Fabrication of CaCO3-Coated Vesicles by Biomineralization and Their Application as Carriers of Drug Delivery Systems
Abstract
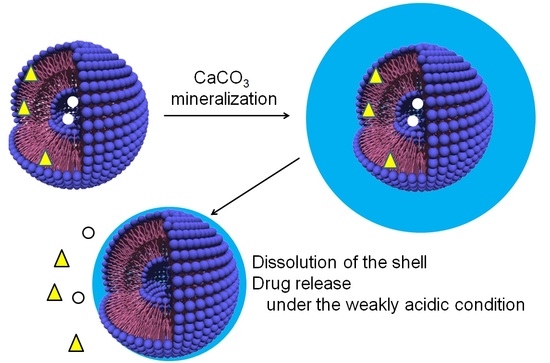
:1. Introduction
2. Results and Discussion
2.1. Structure of CaCO3-Coated Vesicles Fabricated by the Peptide Lipids Induced Minerarlzation
2.1.1. CaCO3-Coating of the Vesicle Surface
2.1.2. Structure of CaCO3-Coated Vesicles
2.2. Drug Release Properties of CaCO3-Coated Vesicles
2.2.1. Dissolution Behaviors of CaCO3 Shells
2.2.2. Hydrophilic and Hydrophobic Drug Release Properties
3. Materials and Methods
3.1. Materials
3.1.1. Peptide Lipid
3.1.2. Vesicles
3.1.3. CaCO3 Coating on the Vesicle Surface by Mineralization
3.2. Methods
3.2.1. Transmission Electron Microscopic Observations
3.2.2. Dynamic Light Scattering Measurements
3.2.3. X-ray Diffraction Measurements
3.2.4. Dissolution Behaviors of Shells on the CaCO3-Coated Vesicles
3.2.5. Drug Release Experiments
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A


| Experiment | Standard (Calcite) | ||||
|---|---|---|---|---|---|
| No. | Crystal Face | 2θ/deg | Intensity | 2θ/deg | Intensity |
| 1 | (0 1 2) | 23.02 | 7.25 | 23.07 | 8.10 |
| 2 | (1 0 4) | 29.36 | 100 | 29.42 | 100 |
| 3 | (0 0 6) | 31.42 | 2.19 | 31.46 | 2.43 |
| 4 | (1 1 0) | 35.96 | 16.9 | 36.00 | 13.7 |
| 5 | (1 1 3) | 39.6 | 0.915 | 39.44 | 20.2 |
| 6 | (2 0 2) | 43.14 | 21.1 | 43.19 | 14.3 |
| 7 | (0 2 4) | 47.44 | 22.3 | 47.15 | 6.16 |
| 8 | (0 1 8) | 47.54 | 7.99 | 47.54 | 19.7 |
| 9 | (1 1 6) | 48.46 | 24.1 | 48.54 | 20.0 |
| 10 | (2 1 1) | 56.56 | 4.93 | 56.61 | 3.73 |
| 11 | (1 2 2) | 57.38 | 11.4 | 57.44 | 9.18 |
| 12 | (2 1 4) | 60.64 | 7.63 | 60.72 | 5.28 |
| 13 | (2 0 8) | 60.94 | 3.08 | 61.05 | 2.41 |
| 14 | (1 1 9) | 61.28 | 2.41 | 61.43 | 3.18 |
| 15 | (1 2 5) | 63.00 | 2.38 | 63.10 | 2.29 |
| 16 | (3 0 0) | 64.64 | 7.71 | 64.71 | 6.84 |
| 17 | (0 0 12) | 65.54 | 5.33 | 65.67 | 3.91 |
| 18 | (2 1 7) | 69.12 | 1.95 | 69.24 | 1.51 |
| 19 | (0 2 10) | 70.20 | 1.79 | 70.30 | 2.13 |
| 20 | (1 2 8) | 72.84 | 3.60 | 72.95 | 2.69 |
| 21 | (1 1 12) | 77.02 | 2.72 | 77.23 | 1.93 |
| 22 | (2 1 10) | 81.44 | 2.57 | 81.60 | 2.24 |
| 23 | (1 3 4) | 83.74 | 5.25 | 83.84 | 1.57 |
References
- Lee, Y.T.; Tan, Y.J.; Oon, C.E. Molecular targeted therapy: Treating cancer with specificity drugs. Eur. J. Pharmacol. 2018, 834, 188–196. [Google Scholar] [CrossRef] [PubMed]
- Kimura, M.; Yasui, F.; Usami, E.; Kawachi, S.; Iwai, M.; Go, M.; Ikeda, Y.; Yoshimura, T. Cost-effectiveness and safety of the molecular targeted drugs afatinib, gefitinib and erlotinib as first-line treatments for patients with advanced EGFR mutation-postive non-small cell lung cancer. Mol. Clin. Oncol. 2018, 9, 201–206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Delaunay, M.; Cadranel, J.; Lusque, A.; Meyer, N.; Gounant, V.; Moro-Sibilot, D.; Michot, J.-M.; Raimboug, J.; Girard, N.; Guisier, F.; et al. Immune-checkpoint inhibitors associated with interstitial lung disease in cancer patients. Eur. Respir. J. 2017, 50, 1700050. [Google Scholar] [CrossRef] [PubMed]
