Insulin Clearance in Obesity and Type 2 Diabetes
Abstract
:1. Introduction
1.1. Overview
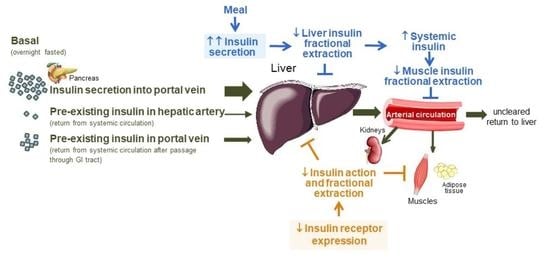
1.2. Insulin Production and Delivery to Tissues That Take Up Insulin
1.3. Cellular Mechanisms of Insulin Removal from Plasma
1.4. Assessment of Insulin Removal from Plasma In Vivo: Units of Measurement
1.5. Effect of Insulin Dose on Insulin Removal by Tissues In Vivo
1.6. Study Protocols to Evaluate Plasma Insulin Clearance and Their Clinical Relevance
2. Effects of Obesity and Type 2 Diabetes on Insulin Clearance
2.1. Effects of Obesity and T2D on Insulin Receptors and CEACAM 1
2.2. Effects of Obesity and T2D on Plasma Insulin Clearance
2.2.1. Transendothelial Insulin Transport
2.2.2. Plasma Insulin Clearance during Constant Intravenous Insulin Infusion Protocols
2.2.3. Plasma Insulin Clearance during Glucose Infusion Protocols
2.2.4. Plasma Insulin Clearance during an Oral Glucose Tolerance or Meal Test
2.2.5. Integrated Multi-Modal Modelling Assessment of Plasma Insulin Clearance
2.3. Non-Alcoholic Fatty Liver Disease and Insulin Clearance in People with Obesity
3. Summary and Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ferrannini, E. A Journey in Diabetes: From Clinical Physiology to Novel Therapeutics: The 2020 Banting Medal for Scientific Achievement Lecture. Diabetes 2021, 70, 338–346. [Google Scholar] [CrossRef]
- DeFronzo, R.A. Banting Lecture. From the triumvirate to the ominous octet: A new paradigm for the treatment of type 2 diabetes mellitus. Diabetes 2009, 58, 773–795. [Google Scholar] [CrossRef] [Green Version]
- Mittendorfer, B.; Patterson, B.W.; Smith, G.I.; Yoshino, M.; Klein, S. Beta-cell function and plasma insulin clearance in people with obesity and different glycemic status. J. Clin. Investig. 2021, e154068, online ahead of print. [Google Scholar] [CrossRef]
- Esser, N.; Utzschneider, K.M.; Kahn, S.E. Early beta cell dysfunction vs. insulin hypersecretion as the primary event in the pathogenesis of dysglycaemia. Diabetologia 2020, 63, 2007–2021. [Google Scholar] [CrossRef]
- Bergman, R.N.; Finegood, D.T.; Kahn, S.E. The evolution of beta-cell dysfunction and insulin resistance in type 2 diabetes. Eur. J. Clin. Investig. 2002, 32 (Suppl. S3), 35–45. [Google Scholar] [CrossRef]
- Czech, M.P. Insulin action and resistance in obesity and type 2 diabetes. Nat. Med. 2017, 23, 804–814. [Google Scholar] [CrossRef] [PubMed]
- Piccinini, F.; Bergman, R.N. The Measurement of Insulin Clearance. Diabetes Care 2020, 43, 2296–2302. [Google Scholar] [CrossRef]
- Gastaldelli, A.; Abdul Ghani, M.; DeFronzo, R.A. Adaptation of Insulin Clearance to Metabolic Demand Is a Key Determinant of Glucose Tolerance. Diabetes 2021, 70, 377–385. [Google Scholar] [CrossRef] [PubMed]
- Bergman, R.N.; Ader, M.; Huecking, K.; Van Citters, G. Accurate assessment of beta-cell function: The hyperbolic correction. Diabetes 2002, 51 (Suppl. S1), S212–S220. [Google Scholar] [CrossRef] [Green Version]
- Madison, L.L.; Uger, R.H.; Rencz, K. The physiologic significance of secretion of insulin into portal circulation: II. Effect of rate of administration of glucagon-free insulin on magnitude of peripheral and hepatic actions. Metabolism 1960, 9, 97–108. [Google Scholar] [PubMed]
- Samols, E.; Ryder, J.A. Studies on tissue uptake of insulin in man using a differential immunoassay for endogenous and exogenous insulin. J. Clin. Investig. 1961, 40, 2092–2102. [Google Scholar] [CrossRef] [Green Version]
- Ferrannini, E.; Wahren, J.; Faber, O.K.; Felig, P.; Binder, C.; DeFronzo, R.A. Splanchnic and renal metabolism of insulin in human subjects: A dose-response study. Am. J. Physiol. 1983, 244, E517–E527. [Google Scholar] [CrossRef]
- Eggleston, E.M.; Jahn, L.A.; Barrett, E.J. Hyperinsulinemia rapidly increases human muscle microvascular perfusion but fails to increase muscle insulin clearance: Evidence that a saturable process mediates muscle insulin uptake. Diabetes 2007, 56, 2958–2963. [Google Scholar] [CrossRef] [Green Version]
- Bratusch-Marrain, P.R.; Waldhausl, W.K.; Gasic, S.; Hofer, A. Hepatic disposal of biosynthetic human insulin and porcine C-peptide in humans. Metabolism 1984, 33, 151–157. [Google Scholar] [CrossRef]
- Chamberlain, M.J.; Stimmler, L. The renal handling of insulin. J. Clin. Investig. 1967, 46, 911–919. [Google Scholar] [CrossRef] [PubMed]
- Rabkin, R.; Simon, N.M.; Steiner, S.; Colwell, J.A. Effect of renal disease on renal uptake and excretion of insulin in man. N. Engl. J. Med. 1970, 282, 182–187. [Google Scholar] [CrossRef]
- Majumdar, S.; Genders, A.J.; Inyard, A.C.; Frison, V.; Barrett, E.J. Insulin entry into muscle involves a saturable process in the vascular endothelium. Diabetologia 2012, 55, 450–456. [Google Scholar] [CrossRef] [Green Version]
- Polidori, D.C.; Bergman, R.N.; Chung, S.T.; Sumner, A.E. Hepatic and Extrahepatic Insulin Clearance Are Differentially Regulated: Results From a Novel Model-Based Analysis of Intravenous Glucose Tolerance Data. Diabetes 2016, 65, 1556–1564. [Google Scholar] [CrossRef] [Green Version]
- Matthews, D.R.; Rudenski, A.S.; Burnett, M.A.; Darling, P.; Turner, R.C. The half-life of endogenous insulin and C-peptide in man assessed by somatostatin suppression. Clin. Endocrinol. 1985, 23, 71–79. [Google Scholar] [CrossRef]
