Pharmacological and Therapeutic Applications of Esculetin
Abstract
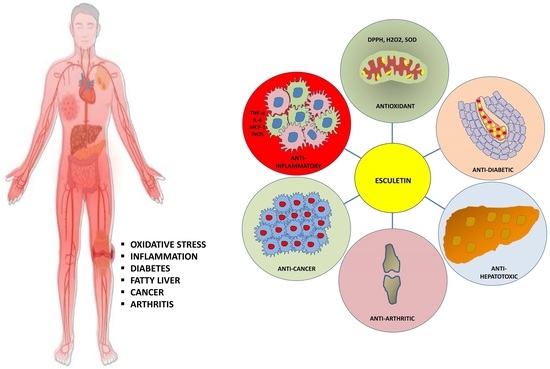
:1. Introduction
2. Therapeutic Applications of Esculetin
2.1. The Role of Esculetin in Cancer Treatment
2.2. The Role of Esculetin in Oxidative Stress Treatment
2.3. The Role of Esculetin in Inflammation Treatment
2.4. The Role of Esculetin in Arthritis Treatment
2.5. The Role of Esculetin in Diabetes Treatment and Its Associated Complication
2.6. The Role of Esculetin in Hepatic Failure Treatment
3. Synthesis of Esculetin
4. Detection, Pharmacokinetic and Metabolic Studies of Esculetin
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Vuolo, M.M.; Lima, V.S.; Junior, M.R.M. Chapter 2—Phenolic Compounds: Structure, Classification, and Antioxidant Power. In Bioactive Compounds: Health Benefits and Potential Applications; Woodhead Publishing: Sawston, UK, 2019; pp. 33–50. [Google Scholar]
- Garg, S.S.; Gupta, J.; Sharma, S.; Sahu, D. An insight into the therapeutic applications of coumarin compounds and their mechanism of action. Eur. J. Pharm. Sci. 2020, 152, 105424. [Google Scholar] [CrossRef] [PubMed]
- Aykin-Burns, N.; Ahmad, I.M.; Zhu, Y.; Oberley, L.W.; Spitz, D.R. Increased levels of superoxide and H2O2 mediate the differential susceptibility of cancer cells versus normal cells to glucose deprivation. Biochem. J. 2009, 418, 29–37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, A.; Misra, V.; Thimmulappa, R.K.; Lee, H.; Ames, S.; Hoque, M.O.; Herman, J.G.; Baylin, S.B.; Sidransky, D.; Gabrielson, E.; et al. Dysfunctional KEAP1-NRF2 interaction in non-small-cell lung cancer. PLoS Med. 2006, 3, e420. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kotamraju, S.; Chitambar, C.R.; Kalivendi, S.V.; Joseph, J.; Kalyanaraman, B. Transferrin receptor-dependent iron uptake is responsible for doxorubicin-mediated apoptosis in endothelial cells: Role of oxidant-induced iron signaling in apoptosis. J. Biol. Chem. 2002, 277, 17179–17187. [Google Scholar] [CrossRef] [Green Version]
- Djavaheri-Mergny, M.; Wietzerbin, J.; Besançon, F. 2-Methoxyestradiol induces apoptosis in Ewing sarcoma cells through mitochondrial hydrogen peroxide production. Oncogene 2003, 22, 2558–2567. [Google Scholar] [CrossRef] [Green Version]
- Kachadourian, R.; Liochev, S.I.; Cabelli, D.E.; Patel, M.N.; Fridovich, I.; Day, B.J. 2-methoxyestradiol does not inhibit superoxide dismutase. Arch. Biochem. Biophys. 2001, 392, 349–353. [Google Scholar] [CrossRef]
- Mooberry, S.L. Mechanism of action of 2-methoxyestradiol: New developments. Drug Resist. Updates 2003, 6, 355–361. [Google Scholar] [CrossRef]
- Heo, J.R.; Kim, S.M.; Hwang, K.A.; Kang, J.H.; Choi, K.C. Resveratrol induced reactive oxygen species and endoplasmic reticulum stress-mediated apoptosis, and cell cycle arrest in the A375SM malignant melanoma cell line. Int. J. Mol. Med. 2018, 42, 1427–1435. [Google Scholar] [CrossRef] [Green Version]
- Forrester, S.J.; Kikuchi, D.S.; Hernandes, M.S.; Xu, Q.; Griendling, K.K. Reactive Oxygen Species in Metabolic and Inflammatory Signaling. Circ. Res. 2018, 122, 877–902. [Google Scholar] [CrossRef]
- Mittal, M.; Siddiqui, M.R.; Tran, K.; Reddy, S.P.; Malik, A.B. Reactive oxygen species in inflammation and tissue injury. Antioxid. Redox Signal. 2014, 20, 1126–1167. [Google Scholar] [CrossRef]
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.; Mazur, M.; Telser, J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007, 39, 44–84. [Google Scholar] [CrossRef] [PubMed]
- Kanazawa, H.; Kurihara, N.; Hirata, K.; Takeda, T. The role of free radicals in airway obstruction in asthmatic patients. Chest 1991, 100, 1319–1322. [Google Scholar] [CrossRef] [PubMed]
- Shanmugasundaram, K.R.; Kumar, S.S.; Rajajee, S. Excessive free radical generation in the blood of children suffering from asthma. Clin. Chim. Acta 2001, 305, 107–114. [Google Scholar] [CrossRef]
- Samimi, L.N.; Farhadi, E.; Tahmasebi, M.N.; Jamshidi, A.; Vaziri, A.S.; Mahmoudi, M. NF-κB signaling in rheumatoid arthritis with focus on fibroblast-like synoviocytes. Auto. Immun. Highlights 2020, 11, 11. [Google Scholar] [CrossRef]
