Walnut Shell Biowaste Valorization via HTC Process for the Removal of Some Emerging Pharmaceutical Pollutants from Aqueous Solutions
Abstract
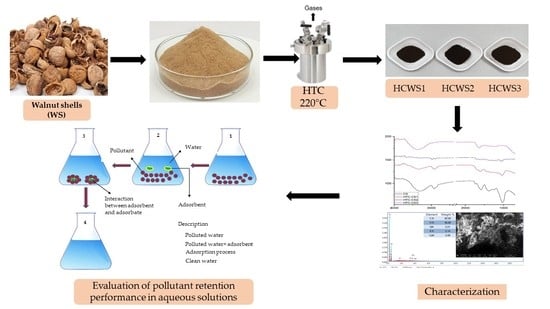
:1. Introduction
2. Results and Discussion
2.1. Characterization of the HCWS Eco-Materials
2.2. Removal Efficiency Tests
2.2.1. Effect of Contact Time on Removal Efficiency
2.2.2. Effect of Adsorbent Amount on Removal Efficiency
2.2.3. Effect of the Initial Pollutant Concentration on the Removal Efficiency
2.3. Kinetic and Adsorption Studies
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Preparation of Hydrochars
3.3. Characterization of Hydrochars
3.4. Removal Efficiency and Kinetic Studies
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Leong, H.Y.; Chang, C.-K.; Khoo, K.S.; Chew, K.W.; Chia, S.R.; Lim, J.W.; Chang, J.-S.; Show, P.L. Waste biorefinery towards a sustainable circular bioeconomy: A solution to global issues. Biotechnol. Biofuels 2021, 14, 87. [Google Scholar] [CrossRef] [PubMed]
- Stegmann, P.; Londo, M.; Junginger, M. The circular bioeconomy: Its elements and role in European bioeconomy clusters. Resour. Conserv. Recycl. X 2020, 6, 100029. [Google Scholar] [CrossRef]
- Akubo, K.; Nahil, M.A.; Williams, P.T. Pyrolysis-catalytic steam reforming of agricultural biomass wastes and biomass components for production of hydrogen/syngas. J. Energy Inst. 2019, 92, 1987–1996. [Google Scholar] [CrossRef]
- Lee, S.Y.; Sankaran, R.; Chew, K.W.; Tan, C.H.; Krishnamoorthy, R.; Chu, D.-T.; Show, P.-L. Waste to bioenergy: A review on the recent conversion technologies. BMC Energy 2019, 1, 4. [Google Scholar] [CrossRef]
- Stefan, D.; Matei, E.; Turcanu, A.A.; Predescu, A.M.; Coman, G.; Rapa, M.; Predescu, C. The use of digestate in green agriculture. Univ. Politeh. Buchar. Sci. Bull. Ser. B Chem. Mater. Sci. 2021, 83, 295–306. [Google Scholar]
- Horvathova, V.; Novakova, R.; Vadkertiova, A. The Possibility of Processing Waste Wood Biomass for Fermentation Purposes. Sustain. For. Based Ind. Glob. Econ. 2020, 84, 349–353. [Google Scholar]
- Alonso, D.M.; Bond, J.Q.; Dumesic, J.A. Catalytic conversion of biomass to biofuels. Green Chem. 2010, 12, 1493–1513. [Google Scholar] [CrossRef]
- Atallah, E.; Kwapinski, W.; Ahmad, M.N.; Leahy, J.J.; Al-Muhtaseb, A.H.; Zeaiter, J. Hydrothermal carbonization of olive mill wastewater: Liquid phase product analysis. J. Environ. Chem. Eng. 2019, 7, 8. [Google Scholar] [CrossRef]
- Li, P.; Shi, X.P.; Wang, X.H.; Song, J.D.; Fang, S.Q.; Bai, J.; Zhang, G.J.; Chang, C.; Pang, S.S. Bio-oil from biomass fast pyrolysis: Yields, related properties and energy consumption analysis of the pyrolysis system. J. Clean. Prod. 2021, 328, 129613. [Google Scholar] [CrossRef]
- Maniscalco, M.P.; Volpe, M.; Messineo, A. Hydrothermal Carbonization as a Valuable Tool for Energy and Environmental Applications: A Review. Energies 2020, 13, 4098. [Google Scholar] [CrossRef]
- Hu, Z.T.; Huo, W.Z.; Chen, Y.; Zhang, Q.; Hu, M.; Zheng, W.C.; Shao, Y.C.; Pan, Z.Y.; Li, X.N.; Zhao, J. Humic Substances Derived From Biomass Waste During Aerobic Composting and Hydrothermal Treatment: A Review. Front. Bioeng. Biotechnol. 2022, 10, 878686. [Google Scholar] [CrossRef]