- Sherwin, R.S.; Kramer, K.J.; Tobin, J.D.; Insel, P.A.; Liljenquist, J.E.; Berman, M.; Andres, R. A model of the kinetics of insulin in man. J. Clin. Investig. 1974, 53, 1481–1492. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Turner, R.C.; Grayburn, J.A.; Newman, G.B.; Nabarro, J.D. Measurement of the insulin delivery rate in man. J. Clin. Endocrinol. Metab. 1971, 33, 279–286. [Google Scholar] [CrossRef] [PubMed]
- Williams, R.F.; Gleason, R.E.; Soeldner, J.S. The half-life of endogenous serum immunoreactive insulin in man. Metabolism 1968, 17, 1025–1029. [Google Scholar] [CrossRef]
- Tomasi, T.; Sledz, D.; Wales, J.K.; Recant, L. Insulin half-life in normal and diabetic subjects. Rev. Neuropsychiatr. Infant 1966, 14, 315–317. [Google Scholar] [CrossRef] [PubMed]
- Silvers, A.; Swenson, R.S.; Farquhar, J.W.; Reaven, G.M. Derivation of a three compartment model describing disappearance of plasma insulin-131-I in man. J. Clin. Investig. 1969, 48, 1461–1469. [Google Scholar] [CrossRef] [Green Version]
- Lauritzen, T.; Pramming, S.; Deckert, T.; Binder, C. Pharmacokinetics of continuous subcutaneous insulin infusion. Diabetologia 1983, 24, 326–329. [Google Scholar] [CrossRef] [Green Version]
- Caumo, A.; Florea, I.; Luzi, L. Effect of a variable hepatic insulin clearance on the postprandial insulin profile: Insights from a model simulation study. Acta Diabetol. 2007, 44, 23–29. [Google Scholar] [CrossRef] [PubMed]
- van Vliet, S.; Koh, H.E.; Patterson, B.W.; Yoshino, M.; LaForest, R.; Gropler, R.J.; Klein, S.; Mittendorfer, B. Obesity Is Associated With Increased Basal and Postprandial beta-Cell Insulin Secretion Even in the Absence of Insulin Resistance. Diabetes 2020, 69, 2112–2119. [Google Scholar] [CrossRef]
- Braet, F.; Wisse, E. Structural and functional aspects of liver sinusoidal endothelial cell fenestrae: A review. Comp. Hepatol. 2002, 1, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jaldin-Fincati, J.R.; Pereira, R.V.S.; Bilan, P.J.; Klip, A. Insulin uptake and action in microvascular endothelial cells of lymphatic and blood origin. Am. J. Physiol. Endocrinol. Metab. 2018, 315, E204–E217. [Google Scholar] [CrossRef] [PubMed]
- Williams, I.M.; Valenzuela, F.A.; Kahl, S.D.; Ramkrishna, D.; Mezo, A.R.; Young, J.D.; Wells, K.S.; Wasserman, D.H. Insulin exits skeletal muscle capillaries by fluid-phase transport. J. Clin. Investig. 2018, 128, 699–714. [Google Scholar] [CrossRef] [Green Version]
- Steil, G.M.; Ader, M.; Moore, D.M.; Rebrin, K.; Bergman, R.N. Transendothelial insulin transport is not saturable in vivo. No evidence for a receptor-mediated process. J. Clin. Investig. 1996, 97, 1497–1503. [Google Scholar] [CrossRef] [Green Version]
- Navalesi, R.; Pilo, A.; Ferrannini, E. Kinetic analysis of plasma insulin disappearance in nonketotic diabetic patients and in normal subjects. A tracer study with 125I-insulin. J. Clin. Investig. 1978, 61, 197–208. [Google Scholar] [CrossRef] [PubMed]
- Bergman, R.N. Origins and History of the Minimal Model of Glucose Regulation. Front. Endocrinol. 2020, 11, 583016. [Google Scholar] [CrossRef]
- Leissring, M.A.; Gonzalez-Casimiro, C.M.; Merino, B.; Suire, C.N.; Perdomo, G. Targeting Insulin-Degrading Enzyme in Insulin Clearance. Int. J. Mol. Sci. 2021, 22, 2235. [Google Scholar] [CrossRef] [PubMed]
- Krupp, M.N.; Knutson, V.P.; Ronnett, G.V.; Lane, M.D. Use of the heavy-isotope density-shift method to investigate insulin receptor synthesis, turnover, and processing. Methods Enzymol. 1983, 96, 423–433. [Google Scholar] [CrossRef]
- Berhanu, P.; Kolterman, O.G.; Baron, A.; Tsai, P.; Olefsky, J.M.; Brandenburg, D. Insulin receptors in isolated human adipocytes. Characterization by photoaffinity labeling and evidence for internalization and cellular processing. J. Clin. Investig. 1983, 72, 1958–1970. [Google Scholar] [CrossRef] [Green Version]
- Fehlmann, M.; Carpentier, J.L.; Van Obberghen, E.; Freychet, P.; Thamm, P.; Saunders, D.; Brandenburg, D.; Orci, L. Internalized insulin receptors are recycled to the cell surface in rat hepatocytes. Proc. Natl. Acad. Sci. USA 1982, 79, 5921–5925. [Google Scholar] [CrossRef] [Green Version]
- McClain, D.A.; Olefsky, J.M. Evidence for two independent pathways of insulin-receptor internalization in hepatocytes and hepatoma cells. Diabetes 1988, 37, 806–815. [Google Scholar] [CrossRef]
- Sonne, O.; Simpson, I.A. Internalization of insulin and its receptor in the isolated rat adipose cell. Time-course and insulin concentration dependency. Biochim. Biophys. Acta 1984, 804, 404–413. [Google Scholar] [CrossRef]
- Marshall, S. Kinetics of insulin receptor internalization and recycling in adipocytes. Shunting of receptors to a degradative pathway by inhibitors of recycling. J. Biol. Chem. 1985, 260, 4136–4144. [Google Scholar] [CrossRef]
- Najjar, S.M.; Perdomo, G. Hepatic Insulin Clearance: Mechanism and Physiology. Physiology 2019, 34, 198–215. [Google Scholar] [CrossRef] [PubMed]
- Rabkin, R.; Petersen, J. Peritubular uptake and processing of insulin. Contrib. Nephrol. 1984, 42, 38–48. [Google Scholar] [CrossRef] [PubMed]
- Rubenstein, A.H.; Spitz, I. Role of the kidney in insulin metabolism and excretion. Diabetes 1968, 17, 161–169. [Google Scholar] [CrossRef]
- Knutson, V.P.; Ronnett, G.V.; Lane, M.D. Rapid, reversible internalization of cell surface insulin receptors. Correlation with insulin-induced down-regulation. J. Biol. Chem. 1983, 258, 12139–12142. [Google Scholar] [CrossRef]