- Liang, Y.; Zhou, Y.; Shen, P. NF-kappa B and its regulation on the immune system. Cell. Mol. Immunol. 2004, 1, 343–350. [Google Scholar]
- Jeong, S.R.; Park, H.Y.; Lee, K.W. Methylglyoxal-derived advanced glycation end products induce matrix metalloproteinases through activation of ERK/JNK/NF-κB pathway in kidney proximal epithelial cells. Food Sci. Biotechnol. 2019, 29, 675–682. [Google Scholar] [CrossRef]
- Xue, M.; McKelvey, K.; Shen, K.; Minhas, N.; March, L.; Park, S.Y.; Jackson, C.J. Endogenous MMP-9 and not MMP-2 promotes rheumatoid synovial fibroblast survival, inflammation and cartilage degradation. Rheumatology 2014, 53, 2270–2279. [Google Scholar] [CrossRef] [Green Version]
- Jacques, C.; Gosset, M.; Berenbaum, F.; Gabay, C. The role of IL-1 and IL-1Ra in joint inflammation and cartilage degradation. Vitamin. Horm. 2006, 74, 371–403. [Google Scholar]
- Ansari, M.Y.; Ahmad, N.; Haqqi, T.M. Oxidative stress and inflammation in osteoarthritis pathogenesis: Role of polyphenols. Biomed. Pharmacother. 2020, 129, 110452. [Google Scholar] [CrossRef]
- Rolo, A.P.; Palmeira, C.M. Diabetes and mitochondrial function: Role of hyperglycemia and oxidative stress. Toxicol. Appl. Pharmacol. 2006, 212, 167–178. [Google Scholar] [CrossRef] [Green Version]
- Haidara, M.A.; Yassin, H.Z.; Rateb, M.; Ammar, H.; Zorkani, M.A. Role of oxidative stress in development of cardiovascular complications in diabetes mellitus. Curr. Vasc. Pharmacol. 2006, 4, 215–227. [Google Scholar] [CrossRef] [PubMed]
- Bajaj, S.; Khan, A. Antioxidants and diabetes. Ind. J. Endocrinol. Metab. 2012, 16, S267–S271. [Google Scholar] [CrossRef] [PubMed]
- Aghadavod, E.; Khodadadi, S.; Baradaran, A.; Nasri, P.; Bahmani, M.; Rafieian-Kopaei, M. Role of Oxidative Stress and Inflammatory Factors in Diabetic Kidney Disease. Iran. J. Kidney Dis. 2016, 10, 337–343. [Google Scholar] [PubMed]
- Sarwar, R.; Pierce, N.; Koppe, S. Obesity and nonalcoholic fatty liver disease: Current perspectives. Diabetes Metab. Syndr. Obes. 2018, 11, 533–542. [Google Scholar] [CrossRef] [Green Version]
- Fabbrini, E.; Sullivan, S.; Klein, S. Obesity and nonalcoholic fatty liver disease: Biochemical, metabolic, and clinical implications. Hepatology 2010, 51, 679–689. [Google Scholar] [CrossRef] [Green Version]
- Bhatt, H.B.; Smith, R.J. Fatty liver disease in diabetes mellitus. Hepatobiliary Surg. Nutr. 2015, 4, 101–108. [Google Scholar]
- Hazlehurst, J.M.; Woods, C.; Marjot, T.; Cobbold, J.F.; Tomlinson, J.W. Non-alcoholic fatty liver disease and diabetes. Metabolism 2016, 65, 1096–1108. [Google Scholar] [CrossRef] [Green Version]
- Tomah, S.; Alkhouri, N.; Hamdy, O. Nonalcoholic fatty liver disease and type 2 diabetes: Where do Diabetologists stand? Clin. Diabetes Endocrinol. 2020, 6, 9. [Google Scholar] [CrossRef]
- Kim, E.J.; Kim, B.H.; Seo, H.S.; Lee, Y.J.; Kim, H.H.; Son, H.H.; Choi, M.H. Cholesterol induced non-alcoholic fatty liver disease and atherosclerosis aggravated by systemic inflammation. PLoS ONE 2014, 9, e97841. [Google Scholar] [CrossRef] [Green Version]
- Enjoji, M.; Yasutake, K.; Kohjima, M.; Nakamuta, M. Nutrition and nonalcoholic Fatty liver disease: The significance of cholesterol. Int. J. Hepatol. 2012, 2012, 925807. [Google Scholar] [CrossRef]
- Malhotra, P.; Gill, R.K.; Saksena, S.; Alrefai, W.A. Disturbances in Cholesterol Homeostasis and Non-alcoholic Fatty Liver Diseases. Front. Med. 2020, 7, 467. [Google Scholar] [CrossRef] [PubMed]
- Masarone, M.; Rosato, V.; Dallio, M.; Gravina, A.G.; Aglitti, A.; Loguercio, C.; Federico, A.; Persico, M. Role of Oxidative Stress in Pathophysiology of Nonalcoholic Fatty Liver Disease. Oxid. Med. Cell Longev. 2018, 2018, 9547613. [Google Scholar] [CrossRef] [PubMed]
- Serviddio, G.; Bellanti, F.; Vendemiale, G. Free radical biology for medicine: Learning from nonalcoholic fatty liver disease. Free Radic. Biol. Med. 2013, 65, 952–968. [Google Scholar] [CrossRef] [PubMed]
- Morita, M.; Ishida, N.; Uchiyama, K.; Yamaguchi, K.; Itoh, Y.; Shichiri, M.; Yoshida, Y.; Hagihara, Y.; Naito, Y.; Yoshikawa, T.; et al. Fatty liver induced by free radicals and lipid peroxidation. Free Radic. Res. 2012, 46, 758–765. [Google Scholar] [CrossRef]