- Saleh, M.; Isik, Z.; Yabalak, E.; Yalvac, M.; Dizge, N. Green production of hydrochar nut group from waste materials in subcritical water medium and investigation of their adsorption performance for crystal violet. Water Environ. Res. 2021, 93, 3075–3089. [Google Scholar] [CrossRef]
- Rasam, S.; Moraveji, M.K.; Soria-Verdugo, A.; Salimi, A. Synthesis, characterization and absorbability of Crocus sativus petals hydrothermal carbonized hydrochar and activated hydrochar. Chem. Eng. Process. Process Intensif. 2021, 159, 108236. [Google Scholar] [CrossRef]
- Chen, T.; Zhang, J.; Li, M.X.; Ge, H.L.; Li, Y.; Duan, T.; Zhu, W.K. Biomass-derived composite aerogels with novel structure for removal/recovery of uranium from simulated radioactive wastewater. Nanotechnology 2019, 30, 455602. [Google Scholar] [CrossRef]
- Queiroz, L.S.; de Souza, L.K.C.; Thomaz, K.T.C.; Lima, E.T.L.; da Rocha, G.N.; do Nascimento, L.A.S.; Pires, L.H.D.; Faial, K.D.F.; da Costa, C.E.F. Activated carbon obtained from amazonian biomass tailings (acai seed): Modification, characterization, and use for removal of metal ions from water. J. Environ. Manag. 2020, 270, 110868. [Google Scholar] [CrossRef]
- Elmouwahidi, A.; Castelo-Quiben, J.; Vivo-Vilches, J.F.; Perez-Cadenas, A.F.; Maldonado-Hodar, F.J.; Carrasco-Marin, F. Activated carbons from agricultural waste solvothermally doped with sulphur as electrodes for supercapacitors. Chem. Eng. J. 2018, 334, 1835–1841. [Google Scholar] [CrossRef]
- Hammud, H.H.; Karnati, R.K.; Al Shafee, M.; Fawaz, Y.; Holail, H. Activated hydrochar from palm leaves as efficient lead adsorbent. Chem. Eng. Commun. 2021, 208, 197–209. [Google Scholar] [CrossRef]
- Pham, V.H.T.; Kim, J.; Chang, S.; Chung, W. Bacterial Biosorbents, an Efficient Heavy Metals Green Clean-Up Strategy: Prospects, Challenges, and Opportunities. Microorganisms 2022, 10, 610. [Google Scholar] [CrossRef]
- Kamali, M.; Appels, L.; Kwon, E.E.; Aminabhavi, T.M.; Dewil, R. Biochar in water and wastewater treatment-a sustainability assessment. Chem. Eng. J. 2021, 420, 129946. [Google Scholar] [CrossRef]
- Mahmood, R.T.; Asad, M.J.; Hadri, S.H.; El-Shorbagy, M.A.; Mousa, A.A.; Dara, R.N.; Awais, M.; Tlili, I. Bioremediation of textile industrial effluents by Fomitopsis pinicola IEBL-4 for environmental sustainability. Hum. Ecol. Risk Assess. 2022, 1–18. [Google Scholar] [CrossRef]
- Brunner, P.H.; Rechberger, H. Handbook of Material Flow Analysis for Environmental, Resource, and Waste Engineers; CRC Press: Boca Raton, FL, USA, 2016. [Google Scholar]
- Turcanu, A.A.; Matei, E.; Rapa, M.; Predescu, A.M.; Coman, G.; Predescu, C. Biowaste Valorization Using Hydrothermal Carbonization for Potential Wastewater Treatment Applications. Water 2022, 14, 2344. [Google Scholar] [CrossRef]
- John, K.I.; Omorogie, M.O. Biomass-based hydrothermal carbons for catalysis and environmental cleanup: A review. Green Chem. Lett. Rev. 2022, 15, 160–184. [Google Scholar] [CrossRef]
- Sivaprasad, S.; Manandhar, A.; Shah, A. Hydrothermal Carbonization: Upgrading Waste Biomass to Char. Available online: https://ohioline.osu.edu/factsheet/fabe-6622 (accessed on 10 June 2022).