- Sbraccia, P.; Wong, K.Y.; Brunetti, A.; Rafaeloff, R.; Trischitta, V.; Hawley, D.M.; Goldfine, I.D. Insulin down-regulates insulin receptor number and up-regulates insulin receptor affinity in cells expressing a tyrosine kinase-defective insulin receptor. J. Biol. Chem. 1990, 265, 4902–4907. [Google Scholar] [CrossRef]
- Ronnett, G.V.; Tennekoon, G.; Knutson, V.P.; Lane, M.D. Kinetics of insulin receptor transit to and removal from the plasma membrane. J. Biol. Chem. 1983, 258, 283–290. [Google Scholar] [CrossRef]
- Rizza, R.A.; Mandarino, L.J.; Gerich, J.E. Dose-response characteristics for effects of insulin on production and utilization of glucose in man. Am. J. Physiol. 1981, 240, E630–E639. [Google Scholar] [CrossRef] [PubMed]
- Lonnroth, P.; DiGirolamo, M.; Smith, U. Influence of ambient glucose and insulin concentrations on adipocyte insulin binding. Metabolism 1983, 32, 609–614. [Google Scholar] [CrossRef]
- Marshall, S.; Olefsky, J.M. Separate intracellular pathways for insulin receptor recycling and insulin degradation in isolated rat adipocytes. J. Cell. Physiol. 1983, 117, 195–203. [Google Scholar] [CrossRef]
- Duckworth, W.C.; Bennett, R.G.; Hamel, F.G. Insulin degradation: Progress and potential. Endocr. Rev. 1998, 19, 608–624. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Knutson, V.P.; Ronnett, G.V.; Lane, M.D. The effects of cycloheximide and chloroquine on insulin receptor metabolism. Differential effects on receptor recycling and inactivation and insulin degradation. J. Biol. Chem. 1985, 260, 14180–14188. [Google Scholar] [CrossRef]
- Bevan, A.P.; Seabright, P.J.; Tikerpae, J.; Posner, B.I.; Smith, G.D.; Siddle, K. The role of insulin dissociation from its endosomal receptor in insulin degradation. Mol. Cell. Endocrinol. 2000, 164, 145–157. [Google Scholar] [CrossRef]
- De Meyts, P.; Whittaker, J. Structural biology of insulin and IGF1 receptors: Implications for drug design. Nat. Rev. Drug Discov. 2002, 1, 769–783. [Google Scholar] [CrossRef]
- White, M.F.; Kahn, C.R. Insulin action at a molecular level—100 years of progress. Mol. Metab. 2021, 52, 101304. [Google Scholar] [CrossRef]
- Belfiore, A.; Malaguarnera, R.; Vella, V.; Lawrence, M.C.; Sciacca, L.; Frasca, F.; Morrione, A.; Vigneri, R. Insulin Receptor Isoforms in Physiology and Disease: An Updated View. Endocr. Rev. 2017, 38, 379–431. [Google Scholar] [CrossRef]
- Eriksson, J.; Lonnroth, P.; Smith, U. Insulin can rapidly increase cell surface insulin binding capacity in rat adipocytes. A novel mechanism related to insulin sensitivity. Diabetes 1992, 41, 707–714. [Google Scholar] [CrossRef]
- Crettaz, M.; Kahn, C.R. Insulin receptor regulation and desensitization in rat hepatoma cells. Concomitant changes in receptor number and in binding affinity. Diabetes 1984, 33, 477–485. [Google Scholar] [CrossRef]
- Muggeo, M.; Bar, R.S.; Roth, J. Change in affinity of insulin receptors following oral glucose in normal adults. J. Clin. Endocrinol. Metab. 1977, 44, 1206–1209. [Google Scholar] [CrossRef]
- Kalant, N.; Ozaki, S.; Maekubo, H.; Mitmaker, B.; Cohen-Khallas, M. Down-regulation of insulin binding by human and rat hepatocytes in primary culture: The possible role of insulin internalization and degradation. Endocrinology 1984, 114, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Marshall, S.; Olefsky, J.M. Effects of insulin incubation on insulin binding, glucose transport, and insulin degradation by isolated rat adipocytes. Evidence for hormone-induced desensitization at the receptor and postreceptor level. J. Clin. Investig. 1980, 66, 763–772. [Google Scholar] [CrossRef] [Green Version]
- Catalano, K.J.; Maddux, B.A.; Szary, J.; Youngren, J.F.; Goldfine, I.D.; Schaufele, F. Insulin resistance induced by hyperinsulinemia coincides with a persistent alteration at the insulin receptor tyrosine kinase domain. PLoS ONE 2014, 9, e108693. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rizza, R.A.; Mandarino, L.J.; Genest, J.; Baker, B.A.; Gerich, J.E. Production of insulin resistance by hyperinsulinaemia in man. Diabetologia 1985, 28, 70–75. [Google Scholar] [CrossRef] [PubMed]
- Insel, J.R.; Kolterman, O.G.; Saekow, M.; Olefsky, J.M. Short-term regulation of insulin receptor affinity in man. Diabetes 1980, 29, 132–139. [Google Scholar] [CrossRef]
- Mandarino, L.; Baker, B.; Rizza, R.; Genest, J.; Gerich, J. Infusion of insulin impairs human adipocyte glucose metabolism in vitro without decreasing adipocyte insulin receptor binding. Diabetologia 1984, 27, 358–363. [Google Scholar] [CrossRef] [Green Version]
- Di Guglielmo, G.M.; Drake, P.G.; Baass, P.C.; Authier, F.; Posner, B.I.; Bergeron, J.J. Insulin receptor internalization and signalling. Mol. Cell. Biochem. 1998, 182, 59–63. [Google Scholar] [CrossRef] [PubMed]
- McClain, D.A. Mechanism and role of insulin receptor endocytosis. Am. J. Med. Sci. 1992, 304, 192–201. [Google Scholar] [CrossRef]
- Hall, C.; Yu, H.; Choi, E. Insulin receptor endocytosis in the pathophysiology of insulin resistance. Exp. Mol. Med. 2020, 52, 911–920. [Google Scholar] [CrossRef] [PubMed]
- Ceresa, B.P.; Kao, A.W.; Santeler, S.R.; Pessin, J.E. Inhibition of clathrin-mediated endocytosis selectively attenuates specific insulin receptor signal transduction pathways. Mol. Cell. Biol. 1998, 18, 3862–3870. [Google Scholar] [CrossRef] [Green Version]
- Tranberg, K.G.; Thorell, J. Variation in the disappearance of unlabeled insulin from plasma: Studies with portal and peripheral infusions. Diabetes 1979, 28, 846–851. [Google Scholar] [CrossRef]