- Basu, S. Carbon tetrachloride-induced lipid peroxidation: Eicosanoid formation and their regulation by antioxidant nutrients. Toxicology 2003, 189, 113–127. [Google Scholar] [CrossRef]
- Von Minden, H.M.; Brandenburg, K.; Seydel, U.; Koch, M.H.; Garamus, V.; Willmeit, R.; Vill, V. Thermotropic and lyotropic properties of long chain alkyl glycopyranosides. Part II. Disaccharide headgroups. Chem. Phys. Lipids 2000, 106, 157–179. [Google Scholar] [CrossRef]
- Traykova, M.; Kostova, I. Coumarin Derivatives and Oxidative Stress. Int. J. Pharmacol. 2005, 1, 29–32. [Google Scholar]
- Kostova, I.; Bhatia, S.; Grigorov, P.; Balkansky, S.; Parmar, V.S.; Prasad, A.K.; Saso, L. Coumarins as antioxidants. Curr. Med. Chem. 2011, 18, 3929–3951. [Google Scholar] [CrossRef]
- Vianna, D.R.; Bubols, G.; Meirelles, G.; Silva, B.V.; da Rocha, A.; Lanznaster, M.; Monserrat, J.M.; Garcia, S.C.; von Poser, G.; Eifler-Lima, V.L. Evaluation of the antioxidant capacity of synthesized coumarins. Int. J. Mol. Sci. 2012, 13, 7260–7270. [Google Scholar] [CrossRef] [Green Version]
- Zhu, J.J.; Jiang, J.G. Pharmacological and Nutritional Effects of Natural Coumarins and Their Structure-Activity Relationships. Mol. Nutr. Food Res. 2018, 62, e1701073. [Google Scholar] [CrossRef]
- Najmanová, I.; Doseděl, M.; Hrdina, R.; Anzenbacher, P.; Filipský, T.; Říha, M.; Mladěnka, P. Cardiovascular effects of coumarins besides their antioxidant activity. Curr. Top. Med. Chem. 2015, 15, 830–849. [Google Scholar] [CrossRef] [PubMed]
- Tien, Y.C.; Liao, J.C.; Chiu, C.S.; Hunag, T.H.; Huang, C.Y.; Chang, W.T.; Peng, W.H. Esculetin ameliorates carbon tetrachloride-mediated hepatic apoptosis in rats. Int. J. Mol. Sci. 2011, 12, 4053–4067. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, M.J.; Chou, F.P.; Tseng, T.H.; Hsieh, M.H.; Lin, M.C.; Wang, C.J. Hibiscus protocatechuic acid or esculetin can inhibit oxidative LDL induced by either copper ion or nitric oxide donor. J. Agric. Food Chem. 2002, 50, 2130–2136. [Google Scholar] [CrossRef] [PubMed]
- Asmat, U.; Abad, K.; Ismail, K. Diabetes mellitus and oxidative stress-A concise review. Saudi Pharm. J. 2016, 24, 547–553. [Google Scholar] [CrossRef] [PubMed]
- Arora, R.; Sawney, S.; Saini, V.; Steffi, C.; Tiwari, M.; Saluja, D. Esculetin induces antiproliferative and apoptotic response in pancreatic cancer cells by directly binding to KEAP1. Mol. Cancer. 2016, 15, 64. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Yang, C.; Zhang, Q.; Wang, C.; Zhou, X.; Zhang, X.; Liu, S. In vitro anticancer effects of esculetin against human leukemia cell lines involves apoptotic cell death, autophagy, G0/G1 cell cycle arrest and modulation of Raf/MEK/ERK signalling pathway. JBUON 2019, 24, 1686–1691. [Google Scholar]
- Park, C.; Jin, C.Y.; Kim, G.Y.; Choi, I.W.; Kwon, T.K.; Choi, B.T.; Lee, S.J.; Lee, W.H.; Choi, Y.H. Induction of apoptosis by esculetin in human leukemia U937 cells through activation of JNK and ERK. Toxicol. Appl. Pharmacol. 2008, 227, 219–228. [Google Scholar] [CrossRef]
- Mortenson, M.M.; Galante, J.G.; Gilad, O.; Schlieman, M.G.; Virudachalam, S.; Kung, H.J.; Bold, R.J. BCL-2 functions as an activator of the AKT signaling pathway in pancreatic cancer. J. Cell. Biochem. 2007, 102, 1171–1179. [Google Scholar] [CrossRef]
- Anand, J.R.; Rijhwani, H.; Malapati, K.; Kumar, P.; Saikia, K.; Lakhar, M. Anticancer activity of esculetin via-modulation of Bcl-2 and NF-κB expression in benzo [a] pyrene induced lung carcinogenesis in mice. Biomed. Prev. Nutr. 2013, 3, 107–112. [Google Scholar] [CrossRef]
- Kim, A.D.; Han, X.; Piao, M.J.; Hewage, S.R.; Hyun, C.L.; Cho, S.J.; Hyun, J.W. Esculetin induces death of human colon cancer cells via the reactive oxygen species-mediated mitochondrial apoptosis pathway. Environ. Toxicol. Pharmacol. 2015, 39, 982–989. [Google Scholar] [CrossRef]