- Yi, W.; Zheng, D.W.N.; Wang, X.H.; Chen, Y.Q.; Hu, J.H.; Yang, H.P.; Shao, J.A.; Zhang, S.H.; Chen, H.P. Biomass hydrothermal conversion under CO2 atmosphere: A way to improve the regulation of hydrothermal products. Sci. Total Environ. 2022, 807, 150900. [Google Scholar] [CrossRef] [PubMed]
- Khushk, S.; Zhang, L.; Pirzada, A.M.; Irfan, M.; Li, A.; Khushk, S.I.A.; Zhang, L.L.B. Cr(VI) Heavy Metal Adsorption from Aqueous Solution by KOH Treated Hydrochar Derived from Agricultural Wastes. In Proceedings of the 5th International Conference on Energy, Environment and Sustainable Development (EESD), Mehran University of Engineering and Technology, Jamshoro, Pakistan, 14–16 November 2018. [Google Scholar]
- Zhou, F.; Li, K.; Hang, F.X.; Zhang, Z.M.; Chen, P.; Wei, L.; Xie, C.F. Efficient removal of methylene blue by activated hydrochar prepared by hydrothermal carbonization and NaOH activation of sugarcane bagasse and phosphoric acid. RSC Adv. 2022, 12, 1885–1896. [Google Scholar] [CrossRef]
- Hammud, H.H.; Shmait, A.; Hourani, N. Removal of Malachite Green from water using hydrothermally carbonized pine needles. RSC Adv. 2015, 5, 7909–7920. [Google Scholar] [CrossRef]
- Espro, C.; Satira, A.; Mauriello, F.; Anajafi, Z.; Moulaee, K.; Iannazzo, D.; Neri, G. Orange peels-derived hydrochar for chemical sensing applications. Sens. Actuators B Chem. 2021, 341, 130016. [Google Scholar] [CrossRef]
- Zhang, B.D.; Heidari, M.; Regmi, B.; Salaudeen, S.; Arku, P.; Thimmannagari, M.; Dutta, A. Hydrothermal Carbonization of Fruit Wastes: A Promising Technique for Generating Hydrochar. Energies 2018, 11, 2022. [Google Scholar] [CrossRef]
- Turcanu, A.A.; Matei, E.; Rapa, M.; Predescu, A.; Coman, G.; Berbecaru, A.; Predescu, C. Valorization of Biomass Waste Using HTC Process for Potential Environmental Applications. Univ. Politeh. Buchar. Sci. Bull. Ser. B Chem. Mater. Sci. 2022, 84, 165–174. [Google Scholar]
- Islam, M.A.; Ahmed, M.J.; Khanday, W.A.; Asif, M.; Hameed, B.H. Mesoporous activated coconut shell-derived hydrochar prepared via hydrothermal carbonization-NaOH activation for methylene blue adsorption. J. Environ. Manag. 2017, 203, 237–244. [Google Scholar] [CrossRef]
- Davies, G.; McGregor, J. Hydrothermal Synthesis of Biomass-Derived Magnetic Carbon Composites for Adsorption and Catalysis. ACS Omega 2021, 6, 33000–33009. [Google Scholar] [CrossRef]
- Weidemann, E.; Niinipuu, M.; Fick, J.; Jansson, S. Using carbonized low-cost materials for removal of chemicals of environmental concern from water. Environ. Sci. Pollut. Res. 2018, 25, 15793–15801. [Google Scholar] [CrossRef]