- Tillil, H.; Shapiro, E.T.; Miller, M.A.; Karrison, T.; Frank, B.H.; Galloway, J.A.; Rubenstein, A.H.; Polonsky, K.S. Dose-dependent effects of oral and intravenous glucose on insulin secretion and clearance in normal humans. Am. J. Physiol. 1988, 254, E349–E357. [Google Scholar] [CrossRef] [PubMed]
- Eaton, R.P.; Allen, R.C.; Schade, D.S. Hepatic removal of insulin in normal man: Dose response to endogenous insulin secretion. J. Clin. Endocrinol. Metab. 1983, 56, 1294–1300. [Google Scholar] [CrossRef] [PubMed]
- Sacca, L.; Orofino, G.; Petrone, A.; Vigorito, C. Direct assessment of splanchnic uptake and metabolic effects of human and porcine insulin. J. Clin. Endocrinol. Metab. 1984, 59, 191–196. [Google Scholar] [CrossRef] [PubMed]
- Waldhausl, W.; Bratusch-Marrain, P.; Gasic, S.; Korn, A.; Nowotny, P. Insulin production rate, hepatic insulin retention and splanchnic carbohydrate metabolism after oral glucose ingestion in hyperinsulinaemic Type 2 (non-insulin-dependent) diabetes mellitus. Diabetologia 1982, 23, 6–15. [Google Scholar] [CrossRef] [Green Version]
- Merovci, A.; Tripathy, D.; Chen, X.; Valdez, I.; Abdul-Ghani, M.; Solis-Herrera, C.; Gastaldelli, A.; DeFronzo, R.A. Effect of Mild Physiologic Hyperglycemia on Insulin Secretion, Insulin Clearance, and Insulin Sensitivity in Healthy Glucose-Tolerant Subjects. Diabetes 2021, 70, 204–213. [Google Scholar] [CrossRef] [PubMed]
- Nestler, J.E.; Clore, J.N.; Blackard, W.G. Reduced insulin clearance in normal subjects due to extreme hyperinsulinemia. Am. J. Med. Sci. 1988, 295, 15–22. [Google Scholar] [CrossRef]
- Polonsky, K.S.; Given, B.D.; Hirsch, L.; Shapiro, E.T.; Tillil, H.; Beebe, C.; Galloway, J.A.; Frank, B.H.; Karrison, T.; Van Cauter, E. Quantitative study of insulin secretion and clearance in normal and obese subjects. J. Clin. Investig. 1988, 81, 435–441. [Google Scholar] [CrossRef]
- Camastra, S.; Manco, M.; Mari, A.; Baldi, S.; Gastaldelli, A.; Greco, A.V.; Mingrone, G.; Ferrannini, E. beta-cell function in morbidly obese subjects during free living: Long-term effects of weight loss. Diabetes 2005, 54, 2382–2389. [Google Scholar] [CrossRef] [Green Version]
- Sohlenius-Sternbeck, A.K. Determination of the hepatocellularity number for human, dog, rabbit, rat and mouse livers from protein concentration measurements. Toxicol. In Vitro 2006, 20, 1582–1586. [Google Scholar] [CrossRef]
- Menuelle, P.; Plas, C. Relationship between insulin binding and glycogenesis in cultured fetal hepatocytes. Diabetologia 1981, 20, 647–653. [Google Scholar] [CrossRef] [Green Version]
- Pringault, E.; Plas, C. Differences in degradation processes for insulin and its receptor in cultured foetal hepatocytes. Biochem. J. 1983, 212, 529–537. [Google Scholar] [CrossRef] [Green Version]
- Simon, J.; Freychet, P.; Rosselin, G. A study of insulin binding sites in the chicken tissues. Diabetologia 1977, 13, 219–228. [Google Scholar] [CrossRef] [Green Version]
- Rogers, A.B.; Dintzis, R.Z. Hepatobiliary System. In Comparative Anatomy and Histology, 2nd ed.; Treuting, P.M., Dintzis, S.M., Montine, K.S., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 229–239. [Google Scholar]
- Bizzotto, R.; Trico, D.; Natali, A.; Gastaldelli, A.; Muscelli, E.; De Fronzo, R.A.; Arslanian, S.; Ferrannini, E.; Mari, A. New Insights on the Interactions Between Insulin Clearance and the Main Glucose Homeostasis Mechanisms. Diabetes Care 2021, 44, 2115–2123. [Google Scholar] [CrossRef] [PubMed]
- Utzschneider, K.M.; Kahn, S.E.; Polidori, D.C. Hepatic Insulin Extraction in NAFLD Is Related to Insulin Resistance Rather Than Liver Fat Content. J. Clin. Endocrinol. Metab. 2019, 104, 1855–1865. [Google Scholar] [CrossRef] [Green Version]
- Ferrannini, E.; Natali, A.; Bell, P.; Cavallo-Perin, P.; Lalic, N.; Mingrone, G. Insulin resistance and hypersecretion in obesity. European Group for the Study of Insulin Resistance (EGIR). J. Clin. Investig. 1997, 100, 1166–1173. [Google Scholar] [CrossRef]
- Flier, J.S.; Minaker, K.L.; Landsberg, L.; Young, J.B.; Pallotta, J.; Rowe, J.W. Impaired in vivo insulin clearance in patients with severe target-cell resistance to insulin. Diabetes 1982, 31, 132–135. [Google Scholar] [CrossRef]
- Gastaldelli, A.; Cusi, K.; Pettiti, M.; Hardies, J.; Miyazaki, Y.; Berria, R.; Buzzigoli, E.; Sironi, A.M.; Cersosimo, E.; Ferrannini, E.; et al. Relationship between hepatic/visceral fat and hepatic insulin resistance in nondiabetic and type 2 diabetic subjects. Gastroenterology 2007, 133, 496–506. [Google Scholar] [CrossRef] [PubMed]
- Jones, C.N.; Abbasi, F.; Carantoni, M.; Polonsky, K.S.; Reaven, G.M. Roles of insulin resistance and obesity in regulation of plasma insulin concentrations. Am. J. Physiol. Endocrinol. Metab. 2000, 278, E501–E508. [Google Scholar] [CrossRef] [PubMed]
- Jones, C.N.; Pei, D.; Staris, P.; Polonsky, K.S.; Chen, Y.D.; Reaven, G.M. Alterations in the glucose-stimulated insulin secretory dose-response curve and in insulin clearance in nondiabetic insulin-resistant individuals. J. Clin. Endocrinol. Metab. 1997, 82, 1834–1838. [Google Scholar] [CrossRef]
- Jung, S.H.; Jung, C.H.; Reaven, G.M.; Kim, S.H. Adapting to insulin resistance in obesity: Role of insulin secretion and clearance. Diabetologia 2018, 61, 681–687. [Google Scholar] [CrossRef] [Green Version]
- Kim, M.K.; Reaven, G.M.; Chen, Y.D.; Kim, E.; Kim, S.H. Hyperinsulinemia in individuals with obesity: Role of insulin clearance. Obesity 2015, 23, 2430–2434. [Google Scholar] [CrossRef] [Green Version]