- Cho, J.H.; Shin, J.C.; Cho, J.J.; Choi, Y.H.; Shim, J.H.; Chae, J.I. Esculetin (6,7-dihydroxycoumarin): A potential cancer chemopreventive agent through suppression of Sp1 in oral squamous cancer cells. Int. J. Oncol. 2015, 46, 265–271. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, G.; Xu, Y.; Zhou, H.F. Esculetin Inhibits Proliferation, Invasion, and Migration of Laryngeal Cancer In Vitro and In Vivo by Inhibiting Janus Kinas (JAK)-Signal Transducer and Activator of Transcription-3 (STAT3) Activation. Med. Sci. Monit. 2019, 25, 7853–7863. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Lu, M.L.; Dai, H.L.; Zhang, S.P.; Wang, H.X.; Wei, N. Esculetin, a coumarin derivative, exerts in vitro and in vivo antiproliferative activity against hepatocellular carcinoma by initiating a mitochondrial-dependent apoptosis pathway. Braz. J. Med. Biol. Res. 2015, 48, 245–253. [Google Scholar] [CrossRef] [Green Version]
- Wang, G.; Lu, M.; Yao, Y.; Wang, J.; Li, J. Esculetin exerts antitumor effect on human gastric cancer cells through IGF-1/PI3K/Akt signaling pathway. Eur. J. Pharmacol. 2017, 814, 207–215. [Google Scholar] [CrossRef]
- Duan, J.; Shi, J.; Ma, X.; Xuan, Y.; Li, P.; Wang, H.; Fan, Y.; Gong, H.; Wang, L.; Pang, Y.; et al. Esculetin inhibits proliferation, migration, and invasion of clear cell renal cell carcinoma cells. Biomed. Pharmacother. 2020, 125, 110031. [Google Scholar] [CrossRef] [PubMed]
- Han, M.H.; Park, C.; Lee, D.S.; Hong, S.H.; Choi, I.W.; Kim, G.Y.; Choi, S.H.; Shim, J.H.; Chae, J.I.; Yoo, Y.H.; et al. Cytoprotective effects of esculetin against oxidative stress are associated with the upregulation of Nrf2-mediated NQO1 expression via the activation of the ERK pathway. Int. J. Mol. Med. 2017, 39, 380–386. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, S.H.; Kang, K.A.; Zhang, R.; Piao, M.J.; Ko, D.O.; Wang, Z.H.; Chae, S.W.; Kang, S.S.; Lee, K.H.; Kang, H.K.; et al. Protective effect of esculetin against oxidative stress-induced cell damage via scavenging reactive oxygen species. Acta. Pharmacol. Sin. 2008, 29, 1319–1326. [Google Scholar] [CrossRef] [Green Version]
- Bilgin, H.M.; Atmaca, M.; Deniz Obay, B.; Ozekinci, S.; Taşdemir, E.; Ketani, A. Protective effects of coumarin and coumarin derivatives against carbon tetrachloride induced acute hepatotoxicity in rats. Exp. Toxicol. Pathol. 2011, 63, 325–330. [Google Scholar] [CrossRef]
- Pruccoli, L.; Morroni, F.; Sita, G.; Hrelia, P.; Tarozzi, A. Esculetin as a Bifunctional Antioxidant Prevents and Counteracts the Oxidative Stress and Neuronal Death Induced by Amyloid Protein in SH-SY5Y Cells. Antioxidants 2020, 9, 551. [Google Scholar] [CrossRef]
- Lee, B.C.; Lee, S.Y.; Lee, H.J.; Sim, G.S.; Kim, J.H.; Kim, J.H.; Cho, Y.H.; Lee, D.H.; Pyo, H.B.; Choe, T.B.; et al. Anti-oxidative and photoprotective effects of coumarins isolated from Fraxinus chinensis. Arch. Pharm. Res. 2007, 30, 1293–1301. [Google Scholar] [CrossRef]
- Zhe, A.X.; Piao, M.J.; Kang, K.A.; Fernando, P.D.S.M.; Kang, H.K.; Koh, Y.S.; Hyun, J.W. Esculetin Prevents the Induction of Matrix Metalloproteinase-1 by Hydrogen Peroxide in Skin Keratinocytes. J. Cancer. Prev. 2019, 24, 123–128. [Google Scholar]
- Kaneko, T.; Tahara, S.; Takabayashi, F. Suppression of lipid hydroperoxide-induced oxidative damage to cellular DNA by esculetin. Biol. Pharm. Bull. 2003, 26, 840–844. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abdulkhaleq, L.A.; Assi, M.A.; Abdullah, R.; Zamri-Saad, M.; Taufiq-Yap, Y.H.; Hezmee, M.N.M. The crucial roles of inflammatory mediators in inflammation: A review. Vet. World 2018, 11, 627–635. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ricciotti, E.; FitzGerald, G.A. Prostaglandins and inflammation. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 986–1000. [Google Scholar] [CrossRef]
- Zinatizadeh, M.R.; Schock, B.; Chalbatani, G.M.; Zarandi, P.K.; Jalali, S.A.; Miri, S.R. The Nuclear Factor Kappa B (NFκB) signaling in cancer development and immune diseases. Genes Dis. 2020, 8, 287–297. [Google Scholar] [CrossRef]
- Cheng, Y.J.; Tian, X.L.; Zeng, Y.Z.; Lan, N.; Guo, L.F.; Liu, K.F.; Fang, H.L.; Fan, H.Y.; Peng, Z.L. Esculetin protects against early sepsis via attenuating inflammation by inhibiting NF-κB and STAT1/STAT3 signaling. Chin. J. Nat. Med. 2021, 19, 432–441. [Google Scholar] [CrossRef]
- Hong, S.H.; Jeong, H.K.; Han, M.H.; Park, C.; Choi, Y.H. Esculetin suppresses lipopolysaccharide-induced inflammatory mediators and cytokines by inhibiting nuclear factor-κB translocation in RAW 264.7 macrophages. Mol. Med. Rep. 2014, 10, 3241–3246. [Google Scholar] [CrossRef] [Green Version]