- Spataru, A.; Jain, R.; Chung, J.W.; Gerner, G.; Krebs, R.; Lens, P.N.L. Enhanced adsorption of orthophosphate and copper onto hydrochar derived from sewage sludge by KOH activation. RSC Adv. 2016, 6, 101827–101834. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, F.Y.; Huang, Q. Highly effective removal of malachite green from aqueous solution by hydrochar derived from phycocyanin-extracted algal bloom residues through hydrothermal carbonization. RSC Adv. 2017, 7, 5790–5799. [Google Scholar] [CrossRef] [Green Version]
- Suhaimi, N.; Kooh, M.R.R.; Lim, C.M.; Chao, C.T.C.; Chau, Y.F.C.; Mahadi, A.H.; Chiang, H.P.; Hassan, N.H.H.; Thotagamuge, R. The Use of Gigantochloa Bamboo-Derived Biochar for the Removal of Methylene Blue from Aqueous Solution. Adsorpt. Sci. Technol. 2022, 2022, 8245797. [Google Scholar] [CrossRef]
- Li, H.Z.; Zhang, Y.N.; Guo, J.Z.; Lv, J.Q.; Huan, W.W.; Li, B. Preparation of hydrochar with high adsorption performance for methylene blue by co-hydrothermal carbonization of polyvinyl chloride and bamboo. Bioresour. Technol. 2021, 337, 125442. [Google Scholar] [CrossRef]
- Han, X.B.; Li, R.; Miao, P.P.; Gao, J.; Hu, G.W.; Zhao, Y.; Chen, T. Design, Synthesis and Adsorption Evaluation of Bio-Based Lignin/Chitosan Beads for Congo Red Removal. Materials 2022, 15, 2310. [Google Scholar] [CrossRef]
- Akpomie, K.G.; Conradie, J. Banana peel as a biosorbent for the decontamination of water pollutants. A review. Environ. Chem. Lett. 2020, 18, 1085–1112. [Google Scholar] [CrossRef]
- Wei, J.; Liu, Y.T.; Li, J.; Yu, H.; Peng, Y.Z. Removal of organic contaminant by municipal sewage sludge-derived hydrochar: Kinetics, thermodynamics and mechanisms. Water Sci. Technol. 2018, 78, 947–956. [Google Scholar] [CrossRef]
- Moreira, W.M.; Viotti, P.V.; Vieira, M.G.A.; Baptista, C.M.; Scaliante, M.H.; Gimenes, M.L. Hydrothermal synthesis of biobased carbonaceous composite from a blend of kraft black liquor and tannin and its application to aspirin and paracetamol removal. Colloids Surf. A Physicochem. Eng. Asp. 2021, 608, 125597. [Google Scholar] [CrossRef]
- Qureshi, T.; Memon, N.; Memon, S.Q.; Yavuz, H.; Lachgar, A.; Denizli, A. Evaluation of hydrochar efficiency for simultaneous removal of diclofenac and ibuprofen from aqueous system using surface response methodology. Environ. Sci. Pollut. Res. 2019, 26, 9796–9804. [Google Scholar] [CrossRef]
- Deng, J.; Li, X.; Wei, X.; Liu, Y.; Liang, J.; Song, B.; Shao, Y.; Huang, W. Hybrid silicate-hydrochar composite for highly efficient removal of heavy metal and antibiotics: Coadsorption and mechanism. Chem. Eng. J. 2020, 387, 124097. [Google Scholar] [CrossRef]
- He, H.; Zhang, N.; Chen, N.; Lei, Z.; Shimizu, K.; Zhang, Z. Efficient phosphate removal from wastewater by MgAl-LDHs modified hydrochar derived from tobacco stalk. Bioresour. Technol. Rep. 2019, 8, 100348. [Google Scholar] [CrossRef]
- bursa.ro. Available online: https://www.bursa.ro/tara-noastra-este-campioana-la-productia-de-nuci-in-ue-cu-60-de-mii-de-tone-pe-an-88029649 (accessed on 2 June 2022).