- Kim, M.K.; Reaven, G.M.; Kim, S.H. Dissecting the relationship between obesity and hyperinsulinemia: Role of insulin secretion and insulin clearance. Obesity 2017, 25, 378–383. [Google Scholar] [CrossRef] [Green Version]
- Marini, M.A.; Frontoni, S.; Succurro, E.; Arturi, F.; Fiorentino, T.V.; Sciacqua, A.; Perticone, F.; Sesti, G. Differences in insulin clearance between metabolically healthy and unhealthy obese subjects. Acta Diabetol. 2014, 51, 257–261. [Google Scholar] [CrossRef]
- Peiris, A.N.; Mueller, R.A.; Smith, G.A.; Struve, M.F.; Kissebah, A.H. Splanchnic insulin metabolism in obesity. Influence of body fat distribution. J. Clin. Investig. 1986, 78, 1648–1657. [Google Scholar] [CrossRef] [Green Version]
- Smith, G.I.; Polidori, D.C.; Yoshino, M.; Kearney, M.L.; Patterson, B.W.; Mittendorfer, B.; Klein, S. Influence of adiposity, insulin resistance, and intrahepatic triglyceride content on insulin kinetics. J. Clin. Investig. 2020, 130, 3305–3314. [Google Scholar] [CrossRef] [Green Version]
- van Raalte, D.H.; van der Palen, E.; Idema, P.; Wong, L.; Keet, S.W.M.; Vlot, M.; Tukkie, R.; van Vlies, B.; Serne, E.H.; Ten Kate, R.W. Peripheral Insulin Extraction in Non-Diabetic Subjects and Type 2 Diabetes Mellitus Patients. Exp. Clin. Endocrinol. Diabetes 2020, 128, 520–527. [Google Scholar] [CrossRef] [PubMed]
- Yoshino, M.; Kayser, B.D.; Yoshino, J.; Stein, R.I.; Reeds, D.; Eagon, J.C.; Eckhouse, S.R.; Watrous, J.D.; Jain, M.; Knight, R.; et al. Effects of Diet versus Gastric Bypass on Metabolic Function in Diabetes. N. Engl. J. Med. 2020, 383, 721–732. [Google Scholar] [CrossRef] [PubMed]
- Koh, H.E.; Patterson, B.W.; Reeds, D.; Mittendorfer, B. Insulin sensitivity and insulin kinetics in African American and Non-Hispanic White people with obesity: Insights from different experimental protocols. Obesity 2022, in press. [Google Scholar] [CrossRef]
- Martinussen, C.; Bojsen-Moller, K.N.; Dirksen, C.; Jacobsen, S.H.; Jorgensen, N.B.; Kristiansen, V.B.; Holst, J.J.; Madsbad, S. Immediate enhancement of first-phase insulin secretion and unchanged glucose effectiveness in patients with type 2 diabetes after Roux-en-Y gastric bypass. Am. J. Physiol. Endocrinol. Metab. 2015, 308, E535–E544. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Piccinini, F.; Polidori, D.C.; Gower, B.A.; Bergman, R.N. Hepatic but Not Extrahepatic Insulin Clearance Is Lower in African American Than in European American Women. Diabetes 2017, 66, 2564–2570. [Google Scholar] [CrossRef] [Green Version]
- Trico, D.; Natali, A.; Arslanian, S.; Mari, A.; Ferrannini, E. Identification, pathophysiology, and clinical implications of primary insulin hypersecretion in nondiabetic adults and adolescents. JCI Insight 2018, 3, e124912. [Google Scholar] [CrossRef] [Green Version]
- Salehi, M.; Prigeon, R.L.; D’Alessio, D.A. Gastric bypass surgery enhances glucagon-like peptide 1-stimulated postprandial insulin secretion in humans. Diabetes 2011, 60, 2308–2314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Asare-Bediako, I.; Paszkiewicz, R.L.; Kim, S.P.; Woolcott, O.O.; Kolka, C.M.; Burch, M.A.; Kabir, M.; Bergman, R.N. Variability of Directly Measured First-Pass Hepatic Insulin Extraction and Its Association with Insulin Sensitivity and Plasma Insulin. Diabetes 2018, 67, 1495–1503. [Google Scholar] [CrossRef] [Green Version]
- Cavallo-Perin, P.; Bruno, A.; Scaglione, L.; Gruden, G.; Cassader, M.; Pagano, G. Feedback inhibition of insulin and glucagon secretion by insulin is altered in abdominal obesity with normal or impaired glucose tolerance. Acta Diabetol. 1993, 30, 154–158. [Google Scholar] [CrossRef]
- DeFronzo, R.A.; Binder, C.; Wahren, J.; Felig, P.; Ferrannini, E.; Faber, O.K. Sensitivity of insulin secretion to feedback inhibition by hyperinsulinaemia. Acta Endocrinol. 1981, 98, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Pincelli, A.I.; Brunani, A.; Caumo, A.; Scacchi, M.; Pasqualinotto, L.; Tibaldi, A.; Dubini, A.; Bonadonna, S.; Cavagnini, F. Hyperinsulinemia in the physiologic range is not superior to short-term fasting in suppressing insulin secretion in obese men. Metabolism 2001, 50, 107–111. [Google Scholar] [CrossRef]
- Tura, A.; Bizzotto, R.; Yamada, Y.; Seino, Y.; Pacini, G.; Ahren, B. Increased insulin clearance in mice with double deletion of glucagon-like peptide-1 and glucose-dependent insulinotropic polypeptide receptors. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2018, 314, R639–R646. [Google Scholar] [CrossRef]
- Ahren, B.; Thomaseth, K.; Pacini, G. Reduced insulin clearance contributes to the increased insulin levels after administration of glucagon-like peptide 1 in mice. Diabetologia 2005, 48, 2140–2146. [Google Scholar] [CrossRef] [Green Version]
- Mingrone, G.; Panunzi, S.; De Gaetano, A.; Ahlin, S.; Spuntarelli, V.; Bondia-Pons, I.; Barbieri, C.; Capristo, E.; Gastaldelli, A.; Nolan, J.J. Insulin sensitivity depends on the route of glucose administration. Diabetologia 2020, 63, 1382–1395. [Google Scholar] [CrossRef]
- Shah, A.; Holter, M.M.; Rimawi, F.; Mark, V.; Dutia, R.; McGinty, J.; Levin, B.; Laferrere, B. Insulin Clearance After Oral and Intravenous Glucose Following Gastric Bypass and Gastric Banding Weight Loss. Diabetes Care 2019, 42, 311–317. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guerra, S.; Gastaldelli, A. The role of the liver in the modulation of glucose and insulin in non alcoholic fatty liver disease and type 2 diabetes. Curr. Opin. Pharmacol. 2020, 55, 165–174. [Google Scholar] [CrossRef]