- Jayakumar, T.; Huang, C.J.; Yen, T.L.; Hsia, C.W.; Sheu, J.R.; Bhavan, P.S.; Huang, W.C.; Hsieh, C.Y.; Hsia, C.H. Activation of Nrf2 by Esculetin Mitigates Inflammatory Responses through Suppression of NF-kB Signaling Cascade in RAW 264.7 Cells. Molecules 2022, 27, 5143. [Google Scholar] [CrossRef]
- Soufli, I.; Toumi, R.; Rafa, H.; Touil-Boukoffa, C. Overview of cytokines and nitric oxide involvement in immuno-pathogenesis of inflammatory bowel diseases. World J. Gastrointest. Pharmacol. Ther. 2016, 7, 353–360. [Google Scholar] [CrossRef]
- Andrew, P.J.; Mayer, B. Enzymatic function of nitric oxide synthases. Cardiovasc. Res. 1999, 43, 521–531. [Google Scholar] [CrossRef]
- Zhu, L.; Nang, C.; Luo, F.; Pan, H.; Zhang, K.; Liu, J.; Zhou, R.; Gao, J.; Chang, X.; He, H.; et al. Esculetin attenuates lipopolysaccharide (LPS)-induced neuroinflammatory processes and depressive-like behavior in mice. Physiol. Behav. 2016, 163, 184–192. [Google Scholar] [CrossRef] [PubMed]
- Leung, K.N.; Leung, P.Y.; Kong, L.P.; Leung, P.K. Immunomodulatory effects of esculetin (6,7-dihydroxycoumarin) on murine lymphocytes and peritoneal macrophages. Cell. Mol. Immunol. 2005, 2, 181–188. [Google Scholar]
- Liu, S.Q.; He, L.; Peng, H. Effect of esculetin on osteoarthritis in rabbit. Med. J. Wuhan Univ. 2004, 9, 567–570. [Google Scholar]
- Kim, Y.; Park, Y.; Namkoong, S.; Lee, J. Esculetin inhibits the inflammatory response by inducing heme oxygenase-1 in cocultured macrophages and adipocytes. Food Funct. 2014, 5, 2371–2377. [Google Scholar] [CrossRef] [PubMed]
- Koelman, L.; Pivovarova-Ramich, O.; Pfeiffer, A.F.H.; Grune, T.; Aleksandrova, K. Cytokines for evaluation of chronic inflammatory status in ageing research: Reliability and phenotypic characterisation. Immun. Ageing 2019, 16, 11. [Google Scholar] [CrossRef] [Green Version]
- Witaicenis, A.; Luchini, A.C.; Hiruma-Lima, C.A.; Felisbino, S.L.; Justulin, L.A., Jr.; Garrido-Mesa, N.; Utrilla, P.; Gálvez, J.; Di Stasi, L.C. Mechanism and effect of esculetin in an experimental animal model of inflammatory bowel disease. Eur. J. Inflamm. 2013, 11, 433–446. [Google Scholar] [CrossRef] [Green Version]
- Singh, L.; Kaur, A.; Garg, S.; Singh, A.P.; Bhatti, R. Protective Effect of Esculetin, Natural Coumarin in Mice Model of Fibromyalgia: Targeting Pro-Inflammatory Cytokines and MAO-A. Neurochem. Res. 2020, 45, 2364–2374. [Google Scholar] [CrossRef]
- Sun, B.; Wang, B.; Xu, M. Esculetin inhibits histamine-induced expression of inflammatory cytokines and mucin in nasal epithelial cells. Clin. Exp. Pharmacol. Physiol. 2019, 46, 821–827. [Google Scholar] [CrossRef]
- Jeong, N.H.; Yang, E.J.; Jin, M.; Lee, J.Y.; Choi, Y.A.; Park, P.H.; Lee, S.R.; Kim, S.U.; Shin, T.Y.; Kwon, T.K.; et al. Esculetin from Fraxinus rhynchophylla attenuates atopic skin inflammation by inhibiting the expression of inflammatory cytokines. Int. Immunopharmacol. 2018, 59, 209–216. [Google Scholar] [CrossRef]
- Ozal, S.A.; Turkekul, K.; Gurlu, V.; Guclu, H.; Erdogan, S. Esculetin Protects Human Retinal Pigment Epithelial Cells from Lipopolysaccharide-induced Inflammation and Cell Death. Curr. Eye Res. 2018, 43, 1169–1176. [Google Scholar] [CrossRef]
- Chen, T.; Guo, Q.; Wang, H.; Zhang, H.; Wang, C.; Zhang, P.; Meng, S.; Li, Y.; Ji, H.; Yan, T. Effects of esculetin on lipopolysaccharide (LPS)-induced acute lung injury via regulation of RhoA/Rho Kinase/NF-κB pathways in vivo and in vitro. Free Radic. Res. 2015, 49, 1459–1468. [Google Scholar] [CrossRef] [PubMed]
- Yum, S.; Jeong, S.; Lee, S.; Kim, W.; Nam, J.; Jung, Y. HIF-prolyl hydroxylase is a potential molecular target for esculetin-mediated anti-colitic effects. Fitoterapia 2015, 103, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhang, Q.; Liu, H.; Lu, C.; Liang, C.L.; Qiu, F.; Han, L.; Dai, Z. Esculetin Ameliorates Psoriasis-Like Skin Disease in Mice by Inducing CD4+Foxp3+ Regulatory T Cells. Front. Immunol. 2018, 9, 2092. [Google Scholar] [CrossRef]