- Acikalin, K.; Karaca, F. Fixed-bed pyrolysis of walnut shell: Parameter effects on yields and characterization of products. J. Anal. Appl. Pyrolysis 2017, 125, 234–242. [Google Scholar] [CrossRef]
- Gilca, I.A.; Ghitescu, R.E.; Puitel, A.C.; Popa, V.I. Preparation of lignin nanoparticles by chemical modification. Iran. Polym. J. 2014, 23, 355–363. [Google Scholar] [CrossRef]
- Yeganeh, F.; Chiewchan, N.; Chonkaew, W. Hydrothermal pretreatment of biomass-waste-garlic skins in the cellulose nanofiber production process. Cellulose 2022, 29, 2333–2349. [Google Scholar] [CrossRef]
- Rangan, A.; Manchiganti, M.V.; Thilaividankan, R.M.; Kestur, S.G.; Menon, R. Novel method for the preparation of lignin-rich nanoparticles from lignocellulosic fibers. Ind. Crops Prod. 2017, 103, 152–160. [Google Scholar] [CrossRef]
- Hu, B.; Wang, K.; Wu, L.H.; Yu, S.H.; Antonietti, M.; Titirici, M.M. Engineering Carbon Materials from the Hydrothermal Carbonization Process of Biomass. Adv. Mater. 2010, 22, 813–828. [Google Scholar] [CrossRef]
- Ferreira, A.F.; Ribau, J.P.; Costa, M. A decision support method for biochars characterization from carbonization of grape pomace. Biomass Bioenergy 2021, 145, 105946. [Google Scholar] [CrossRef]
- Anastopoulos, I.; Pashalidis, I.; Hosseini-Bandegharaei, A.; Giannakoudakis, D.A.; Robalds, A.; Usman, M.; Belen Escudero, L.; Zhou, Y.; Colmenares, J.C.; Nunez-Delgado, A.; et al. Agricultural biomass/waste as adsorbents for toxic metal decontamination of aqueous solutions. J. Mol. Liq. 2019, 295, 111684. [Google Scholar] [CrossRef]
- Duan, X.H.; Hong, W.; Srinivasakannan, C.; Wang, X. Hydrochar silicate composite sorbent via simple hydrothermal carbonization and its application to methylene blue removal. Mater. Res. Express 2019, 6, 035601. [Google Scholar] [CrossRef]
- Yedro, F.M.; Garcia-Serna, J.; Cantero, D.A.; Sobron, F.; Cocero, M.J. Hydrothermal hydrolysis of grape seeds to produce bio-oil. RSC Adv. 2014, 4, 30332–30339. [Google Scholar] [CrossRef]
- Nguyen, D.T.; Tran, H.N.; Juang, R.S.; Dat, N.D.; Tomul, F.; Ivanets, A.; Woo, S.H.; Hosseini-Bandegharaei, A.; Nguyen, V.P.; Chao, H.P. Adsorption process and mechanism of acetaminophen onto commercial activated carbon. J. Environ. Chem. Eng. 2020, 8, 104408. [Google Scholar] [CrossRef]
- Paton-Carrero, A.; Sanchez, P.; Sanchez-Silva, L.; Romero, A. Graphene-based materials behaviour for dyes adsorption. Mater. Today Commun. 2022, 30, 103033. [Google Scholar] [CrossRef]
- Maruthapandi, M.; Kumar, V.B.; Luong, J.H.T.; Gedanken, A. Kinetics, Isotherm, and Thermodynamic Studies of Methylene Blue Adsorption on Polyaniline and Polypyrrole Macro-Nanoparticles Synthesized by C-Dot-Initiated Polymerization. ACS Omega 2018, 3, 7196–7203. [Google Scholar] [CrossRef]
- Wawrzkiewicz, M.; Podkoscielna, B. Innovative Polymer Microspheres with Chloride Groups Synthesis, Characterization and Application for Dye Removal. Processes 2022, 10, 1568. [Google Scholar] [CrossRef]