- Campbell, J.E.; Newgard, C.B. Mechanisms controlling pancreatic islet cell function in insulin secretion. Nat. Rev. Mol. Cell Biol. 2021, 22, 142–158. [Google Scholar] [CrossRef]
- Nauck, M.A.; Meier, J.J. The incretin effect in healthy individuals and those with type 2 diabetes: Physiology, pathophysiology, and response to therapeutic interventions. Lancet Diabetes Endocrinol. 2016, 4, 525–536. [Google Scholar] [CrossRef]
- Olefsky, J.M. LIlly lecture 1980. Insulin resistance and insulin action. An in vitro and in vivo perspective. Diabetes 1981, 30, 148–162. [Google Scholar] [CrossRef]
- Olefsky, J.M.; Ciaraldi, T.P.; Kolterman, O.G. Mechanisms of insulin resistance in non-insulin-dependent (type II) diabetes. Am. J. Med. 1985, 79, 12–22. [Google Scholar] [CrossRef]
- Kolterman, O.G.; Reaven, G.M.; Olefsky, J.M. Relationship between in vivo insulin resistance and decreased insulin receptors in obese man. J. Clin. Endocrinol. Metab. 1979, 48, 487–494. [Google Scholar] [CrossRef]
- Kolterman, O.G.; Insel, J.; Saekow, M.; Olefsky, J.M. Mechanisms of insulin resistance in human obesity: Evidence for receptor and postreceptor defects. J. Clin. Investig. 1980, 65, 1272–1284. [Google Scholar] [CrossRef] [Green Version]
- Goodyear, L.J.; Giorgino, F.; Sherman, L.A.; Carey, J.; Smith, R.J.; Dohm, G.L. Insulin receptor phosphorylation, insulin receptor substrate-1 phosphorylation, and phosphatidylinositol 3-kinase activity are decreased in intact skeletal muscle strips from obese subjects. J. Clin. Investig. 1995, 95, 2195–2204. [Google Scholar] [CrossRef]
- Smith, U. Regulation of the number of insulin receptors in human fat cells. Acta Endocrinol. Suppl. 1980, 239, 19–22. [Google Scholar]
- Caro, J.F.; Ittoop, O.; Pories, W.J.; Meelheim, D.; Flickinger, E.G.; Thomas, F.; Jenquin, M.; Silverman, J.F.; Khazanie, P.G.; Sinha, M.K. Studies on the mechanism of insulin resistance in the liver from humans with noninsulin-dependent diabetes. Insulin action and binding in isolated hepatocytes, insulin receptor structure, and kinase activity. J. Clin. Investig. 1986, 78, 249–258. [Google Scholar] [CrossRef]
- Caro, J.F.; Sinha, M.K.; Raju, S.M.; Ittoop, O.; Pories, W.J.; Flickinger, E.G.; Meelheim, D.; Dohm, G.L. Insulin receptor kinase in human skeletal muscle from obese subjects with and without noninsulin dependent diabetes. J. Clin. Investig. 1987, 79, 1330–1337. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arner, P.; Einarsson, K.; Backman, L.; Nilsell, K.; Lerea, K.M.; Livingston, J.N. Studies of liver insulin receptors in non-obese and obese human subjects. J. Clin. Investig. 1983, 72, 1729–1736. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kolterman, O.G.; Gray, R.S.; Griffin, J.; Burstein, P.; Insel, J.; Scarlett, J.A.; Olefsky, J.M. Receptor and postreceptor defects contribute to the insulin resistance in noninsulin-dependent diabetes mellitus. J. Clin. Investig. 1981, 68, 957–969. [Google Scholar] [CrossRef] [Green Version]
- Harrison, L.C.; Martin, F.I.; Melick, R.A. Correlation between insulin receptor binding in isolated fat cells and insulin sensitivity in obese human subjects. J. Clin. Investig. 1976, 58, 1435–1441. [Google Scholar] [CrossRef] [PubMed]
- Borissova, A.M.; Tankova, T.I.; Koev, D.J. Insulin secretion, peripheral insulin sensitivity and insulin-receptor binding in subjects with different degrees of obesity. Diabetes Metab. 2004, 30, 425–431. [Google Scholar] [CrossRef]
- Wigand, J.P.; Blackard, W.G. Downregulation of insulin receptors in obese man. Diabetes 1979, 28, 287–291. [Google Scholar] [CrossRef] [PubMed]
- Bar, R.S.; Gorden, P.; Roth, J.; Siebert, C.W. Insulin receptors in patients with insulinomas: Changes in receptor affinity and concentration. J. Clin. Endocrinol. Metab. 1977, 44, 1210–1213. [Google Scholar] [CrossRef]
- Bar, R.S.; Gorden, P.; Roth, J.; Kahn, C.R.; De Meyts, P. Fluctuations in the affinity and concentration of insulin receptors on circulating monocytes of obese patients: Effects of starvation, refeeding, and dieting. J. Clin. Investig. 1976, 58, 1123–1135. [Google Scholar] [CrossRef] [PubMed]
- Beck-Nielsen, H.; Pedersen, O.; Bagger, J.P.; Sorensen, N.S. The insulin receptor in normal and obese persons. Acta Endocrinol. 1976, 83, 565–575. [Google Scholar] [CrossRef] [PubMed]
- McElduff, A.; Hedo, J.A.; Taylor, S.I.; Roth, J.; Gorden, P. Insulin receptor degradation is accelerated in cultured lymphocytes from patients with genetic syndromes of extreme insulin resistance. J. Clin. Investig. 1984, 74, 1366–1374. [Google Scholar] [CrossRef]
- Kasuga, M.; Kahn, C.R.; Hedo, J.A.; Van Obberghen, E.; Yamada, K.M. Insulin-induced receptor loss in cultured human lymphocytes is due to accelerated receptor degradation. Proc. Natl. Acad. Sci. USA 1981, 78, 6917–6921. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arner, P.; Einarsson, K.; Ewerth, S.; Livingston, J. Studies of the human liver insulin receptor in noninsulin-dependent diabetes mellitus. J. Clin. Investig. 1986, 77, 1716–1718. [Google Scholar] [CrossRef] [Green Version]
- Prince, M.J.; Tsai, P.; Olefsky, J.M. Insulin binding, internalization, and insulin receptor regulation in fibroblasts from type II, non-insulin-dependent diabetic subjects. Diabetes 1981, 30, 596–600. [Google Scholar] [CrossRef]