- Nieminen, P.; Hämäläinen, W.; Savinainen, J.; Lehtonen, M.; Lehtiniemi, S.; Rinta- Paavola, J.; Lehenkari, P.; Kääriäinen, T.; Joukainen, A.; Kröger, H.; et al. Metabolomics of Synovial Fluid and Infrapatellar Fat Pad in Patients with Osteoarthritis or Rheumatoid Arthritis. Inflammation 2022, 45, 1101–1117. [Google Scholar] [CrossRef]
- Ajami, S.; Javaheri, B.; Chang, Y.M.; Maruthainar, N.; Khan, T.; Donaldson, J.; Pitsillides, A.A.; Liu, C. Spatial links between subchondral bone architectural features and cartilage degeneration in osteoarthritic joints. Sci. Rep. 2022, 12, 6694. [Google Scholar] [CrossRef] [PubMed]
- Mariscal, A.; Zamora, C.; Díaz-Torné, C.; Ortiz, M.À.; Agustín, J.J.; Reina, D.; Estrada, P.; Moya, P.; Corominas, H.; Vidal, S. Increase of Circulating Monocyte-Platelet Conjugates in Rheumatoid Arthritis Responders to IL-6 Blockage. Int. J. Mol. Sci. 2022, 23, 5748. [Google Scholar] [CrossRef]
- Carmona-Rivera, C.; Carlucci, P.M.; Goel, R.R.; James, E.; Brooks, S.R.; Rims, C.; Hoffmann, V.; Fox, D.A.; Buckner, J.H.; Kaplan, M.J. Neutrophil extracellular traps mediate articular cartilage damage and enhance cartilage component immunogenicity in rheumatoid arthritis. JCI Insight 2020, 5, e139388. [Google Scholar] [CrossRef]
- Sundaram, M.S.; Neog, M.K.; Rasool, M.; Kumar, G.S.; Hemshekhar, M.; Kemparaju, K.; Girish, K.S. Guggulipid ameliorates adjuvant-induced arthritis and liver oxidative damage by suppressing inflammatory and oxidative stress mediators. Phytomedicine 2019, 64, 152924. [Google Scholar] [CrossRef]
- Rose, B.J.; Kooyman, D.L. A Tale of Two Joints: The Role of Matrix Metalloproteases in Cartilage Biology. Dis. Markers 2016, 2016, 4895050. [Google Scholar] [CrossRef] [Green Version]
- Burrage, P.S.; Mix, K.S.; Brinckerhoff, C.E. Matrix metalloproteinases: Role in arthritis. Front. Biosci. 2006, 11, 529–543. [Google Scholar] [CrossRef] [Green Version]
- Yousefi, B.; Jadidi-Niaragh, F.; Azizi, G.; Hajighasemi, F.; Mirshafiey, A. The role of leukotrienes in immunopathogenesis of rheumatoid arthritis. Mod. Rheumatol. 2014, 24, 225–235. [Google Scholar] [CrossRef] [PubMed]
- Mastbergen, S.C.; Ooms, A.; Turmezei, T.D.; MacKay, J.W.; Van Heerwaarden, R.J.; Spruijt, S.; Lafeber, F.P.J.G.; Jansen, M.P. Subchondral bone changes after joint distraction treatment for end stage knee osteoarthritis. Osteoarthr. Cartil. 2022, 30, 965–972. [Google Scholar] [CrossRef] [PubMed]
- Kuettner, K.E. Biochemistry of articular cartilage in health and disease. Clin. Biochem. 1992, 25, 155–163. [Google Scholar] [CrossRef]
- Yamada, H.; Watanabe, K.; Saito, T.; Hayashi, H.; Niitani, Y.; Kikuchi, T.; Ito, A.; Fujikawa, K.; Lohmander, L.S. Esculetin (dihydroxycoumarin) inhibits the production of matrix metalloproteinases in cartilage explants, and oral administration of its prodrug, CPA-926, suppresses cartilage destruction in rabbit experimental osteoarthritis. J. Rheumatol. 1999, 26, 654–662. [Google Scholar]
- Watanabe, K.; Ito, A.; Sato, T.; Saito, T.; Hayashi, H.; Niitani, Y. Esculetin suppresses proteoglycan metabolism by inhibiting the production of matrix metalloproteinases in rabbit chondrocytes. Eur. J. Pharmacol. 1999, 370, 297–305. [Google Scholar] [CrossRef]
- Rzodkiewicz, P.; Gąsińska, E.; Gajewski, M.; Bujalska-Zadrożny, M.; Szukiewicz, D.; Maśliński, S. Esculetin reduces leukotriene B4 level in plasma of rats with adjuvant induced arthritis. Reumatologia 2016, 54, 161–164. [Google Scholar] [CrossRef]
- Hemshekhar, M.; Sunitha, K.; Thushara, R.M.; Santhosh, M.S.; Sundaram, M.S.; Kemparaju, K.; Girish, K.S. Antiarthritic and antiinflammatory propensity of 4-methylesculetin, a coumarin derivative. Biochimie 2013, 95, 1326–1335. [Google Scholar] [CrossRef]
- Elliott, S.; Rowan, A.D.; Carrère, S.; Koshy, P.; Catterall, J.B.; Cawston, T.E. Esculetin inhibits cartilage resorption induced by interleukin 1alpha in combination with oncostatin M. Ann. Rheum. Dis. 2001, 60, 158–165. [Google Scholar] [CrossRef] [Green Version]
- Singh, A.; Kukreti, R.; Saso, L.; Kukreti, S. Mechanistic Insight into Oxidative Stress- Triggered Signaling Pathways and Type 2 Diabetes. Molecules 2022, 27, 950. [Google Scholar] [CrossRef]
- Maritim, A.C.; Sanders, R.A.; Watkins, J.B. Diabetes, oxidative stress, and antioxidants: A review. J. Biochem. Mol. Toxicol. 2003, 17, 24–38. [Google Scholar] [CrossRef]