- Yamagata, A.; Yokoyama, T.; Fukuda, Y.; Ishida, T. Impact of interstitial lung disease associated with immune checkpoint inhibitors on prognosis in patients with non-small-cell lung cancer. Cancer Chemother. Pharamacol. 2021, 87, 251–258. [Google Scholar] [CrossRef] [PubMed]
- Misha, D.; Kang, H.C.; Bae, Y.H. Reconstitutable charged polymeric (PLGA)(2)-b-PEI micelles for gene therapeutics delivery. Biomaterials 2011, 32, 3845–3854. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yokoyama, M. Polymeric micelles as drug carriers: Their light and shadows. J. Drug Target. 2014, 22, 576–583. [Google Scholar] [CrossRef] [PubMed]
- Morgan, M.T.; Carnahan, M.A.; Finkelstein, S.; Prata, C.A.H.; Degoricijia, L.; Lee, S.J.; Grinstaff, M.W. Dendric supramolecular assemblies for drug delivery. Chem. Commun. 2005, 34, 4309–4311. [Google Scholar] [CrossRef]
- Tomas, T.P.; Joice, M.; Sumit, M.; Silpe, J.E.; Kotlyar, A.; Bharathi, S.; Kukowska-Latallo, J.; Baker, J.R.; Choi, S.K. Design and in vitro validation of multivalent dendrimer methotrexates as a folate-targeting anticancer therapeutic. Curr. Pharm. Des. 2013, 19, 6594–6605. [Google Scholar] [CrossRef] [Green Version]
- Perli, G.; Wang, Q.; Braga, C.B.; Bertuzzi, D.L.; Fontana, L.A.; Soares, M.C.P.; Ruiz, J.; Megiatt, J.D., Jr.; Astruc, D.; Ornelas, C. Self-assembly of a triazolylferrocenyl dendrimer in water yields nontraditional intrinsic green fluorescent vesosomes for nanotheranostic applications. J. Am. Chem. Soc. 2021, 143, 12948–12954. [Google Scholar] [CrossRef] [PubMed]
- Shang, S.; Huang, S.; Zhang, A.; Feng, J.; Yang, S. The binary complex of poly(PEGMA-co-MAA)hydrogel and PLGA nanoparticles as a novel oral drug delivery system for ibuprofen delivery. J. Biomater. Sci. Polym. Ed. 2017, 28, 1874–1887. [Google Scholar] [CrossRef]
- Murai, K.; Kurumisawa, K.; Nomura, Y.; Matsumoto, M. Regulated drug release abilities of calcium carbonate-gelatin hybrid nanocarriers fabricated via a self-organizational process. ChemMedChem 2017, 12, 1595–1599. [Google Scholar] [CrossRef]
- Chander, S.; Kulkarni, G.; Dhiman, N.; Kharkwai, H. Protein-based nanohydrogels for bioactive delivery. Front. Chem. 2021, 9, 573748. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Tachibana, T.; Yamana, K.; Kawasaki, R.; Yabuki, A. Simple formation of cancer drug-containing self-assembled hydrogels with temperature and pH-responsive release. Langmuir 2021, 37, 11269–11275. [Google Scholar] [CrossRef]
- Murai, K.; Higuchi, M.; Kinoshita, T.; Nagata, K.; Kato, K. Design of the nanocarrier having the regulated durug release ability utilizing a reversible conformational transition of peptide responsed to slight pH changes. Phys. Chem. Chem. Phys. 2013, 15, 11454–11460. [Google Scholar] [CrossRef] [PubMed]