- Heinrich, G.; Muturi, H.T.; Rezaei, K.; Al-Share, Q.Y.; DeAngelis, A.M.; Bowman, T.A.; Ghadieh, H.E.; Ghanem, S.S.; Zhang, D.; Garofalo, R.S.; et al. Reduced Hepatic Carcinoembryonic Antigen-Related Cell Adhesion Molecule 1 Level in Obesity. Front. Endocrinol. 2017, 8, 54. [Google Scholar] [CrossRef] [PubMed]
- Lee, W. The CEACAM1 expression is decreased in the liver of severely obese patients with or without diabetes. Diagn. Pathol. 2011, 6, 40. [Google Scholar] [CrossRef] [Green Version]
- Poy, M.N.; Yang, Y.; Rezaei, K.; Fernstrom, M.A.; Lee, A.D.; Kido, Y.; Erickson, S.K.; Najjar, S.M. CEACAM1 regulates insulin clearance in liver. Nat. Genet. 2002, 30, 270–276. [Google Scholar] [CrossRef]
- DeAngelis, A.M.; Heinrich, G.; Dai, T.; Bowman, T.A.; Patel, P.R.; Lee, S.J.; Hong, E.G.; Jung, D.Y.; Assmann, A.; Kulkarni, R.N.; et al. Carcinoembryonic antigen-related cell adhesion molecule 1: A link between insulin and lipid metabolism. Diabetes 2008, 57, 2296–2303. [Google Scholar] [CrossRef] [Green Version]
- Al-Share, Q.Y.; DeAngelis, A.M.; Lester, S.G.; Bowman, T.A.; Ramakrishnan, S.K.; Abdallah, S.L.; Russo, L.; Patel, P.R.; Kaw, M.K.; Raphael, C.K.; et al. Forced Hepatic Overexpression of CEACAM1 Curtails Diet-Induced Insulin Resistance. Diabetes 2015, 64, 2780–2790. [Google Scholar] [CrossRef] [Green Version]
- Smith, G.I.; Mittendorfer, B.; Klein, S. Metabolically healthy obesity: Facts and fantasies. J. Clin. Investig. 2019, 129, 3978–3989. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bergman, R.N.; Piccinini, F.; Kabir, M.; Kolka, C.M.; Ader, M. Hypothesis: Role of Reduced Hepatic Insulin Clearance in the Pathogenesis of Type 2 Diabetes. Diabetes 2019, 68, 1709–1716. [Google Scholar] [CrossRef] [PubMed]
- Bojsen-Moller, K.N.; Lundsgaard, A.M.; Madsbad, S.; Kiens, B.; Holst, J.J. Hepatic Insulin Clearance in Regulation of Systemic Insulin Concentrations-Role of Carbohydrate and Energy Availability. Diabetes 2018, 67, 2129–2136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Douros, J.D.; Tong, J.; D’Alessio, D.A. The Effects of Bariatric Surgery on Islet Function, Insulin Secretion, and Glucose Control. Endocr. Rev. 2019, 40, 1394–1423. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.H.; Reaven, G.M. Insulin clearance: An underappreciated modulator of plasma insulin concentration. J. Investig. Med. 2016, 64, 1162–1165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bonora, E.; Zavaroni, I.; Coscelli, C.; Butturini, U. Decreased hepatic insulin extraction in subjects with mild glucose intolerance. Metabolism 1983, 32, 438–446. [Google Scholar] [CrossRef]
- Consortium, R. Metabolic Contrasts Between Youth and Adults With Impaired Glucose Tolerance or Recently Diagnosed Type 2 Diabetes: I. Observations Using the Hyperglycemic Clamp. Diabetes Care 2018, 41, 1696–1706. [Google Scholar] [CrossRef] [Green Version]
- Faber, O.K.; Christensen, K.; Kehlet, H.; Madsbad, S.; Binder, C. Decreased insulin removal contributes to hyperinsulinemia in obesity. J. Clin. Endocrinol. Metab. 1981, 53, 618–621. [Google Scholar] [CrossRef]
- Lorenzo, C.; Hanley, A.J.; Wagenknecht, L.E.; Rewers, M.J.; Stefanovski, D.; Goodarzi, M.O.; Haffner, S.M. Relationship of insulin sensitivity, insulin secretion, and adiposity with insulin clearance in a multiethnic population: The insulin Resistance Atherosclerosis study. Diabetes Care 2013, 36, 101–103. [Google Scholar] [CrossRef] [Green Version]
- Meistas, M.T.; Margolis, S.; Kowarski, A.A. Hyperinsulinemia of obesity is due to decreased clearance of insulin. Am. J. Physiol. 1983, 245, E155–E159. [Google Scholar] [CrossRef] [PubMed]
- Robertson, D.A.; Singh, B.M.; Hale, P.J.; Nattrass, M. Effects of morbid obesity on insulin clearance and insulin sensitivity in several aspects of metabolism as assessed by low-dose insulin infusion. Metabolism 1992, 41, 604–612. [Google Scholar] [CrossRef]
- Davidson, M.B.; Harris, M.D.; Rosenberg, C.S. Inverse relationship of metabolic clearance rate of insulin to body mass index. Metabolism 1987, 36, 219–222. [Google Scholar] [CrossRef]
- Zuniga-Guajardo, S.; Jimenez, J.; Angel, A.; Zinman, B. Effects of massive obesity on insulin sensitivity and insulin clearance and the metabolic response to insulin as assessed by the euglycemic clamp technique. Metabolism 1986, 35, 278–282. [Google Scholar] [CrossRef]
- Polonsky, K.; Frank, B.; Pugh, W.; Addis, A.; Karrison, T.; Meier, P.; Tager, H.; Rubenstein, A. The limitations to and valid use of C-peptide as a marker of the secretion of insulin. Diabetes 1986, 35, 379–386. [Google Scholar] [CrossRef]
- Jansson, P.A.; Fowelin, J.P.; von Schenck, H.P.; Smith, U.P.; Lonnroth, P.N. Measurement by microdialysis of the insulin concentration in subcutaneous interstitial fluid. Importance of the endothelial barrier for insulin. Diabetes 1993, 42, 1469–1473. [Google Scholar] [CrossRef]
- Sjostrand, M.; Gudbjornsdottir, S.; Holmang, A.; Lonn, L.; Strindberg, L.; Lonnroth, P. Delayed transcapillary transport of insulin to muscle interstitial fluid in obese subjects. Diabetes 2002, 51, 2742–2748. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barrett, E.J.; Wang, H.; Upchurch, C.T.; Liu, Z. Insulin regulates its own delivery to skeletal muscle by feed-forward actions on the vasculature. Am. J. Physiol. Endocrinol. Metab. 2011, 301, E252–E263. [Google Scholar] [CrossRef] [Green Version]