- Rains, J.L.; Jain, S.K. Oxidative stress, insulin signaling, and diabetes. Free Radic. Biol. Med. 2011, 50, 567–575. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prabakaran, D.; Ashokkumar, N. Protective effect of esculetin on hyperglycemia-mediated oxidative damage in the hepatic and renal tissues of experimental diabetic rats. Biochimie 2013, 95, 366–373. [Google Scholar] [CrossRef] [PubMed]
- Kadakol, A.; Malek, V.; Goru, S.K.; Pandey, A.; Bagal, S.; Gaikwad, A.B. Esculetin attenuates alterations in Ang II and acetylcholine mediated vascular reactivity associated with hyperinsulinemia and hyperglycemia. Biochem. Biophys. Res. Commun. 2015, 461, 342–347. [Google Scholar] [CrossRef] [PubMed]
- Surse, V.M.; Gupta, J.; Tikoo, K. Esculetin induced changes in Mmp13 and Bmp6 gene expression and histone H3 modifications attenuate development of glomerulosclerosis in diabetic rats. J. Mol. Endocrinol. 2011, 46, 245–254. [Google Scholar] [CrossRef] [Green Version]
- Kadakol, A.; Malek, V.; Goru, S.K.; Pandey, A.; Gaikwad, A.B. Esculetin reverses histone H2A/H2B ubiquitination, H3 dimethylation, acetylation and phosphorylation in preventing type 2 diabetic cardiomyopathy. J. Funct. Foods 2015, 17, 127–136. [Google Scholar] [CrossRef]
- Kadakol, A.; Malek, V.; Goru, S.K.; Pandey, A.; Sharma, N.; Gaikwad, A.B. Esculetin ameliorates insulin resistance and type 2 diabetic nephropathy through reversal of histone H3 acetylation and H2A lysine 119 monoubiquitination. J. Funct. Foods 2017, 35, 256–266. [Google Scholar] [CrossRef]
- Pandey, A.; Raj, P.; Goru, S.K.; Kadakol, A.; Malek, V.; Sharma, N.; Gaikwad, A.B. Esculetin ameliorates hepatic fibrosis in high fat diet induced non-alcoholic fatty liver disease by regulation of FoxO1 mediated pathway. Pharmacol. Rep. 2017, 69, 666–672. [Google Scholar] [CrossRef]
- Choi, R.Y.; Ham, J.R.; Lee, M.K. Esculetin prevents non-alcoholic fatty liver in diabetic mice fed high-fat diet. Chem. Biol. Interact. 2016, 260, 13–21. [Google Scholar] [CrossRef]
- Park, Y.; Sung, J.; Yang, J.; Ham, H.; Kim, Y.; Jeong, H.S.; Lee, J. Inhibitory effect of esculetin on free-fatty-acid-induced lipid accumulation in human HepG2 cells through activation of AMP-activated protein kinase. Food Sci. Biotechnol. 2017, 26, 263–269. [Google Scholar] [CrossRef]
- Yang, X.J.; Gao, H.H. Study on the synthesis of esculetin under microwave irradiation. Appl. Chem. Ind. 2011, 40, 627–629. [Google Scholar]
- Zhang, T. Synthesis of 6,7-Dihydroxyeoumarin. Master’s Thesis, Nanjing University Science Technology, Nanjing, China, 2007. [Google Scholar]
- Cao, W.Q.; Xue, J.F.; Shi, C.M.; Ding, C.F.; Zhu, X.G.; Liu, F.; Zhou, X.J. Synthesis of 6,7-Dihydroxy Coumarin. Fine Chem. Intermed. 2013, 43, 39–41. [Google Scholar]
- Yang, S.M.; Shim, G.Y.; Kim, B.G.; Ahn, J.H. Biological synthesis of coumarins in Escherichia coli. Microb. Cell Fact. 2015, 14, 65. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palmer, M.E.; Andrews, L.J.; Abbey, T.C.; Dahlquist, A.E.; Wenzler, E. The importance of pharmacokinetics and pharmacodynamics in antimicrobial drug development and their influence on the success of agents developed to combat resistant gram negative pathogens: A review. Front Pharmacol. 2022, 13, 888079. [Google Scholar] [CrossRef]
- Li, Y.Y.; Song, Y.Y.; Liu, C.H.; Huang, X.T.; Zheng, X.; Li, N.; Xu, M.L.; Mi, S.Q.; Wang, N.S. Simultaneous determination of esculin and its metabolite esculetin in rat plasma by LC-ESI-MS/MS and its application in pharmacokinetic study. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2012, 907, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.S.; Ha, T.Y.; Ahn, J.; Kim, S. Analysis and distribution of esculetin in plasma and tissues of rats after oral administration. Prev. Nutr. Food Sci. 2014, 19, 321–326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mei, Y.; Dong, B.; Geng, Z.; Xu, L. Excess Uric Acid Induces Gouty Nephropathy Through Crystal Formation: A Review of Recent Insights. Front. Endocrinol. 2022, 13, 911968. [Google Scholar] [CrossRef]