- Beagan, A.M.; Alghamdi, A.A.; Lahmadi, S.S.; Halwani, M.A.; Almeataq, M.S.; Alhazaa, A.N.; Alotaibi, K.M.; Alswieleh, A.M. Folic acid-terminated poly(2-diethyl amino ethyl methacrylate) brush-gated magnetic mesoporous nanoparticles as a smart drug delivery system. Polymers 2021, 13, 59. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Edo, G.; Xue, E.Y.; Ha, S.Y.Y.; Pontón, I.; González-Delgado, J.A.; Borrós, S.; Torres, T.; Ng, D.K.P.; Sanchez-Garcia, D. Nanoparticles for triple drug release for combined chemo- and photodynamic therapy. Chem.-A Eur. J. 2021, 27, 14610–14618. [Google Scholar] [CrossRef]
- Arai, M.; Miura, T.; Ito, Y.; Kinoshita, T.; Higuchi, M. Protein sensing device with multi-recognition ability composed of self-organized glycopeptide bundle. Int. J. Mol. Sci. 2021, 22, 366. [Google Scholar] [CrossRef]
- Sato, Y.; Asawa, K.; Hung, T.; Noiri, M.; Nakamura, N.; Ekdahl, K.N.; Nilson, B.; Ishihara, K.; Teramura, Y. Induction of spontaneous liposome adsorption by exogenous surface modification with cell-penetrating peptide-conjugated lipids. Langmuir 2021, 37, 9711–9723. [Google Scholar] [CrossRef]
- Maruyama, K.; Ishida, O.; Kasaoka, S.; Takizawa, T.; Utoguchi, N.; Shinohara, A.; Chiba, M.; Kobayashi, H.; Eriguchi, M.; Yanagie, H. Intercellular targeting of sodium mercaptoundecahydrododecaborate (BSH) to solid tumors by transferrin-PEG liposomes, for boron neutron-capture therapy (BNCT). J. Control. Release 2004, 98, 195–207. [Google Scholar] [CrossRef]
- Joanitti, G.A.; Sawant, R.S.; Torchilin, V.P.; Freitas, S.M.; Azevedo, R.B. Optimizing liposome for delivery of Bowman-Birk protease inhibitors—Platforms for multiple biomedical applications. Colloids Surf. B Biointerfaces 2018, 167, 474–482. [Google Scholar] [CrossRef]
- Norouzi, M.; Amerian, M.; Amerian, M.; Atyabi, F. Clinical applications of nanomedicine in cancer therapy. Drug Discov. Today 2020, 25, 107–125. [Google Scholar] [CrossRef] [PubMed]
- Matsumura, Y.; Maeda, H. A new concept for macromolecular therapeutics in cancer chemotherapy: Mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs. Cancer Res. 1986, 46 Pt 1, 6387–6392. [Google Scholar]
- Allen, T.M.; Cullis, P.R. Drug delivery systems: Entering the mainstream. Science 2004, 303, 1818–1822. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, H.; Rho, S.; Stiles, W.R.; Hu, S.; Baek, Y.; Hwang, D.W.; Kashiwagi, S.; Kim, M.S.; Choi, H.S. Size-dependent EPR effect of polymeric nanoparticles on tumor targeting. Adv. Healthc. Mater. 2020, 9, 1901203. [Google Scholar] [CrossRef]
- Swietach, P.; Vaughan-Jones, R.D.; Harris, A.L.; Hulikova, A. The chemistry, physiology and pathology of pH in cancer. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2014, 369, 20130099. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ward, C.; Meehan, J.; Gray, M.E.; Murray, A.F.; Argyle, D.J.; Kunkler, I.H.; Langdon, S.P. The impact of tumor pH on cancer progression: Strategies for clinical intervention. Explor. Target. Anti-tumor Ther. 2020, 1, 71–100. [Google Scholar] [CrossRef] [Green Version]
- Koltai, T. The pH paradigm in cancer. Eur. J. Clin. Nutr. 2020, 74, 14–19. [Google Scholar] [CrossRef]