- Paavonsalo, S.; Hariharan, S.; Lackman, M.H.; Karaman, S. Capillary Rarefaction in Obesity and Metabolic Diseases-Organ-Specificity and Possible Mechanisms. Cells 2020, 9, 2683. [Google Scholar] [CrossRef] [PubMed]
- Clark, M.G. Impaired microvascular perfusion: A consequence of vascular dysfunction and a potential cause of insulin resistance in muscle. Am. J. Physiol. Endocrinol. Metab. 2008, 295, E732–E750. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Love, K.M.; Liu, J.; Regensteiner, J.G.; Reusch, J.E.B.; Liu, Z. GLP-1 and insulin regulation of skeletal and cardiac muscle microvascular perfusion in type 2 diabetes. J. Diabetes 2020, 12, 488–498. [Google Scholar] [CrossRef]
- Williams, I.M.; McClatchey, P.M.; Bracy, D.P.; Bonner, J.S.; Valenzuela, F.A.; Wasserman, D.H. Transendothelial Insulin Transport is Impaired in Skeletal Muscle Capillaries of Obese Male Mice. Obesity 2020, 28, 303–314. [Google Scholar] [CrossRef] [Green Version]
- Broussard, J.L.; Castro, A.V.; Iyer, M.; Paszkiewicz, R.L.; Bediako, I.A.; Szczepaniak, L.S.; Szczepaniak, E.W.; Bergman, R.N.; Kolka, C.M. Insulin access to skeletal muscle is impaired during the early stages of diet-induced obesity. Obesity 2016, 24, 1922–1928. [Google Scholar] [CrossRef] [Green Version]
- Sandqvist, M.; Strindberg, L.; Schmelz, M.; Lonnroth, P.; Jansson, P.A. Impaired delivery of insulin to adipose tissue and skeletal muscle in obese women with postprandial hyperglycemia. J. Clin. Endocrinol. Metab. 2011, 96, E1320–E1324. [Google Scholar] [CrossRef] [Green Version]
- Bouskela, E.; Bottino, D.A.; Tavares, J.C. Microvascular Permeability in Diabetes. In Molecular Basis for Microcirculatory Disorders; Springer: Paris, France, 2003. [Google Scholar]
- Rask-Madsen, C.; King, G.L. Vascular complications of diabetes: Mechanisms of injury and protective factors. Cell Metab. 2013, 17, 20–33. [Google Scholar] [CrossRef] [Green Version]
- Solis-Herrera, C.; Triplitt, C.; Cersosimo, E.; DeFronzo, R.A. Pathogenesis of Type 2 Diabetes Mellitus. In Endotext; Feingold, K.R., Anawalt, B., Boyce, A., Chrousos, G., de Herder, W.W., Dhatariya, K., Dungan, K., Grossman, A., Hershman, J.M., Hofland, J., et al., Eds.; MDText.com, Inc.: South Dartmouth, MA, USA, 2000. [Google Scholar]
- Wang, X.; Hansen, B.C.; Shi, D.; Fang, Y.; Du, F.; Wang, B.; Chen, Y.M.; Gregoire, F.M.; Wang, Y.X. Quantification of beta-cell insulin secretory function using a graded glucose infusion with C-peptide deconvolution in dysmetabolic, and diabetic cynomolgus monkeys. Diabetol. Metab. Syndr. 2013, 5, 40. [Google Scholar] [CrossRef] [Green Version]
- Erdmann, J.; Pohnl, K.; Mayr, M.; Sypchenko, O.; Naumann, A.; Wagenpfeil, S.; Schusdziarra, V. Disturbances of basal and postprandial insulin secretion and clearance in obese patients with type 2 diabetes mellitus. Horm. Metab. Res. 2012, 44, 60–69. [Google Scholar] [CrossRef] [PubMed]
- Erdmann, J.; Kallabis, B.; Oppel, U.; Sypchenko, O.; Wagenpfeil, S.; Schusdziarra, V. Development of hyperinsulinemia and insulin resistance during the early stage of weight gain. Am. J. Physiol. Endocrinol. Metab. 2008, 294, E568–E575. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, G.I.; Shankaran, M.; Yoshino, M.; Schweitzer, G.G.; Chondronikola, M.; Beals, J.W.; Okunade, A.L.; Patterson, B.W.; Nyangau, E.; Field, T.; et al. Insulin resistance drives hepatic de novo lipogenesis in nonalcoholic fatty liver disease. J. Clin. Investig. 2020, 130, 1453–1460. [Google Scholar] [CrossRef] [PubMed]
- Bril, F.; Barb, D.; Portillo-Sanchez, P.; Biernacki, D.; Lomonaco, R.; Suman, A.; Weber, M.H.; Budd, J.T.; Lupi, M.E.; Cusi, K. Metabolic and histological implications of intrahepatic triglyceride content in nonalcoholic fatty liver disease. Hepatology 2017, 65, 1132–1144. [Google Scholar] [CrossRef] [Green Version]
- Bril, F.; Lomonaco, R.; Orsak, B.; Ortiz-Lopez, C.; Webb, A.; Tio, F.; Hecht, J.; Cusi, K. Relationship between disease severity, hyperinsulinemia, and impaired insulin clearance in patients with nonalcoholic steatohepatitis. Hepatology 2014, 59, 2178–2187. [Google Scholar] [CrossRef]
- Kotronen, A.; Juurinen, L.; Tiikkainen, M.; Vehkavaara, S.; Yki-Jarvinen, H. Increased liver fat, impaired insulin clearance, and hepatic and adipose tissue insulin resistance in type 2 diabetes. Gastroenterology 2008, 135, 122–130. [Google Scholar] [CrossRef]
- Lamprinou, A.; Willmann, C.; Machann, J.; Schick, F.; Eckstein, S.S.; Dalla Man, C.; Visentin, R.; Birkenfeld, A.L.; Peter, A.; Stefan, N.; et al. Determinants of hepatic insulin clearance—Results from a Mendelian Randomization study. Metabolism 2021, 119, 154776. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koh, H.-C.E.; Cao, C.; Mittendorfer, B. Insulin Clearance in Obesity and Type 2 Diabetes. Int. J. Mol. Sci. 2022, 23, 596. https://doi.org/10.3390/ijms23020596
Koh H-CE, Cao C, Mittendorfer B. Insulin Clearance in Obesity and Type 2 Diabetes. International Journal of Molecular Sciences. 2022; 23(2):596. https://doi.org/10.3390/ijms23020596
Chicago/Turabian StyleKoh, Han-Chow E., Chao Cao, and Bettina Mittendorfer. 2022. "Insulin Clearance in Obesity and Type 2 Diabetes" International Journal of Molecular Sciences 23, no. 2: 596. https://doi.org/10.3390/ijms23020596
APA StyleKoh, H. -C. E., Cao, C., & Mittendorfer, B. (2022). Insulin Clearance in Obesity and Type 2 Diabetes. International Journal of Molecular Sciences, 23(2), 596. https://doi.org/10.3390/ijms23020596