, D.; Li, J.; Kouzmanova, M.; Goltsev, V. Influence of Cuscuta campestris Yunck. on the photosynthetic activity of Ipomoea tricolor Cav.–in vivo chlorophyll a fluorescence assessment. Photosynthetica 2020, 58, 422–432. [Google Scholar] [CrossRef]
- Chen, W.; Jia, B.; Chen, J.; Feng, Y.; Li, Y.; Chen, M.; Liu, H.; Yin, Z. Effects of different planting densities on photosynthesis in maize determined via prompt fluorescence, delayed fluorescence and P700 signals. Plants 2021, 10, 276. [Google Scholar] [CrossRef] [PubMed]
- Oukarroum, A.; Bussotti, F.; Goltsev, V.; Kalaji, H.M. Correlation between reactive oxygen species production and photochemistry of photosystems I and II in Lemna gibba L. plants under salt stress. Environ. Exp. Bot. 2015, 109, 80–88. [Google Scholar] [CrossRef]
- Zhang, D.; Zhang, Q.S.; Yang, X.Q.; Sheng, Z.T.; Nan, G.N. The alternation between PSII and PSI in ivy (Hedera nepalensis) demonstrated by in vivo chlorophyll a fluorescence and modulated 820 nm reflection. Plant Physiol. Biochem. 2016, 108, 499–506. [Google Scholar] [CrossRef]
- Xiao, F.; Zhang, Y.; Yang, Y.; Zhang, W. Downregulation of PSII activity and increased cyclic electron transport in cotton prevents PSI from photoinhibition due to night chilling. Photosynthetica 2019, 57, 523–532. [Google Scholar] [CrossRef]
- Strasser, R.J.; Tsimilli-Michael, M.; Srivastava, A. Analysis of the chlorophyll a fluorescence transient. In Chlorophyll a Fluorescence: A Signature of Photosynthesis; Papageorgiou, G.C., Govinjee, Eds.; Springer: Dordrecht, The Netherlands, 2004; Volume 19, pp. 321–362. [Google Scholar]
- Mlinarić, S.; Antunović Dunić, J.; Skendrović Babojelić, M.; Cesar, V.; Lepeduš, H. Differential accumulation of photosynthetic proteins regulates diurnal photochemical adjustments of PSII in common fig (Ficus carica L.) leaves. J. Plant Physiol. 2017, 209, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Cabahug, R.A.M.; You, N.H.; Nam, S.Y. Chlorophyll fluorescence and growth evaluation of ornamental foliage plants in response to light intensity levels under continuous lighting conditions. Flower Res. J. 2021, 29, 153–164. [Google Scholar] [CrossRef]
- Chan, T.; Shimizu, Y.; Pospíšil, P.; Nijo, N.; Fujiwara, A.; Taninaka, Y.; Ishikawa, T.; Hori, H.; Nanba, D.; Imai, A. Quality control of photosystem II: Lipid peroxidation accelerates photoinhibition under excessive illumination. PLoS ONE 2012, 7, e52100. [Google Scholar] [CrossRef] [PubMed]
- Pospíšil, P. Production of reactive oxygen species by photosystem II as a response to light and temperature stress. Front. Plant Sci. 2016, 7, 1950. [Google Scholar] [CrossRef]
- Yamamoto, Y. Quality control of photosystem II: The mechanisms for avoidance and tolerance of light and heat stresses are closely linked to membrane fluidity of the thylakoids. Front. Plant Sci. 2016, 7, 1136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sonoike, K. Photoinhibition of photosystem I. Physiol. Plant. 2011, 142, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Legner, N.; Meinen, C.; Rauber, R. Root differentiation of agricultural plant cultivars and proveniences using FTIR spectroscopy. Front. Plant Sci. 2018, 9, 748. [Google Scholar] [CrossRef] [PubMed]