- Matei, E.; Predescu, A.M.; Rapa, M.; Turcanu, A.; Predescu, C.; Vidu, R.; Favier, L.; Covaliu, C.I.; Ignat, D.; Grigore, V. Testing of Alginate/Chitosan/Glass Bubbles Adsorbent for Copper Removal from Wastewater. Mater. Plast. 2021, 58, 19–26. [Google Scholar] [CrossRef]
- Shikuku, V.O.; Mishra, T. Adsorption isotherm modeling for methylene blue removal onto magnetic kaolinite clay: A comparison of two-parameter isotherms. Appl. Water Sci. 2021, 11, 103. [Google Scholar] [CrossRef]









| MB | PRM | ||||||
|---|---|---|---|---|---|---|---|
| Parameters | HCWS1 | HCWS2 | HCWS3 | HCWS1 | HCWS2 | HCWS3 | |
| PFO | R2 | 0.99 | 0.93 | 0.96 | 0.781 | 0.823 | 0.942 |
| qt (mg/g) | 1.66 | 1.69 | 3.13 | 30.619 | 25.585 | 24.945 | |
| qe (mg/g) | 1.44 | 1.71 | 2.26 | 8.2 | 9.255 | 14.807 | |
| k1 (min−1) | 0.08 | 0.07 | 0.23 | 0.089 | 0.079 | 0.061 | |
| PSO | R2 | 0.93 | 0.88 | 0.99 | 0.92 | 0.95 | 0.992 |
| qt (mg/g) | 6.21 | 2.38 | 2.25 | 10.869 | 15.384 | 14.727 | |
| qe (mg/g) | 1.44 | 1.71 | 2.26 | 8.2 | 9.255 | 14.807 | |
| k2 (g/mg × min) | 10.72 | 7.76 | 3.78 | 5.056 | 4.603 | 2.138 | |
| R2 | 0.98 | 0.97 | 0.95 | 0.98 | 0.97 | 0.95 | |
| W–M | C (mg/g) | 0.35 | 0.41 | 0.89 | 5.76 | 4.75 | 4.40 |
| k3 (mg/g × min1/2) | 0.08 | 0.07 | 0.23 | 1.66 | 1.69 | 2.32 | |
| Parameters | MB | PRM | |
|---|---|---|---|
| Langmuir | R2 | 0.975 | 0.997 |
| Q0 (mg/g) | 17.006 | 15.384 | |
| b | 0.004 | 0.009 | |
| RL | 0.806–0.976 | 0.513–0.808 | |
| Freundlich | R2 | 0.999 | 0.999 |
| KF (mg−1) | 4.508 | 2.904 | |
| nF | 1.779 | 1.592 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Țurcanu, A.A.; Matei, E.; Râpă, M.; Predescu, A.M.; Berbecaru, A.-C.; Coman, G.; Predescu, C. Walnut Shell Biowaste Valorization via HTC Process for the Removal of Some Emerging Pharmaceutical Pollutants from Aqueous Solutions. Int. J. Mol. Sci. 2022, 23, 11095. https://doi.org/10.3390/ijms231911095
Țurcanu AA, Matei E, Râpă M, Predescu AM, Berbecaru A-C, Coman G, Predescu C. Walnut Shell Biowaste Valorization via HTC Process for the Removal of Some Emerging Pharmaceutical Pollutants from Aqueous Solutions. International Journal of Molecular Sciences. 2022; 23(19):11095. https://doi.org/10.3390/ijms231911095
Chicago/Turabian StyleȚurcanu, Anca Andreea, Ecaterina Matei, Maria Râpă, Andra Mihaela Predescu, Andrei-Constantin Berbecaru, George Coman, and Cristian Predescu. 2022. "Walnut Shell Biowaste Valorization via HTC Process for the Removal of Some Emerging Pharmaceutical Pollutants from Aqueous Solutions" International Journal of Molecular Sciences 23, no. 19: 11095. https://doi.org/10.3390/ijms231911095
APA StyleȚurcanu, A. A., Matei, E., Râpă, M., Predescu, A. M., Berbecaru, A. -C., Coman, G., & Predescu, C. (2022). Walnut Shell Biowaste Valorization via HTC Process for the Removal of Some Emerging Pharmaceutical Pollutants from Aqueous Solutions. International Journal of Molecular Sciences, 23(19), 11095. https://doi.org/10.3390/ijms231911095