- Murai, K.; Higuchi, M.; Kinoshita, T.; Nagata, K.; Kato, K. Calcium carbonate biomineralization utilizing a multifunctional β-sheet peptide template. Chem. Commun. 2013, 49, 9947–9949. [Google Scholar] [CrossRef]
- Murai, K.; Kinoshita, T.; Nagata, K.; Higuchi, M. Mineralization of calcium carbonate on multifunctional peptide assembly actiong as mineral source supplier and template. Langmuir 2016, 32, 9351–9359. [Google Scholar] [CrossRef]
- Bringer, W.S.; Chao, T.L. Is the serine protease “charge relay system” a charge relay system? Biochem. Biophys. Res. Commun. 1975, 63, 78–83. [Google Scholar] [CrossRef]
- Matsuki, H.; Kaneshina, S. Thermodynamics of bilayer phase transitions of phospholipids. Netsu Sokutei 2006, 33, 74–82. [Google Scholar]
- Plummer, L.N.; Busenberg, E. The solubilites of calcite, aragonite and vaterite in CO2-H2O solutions between 0 and 90°C, and an evaluation of the aqueous model for the system CaCO3-CO2-H2O. Geochim. Cosmochim. Acta 1982, 46, 1011–1040. [Google Scholar] [CrossRef]
- Graft, D.L. Crystallographic tables for the rhombohedral carbonates. Am. Mineral. 1961, 46, 1283–1316. [Google Scholar]
- De Villiers, J.R.P. Crystal structure of aragonite, strontianite, and witherite Am. Mineral. 1971, 56, 758–767. [Google Scholar]
- Wang, J.; Becker, U. Structure and carbonate orientation of vaterite (CaCO3). Am. Mineral. 2009, 94, 380–386. [Google Scholar] [CrossRef]
- Miyoshi, N.; Hara, K.; Kimura, S.; Nakanishi, K.; Fukuda, M. A New Method of Determining Intercellular Free Ca2+ Concentration Using Quin 2-fluorescence, Photochem. Photobiol. 1991, 53, 415–418. [Google Scholar] [CrossRef] [PubMed]
- Carpino, L.A.; Han, G.Y. 9-Fluorenymethoxycarbonyl amino-protecting group. J. Org. Chem. 1972, 37, 3404–3409. [Google Scholar] [CrossRef]









Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miyamaru, C.; Koide, M.; Kato, N.; Matsubara, S.; Higuchi, M. Fabrication of CaCO3-Coated Vesicles by Biomineralization and Their Application as Carriers of Drug Delivery Systems. Int. J. Mol. Sci. 2022, 23, 789. https://doi.org/10.3390/ijms23020789
Miyamaru C, Koide M, Kato N, Matsubara S, Higuchi M. Fabrication of CaCO3-Coated Vesicles by Biomineralization and Their Application as Carriers of Drug Delivery Systems. International Journal of Molecular Sciences. 2022; 23(2):789. https://doi.org/10.3390/ijms23020789
Chicago/Turabian StyleMiyamaru, Chiho, Mao Koide, Nana Kato, Shogo Matsubara, and Masahiro Higuchi. 2022. "Fabrication of CaCO3-Coated Vesicles by Biomineralization and Their Application as Carriers of Drug Delivery Systems" International Journal of Molecular Sciences 23, no. 2: 789. https://doi.org/10.3390/ijms23020789
APA StyleMiyamaru, C., Koide, M., Kato, N., Matsubara, S., & Higuchi, M. (2022). Fabrication of CaCO3-Coated Vesicles by Biomineralization and Their Application as Carriers of Drug Delivery Systems. International Journal of Molecular Sciences, 23(2), 789. https://doi.org/10.3390/ijms23020789