- Türker-Kaya, S.; Huck, C.W. A review of mid-infrared and near-infrared imaging: Principles, concepts and applications in plant tissue analysis. Molecules 2017, 22, 168. [Google Scholar] [CrossRef]
- Wei, Z.; Jiao, D.; Xu, J. Using Fourier transform infrared spectroscopy to study effects of magnetic field treatment on wheat (Triticum aestivum L.) seedlings. J. Spectroscop. 2015, 2015, 570190. [Google Scholar] [CrossRef]
- Strasser, R.J.; Srivastava, A.; Tsimilli-Michael, M. The fluorescence transient as a tool to characterize and screen photosynthetic samples. In Probing Photosynthesis: Mechanism, Regulation & Adaptation, 1st ed.; Yunus, M., Pathre, U., Mohanty, P., Eds.; CRC: New York, NY, USA, 2000; pp. 445–483. [Google Scholar]
- Appenroth, K.-J.; Stöckel, J.; Srivastava, A.; Strasser, R. Multiple effects of chromate on the photosynthetic apparatus of Spirodela polyrhiza as probed by OJIP chlorophyll a fluorescence measurements. Environ. Pollut. 2001, 115, 49–64. [Google Scholar] [CrossRef]
- Goltsev, V.; Kalaji, H.; Paunov, M.; Bąba, W.; Horaczek, T.; Mojski, J.; Kociel, H.; Allakhverdiev, S. Variable chlorophyll fluorescence and its use for assessing physiological condition of plant photosynthetic apparatus. Russ. J. Plant Physiol. 2016, 63, 869–893. [Google Scholar] [CrossRef]
- van Heerden, P.D.R.; Swanepoel, J.W.; Kruger, G.H.J. Modulation of photosynthesis by drought in two desert scrub species exhibiting C3-mode CO2 assimilation. Environ. Exp. Bot. 2007, 61, 124–136. [Google Scholar] [CrossRef]
- Verma, S.; Dubey, R. Lead toxicity induces lipid peroxidation and alters the activities of antioxidant enzymes in growing rice plants. Plant Sci. 2003, 164, 645–655. [Google Scholar] [CrossRef]
- Schlager, S. Chapter 9—Morpho and Rvcg—Shape Analysis in R: R-Packages for Geometric Morphometrics, Shape Analysis and Surface Manipulations. In Statistical Shape and Deformation Analysis; Zheng, G., Li, S., Székely, G., Eds.; Academic Press: Cambridge, MA, USA, 2017; pp. 217–256. [Google Scholar]






| Parameter | Finola | USO31 | ||
|---|---|---|---|---|
| CV1 | CV2 | CV1 | CV2 | |
| ABS/RC | −54.3 | 154.9 | −212.3 | −310.6 |
| DI0/RC | −407.8 | 307.9 | −228.3 | −465.6 |
| TR0/RC | 353.6 | −153.0 | 16.0 | 155.1 |
| ET0/RC | −156.2 | −95.2 | 681.3 | 685.8 |
| RE0/RC | 232.1 | 189.6 | −87.5 | 189.7 |
| φP0 | −1016.8 | −936.9 | −1565.2 | 3258.2 |
| ψE0 | 2373.0 | −5883.1 | −745.5 | 8882.0 |
| φE0 | −3768.5 | 7400.1 | −2460.7 | −13,393.0 |
| δR0 | −408.7 | −256.3 | −1348.0 | 947.1 |
| φR0 | 260.5 | −327.2 | 4068.7 | −2938.0 |
| γRC/(1 − γRC) | 682.7 | 1803.5 | −870.0 | −1354.0 |
| φP0/(1 − φP0) | −24.8 | −0.1 | −76.0 | −78.6 |
| ψE0/(1 − ψE0) | −7.0 | 273.9 | −375.8 | −282.4 |
| PIabs | 153.3 | −197.7 | 323.2 | 314.4 |
| δR0/(1 − δR0) | 14.3 | 35.6 | 48.0 | −109.7 |
| PItotal | −9.1 | 6.2 | −75.1 | 79.2 |
| Finola | |||||
|---|---|---|---|---|---|
| P16/8 | P20/4 | P24/0 | W16/8 | W20/4 | |
| P20/4 | 11.18 * | ||||
| P24/0 | 11.96 ** | 8.36 | |||
| W16/8 | 18.32 *** | 19.88 *** | 17.31 *** | ||
| W20/4 | 12.47 ** | 8.32 | 3.49 | 17.8 *** | |
| W24/0 | 12.98 ** | 12.66 ** | 8.34 | 16.77 *** | 9.12 |
| USO31 | |||||
| P20/4 | 11.1 | ||||
| P24/0 | 9.4 | 13.64 | |||
| W16/8 | 33.91 *** | 42.47 *** | 34.07 *** | ||
| W20/4 | 8.64 | 11.86 | 8.04 | 34.83 *** | |
| W24/0 | 14.44 | 21.75 * | 10.13 | 28.07 ** | 15.04 |
| Recorded Data and Technical Parameters | Description |
|---|---|
| F0 | Minimal fluorescence intensity (20 μs) |
| Fm | Maximal fluorescence intensity |
| VJ = (FJ − F0)/(Fm − F0) | Relative variable fluorescence at 2 ms |
| VI = (FI − F0)/(Fm − F0) | Relative variable fluorescence at 30 ms |
| VK = (FK – F0)/(Fm – F0) | Relative variable fluorescence at 0.3 ms |
| FV = Fm − F0 | Maximal variable fluorescence |
| M0 = (dV/dt)0 | Approximated initial slope of relative variable fluorescence Fv |
| φP0 = TR0/ABS = Fv/Fm | Maximum quantum yield of PSII |
| ψE0 = ET0/TR0 = 1 − VJ | Probability that a trapped exciton moves an electron further than QA |
| φE0 = ET0/ABS = [1 − (F0/Fm)](1 − VJ) | Quantum yield for electron transport |
| δR0 = RE0 − ET0 = (1 − VI)/(1 − VJ) | Probability with which an electron from the intersystem electron carriers moves to reduce end electron acceptors at the PSI acceptor side |
| φR0 = RE0/ABS = [1 − (F0/Fm)]ψE0 δR0 | Quantum yield for reduction of end electron acceptors at the PSI acceptor side |
| %OEC = [1 − (VK/VJ)]treatment/[1 − (VK/VJ)control | Fraction of Oxygen Evolving Complexes (OEC) |
| ABS/RC = M0(1/VJ)[1/φP0] | Absorption flux per active RC |
| TR0/RC = M0(1/VJ) | Trapping flux per active RC |
| ET0/RC = M0(1/VJ)(1 − VJ) | Electron transport flux per active RC |
| DI0/RC = (ABS/RC) − (TR0/RC) | Dissipation flux per active RC |
| γRC = ChlRC/Chltotal = RC/(ABS + RC) | Electron flux reducing end electron acceptors at the PSI acceptor side, per RC |
| RE0/RC = M0(1/VJ)ψE0 δR0 | Probability that a PSII Chl molecule functions as RC |
| PIABS = [γRC/(1 − γRC)][φP0/(1 − φP0)][ψE0/(1 − ψE0)] | Performance index on an absorption basis |
| PItotal = PIABS[δR0/(1 − δR0)] | Performance index for energy conservation from exciton to the reduction of the PSI end acceptor |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Šrajer Gajdošik, M.; Vicić, A.; Gvozdić, V.; Galić, V.; Begović, L.; Mlinarić, S. Effect of Prolonged Photoperiod on Light-Dependent Photosynthetic Reactions in Cannabis. Int. J. Mol. Sci. 2022, 23, 9702. https://doi.org/10.3390/ijms23179702
Šrajer Gajdošik M, Vicić A, Gvozdić V, Galić V, Begović L, Mlinarić S. Effect of Prolonged Photoperiod on Light-Dependent Photosynthetic Reactions in Cannabis. International Journal of Molecular Sciences. 2022; 23(17):9702. https://doi.org/10.3390/ijms23179702
Chicago/Turabian StyleŠrajer Gajdošik, Martina, Antonia Vicić, Vlatka Gvozdić, Vlatko Galić, Lidija Begović, and Selma Mlinarić. 2022. "Effect of Prolonged Photoperiod on Light-Dependent Photosynthetic Reactions in Cannabis" International Journal of Molecular Sciences 23, no. 17: 9702. https://doi.org/10.3390/ijms23179702
APA StyleŠrajer Gajdošik, M., Vicić, A., Gvozdić, V., Galić, V., Begović, L., & Mlinarić, S. (2022). Effect of Prolonged Photoperiod on Light-Dependent Photosynthetic Reactions in Cannabis. International Journal of Molecular Sciences, 23(17), 9702. https://doi.org/10.3390/ijms23179702