- Xu, L.; Lu, L.L.; Gao, J.D. Traditional Chinese Herbal Medicine Plays a Role in the Liver, Kidney, and Intestine to Ameliorate Hyperuricemia according to Experimental Studies. Evid. Based Complement. Altern. Med. 2021, 2021, 4618352. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, M.; Ye, H.; Shao, Y.; Yu, Y.; Wang, M.; Zhao, C. Comparative pharmacokinetic study of the main components of cortex fraxini after oral administration in normal and hyperuricemic rats. Biomed. Chromatogr. 2017, 31, e3934. [Google Scholar] [CrossRef]
- Kwak, J.H.; Kim, Y.; Staatz, C.E.; Baek, I.H. Oral bioavailability and pharmacokinetics of esculetin following intravenous and oral administration in rats. Xenobiotica 2021, 51, 811–817. [Google Scholar] [CrossRef]
- Tsai, T.H.; Huang, C.T.; Shum, A.Y.; Chen, C.F. Simultaneous blood and biliary sampling of esculetin by microdialysis in the rat. Life Sci. 1999, 65, 1647–1655. [Google Scholar] [CrossRef]
- Wang, Z.; Zhu, W.; Liu, H.; Wu, G.; Song, M.; Yang, B.; Yang, D.; Wang, Q.; Kuang, H. Simultaneous Determination of Aesculin, Aesculetin, Fraxetin, Fraxin and Polydatin in Beagle Dog Plasma by UPLC-ESI-MS/MS and Its Application in a Pharmacokinetic Study after Oral Administration Extracts of Ledum palustre L. Molecules 2018, 23, 2285. [Google Scholar] [CrossRef] [PubMed]
- Rehman, S.U.; Kim, I.S.; Kang, K.S.; Yoo, H.H. HPLC Determination of Esculin and Esculetin in Rat Plasma for Pharmacokinetic Studies. J. Chromatogr. Sci. 2015, 53, 1322–1327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Zhao, M.; Ou, Y.; Zeng, B.; Lou, X.; Wang, M.; Zhao, C. Metabolic profile of esculin in rats by ultra high performance liquid chromatography combined with Fourier transform ion cyclotron resonance mass spectrometry. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2016, 1020, 120–128. [Google Scholar] [CrossRef] [PubMed]
- Schimmer, O.; Eschelbach, H. Esculetin in Alchemilla speciosa: Identification and antimutagenic properties. Pharmazie 1997, 52, 476–478. [Google Scholar]
- Zhou, L.; Kang, J.; Fan, L.; Ma, X.C.; Zhao, H.Y.; Han, J.; Wang, B.R.; Guo, D.A. Simultaneous analysis of coumarins and secoiridoids in Cortex Fraxini by high-performance liquid chromatography-diode array detection-electrospray ionization tandem mass spectrometry. J. Pharm. Biomed. Anal. 2008, 47, 39–46. [Google Scholar] [CrossRef]











| Pharmacological Mechanism | Inhibition/Activation/ Downregulation/Upregulation | Model Used | Dosage | Application | Reference |
|---|---|---|---|---|---|
| Cell-cycle arrest at G1-phase Activate ARE pathway and impede binding interactions between Nrf2 and KEAP-1 Attenuate NF-κB pathway | Human PANC-1 cells | 100 µM | In vitro | [45] | |
| Inhibit cell proliferation Induce autophagy by forming autophagic-vesicles Downregulate cyclin D1, D3, DK4 and DK2 Induce cell-cycle arrest at G0/G1-phase Block MEK/ERK phosphorylation by inhibiting Raf/MEK/ERK signaling | Human leukemia cells (HL-60 cells) | 20 µM | In vitro | [47] | |
| Downregulate JNK/ERK signaling | Human leukemia cells (U937 cells) | 30 µM | In vitro | [48] | |
| Downregulate Bcl-2 and NF-κB expressions Induce apoptosis | Benzo[a]pyrene-induced lung carcinogenesis in Swiss-albino mice | 50 mg/kg | In vivo | [50] | |
| Anti-cancer | Activate MAPK signaling Activate caspase-3 and 9 and cause apoptosis Release cytochrome c into cytosol Increase mitochondrial membrane depolarization Increase Bax expression | Human colon cancer cells (HT-29 cells) | 55 µg/mL | In vitro | [51] |
| Suppress SP1, p27, cyclin D1, Mcl-1, survivin expressions Induce apoptosis | Oral squamous cancer (HN22 and HSC4 cells) | 20 µg/mL | In vitro | [52] | |
| Downregulate STAT3 phosphorylation Inhibition of JAK/STAT pathways Induce cell-cycle arrest at G1/S-phase | Laryngeal cancer (Hep2 cells) | 2, 10 µM | In vitro, In vivo | [53] | |
| Cell-cycle arrest at S-phase Elevate caspase-3, 9 expressions Reduce mitochondrial membrane potential Increase Bax expression Downregulate Bcl-2 expression | Hepatocellular carcinoma (C57BL/6 mice were implanted with Hepa1–6 cells and SMMC-7721 cells) | 2.24 mM | In vitro, In vivo | [54] | |
| Suppress IGF-1/PI3K/Akt and IGF-1/MAPK signaling Reduce mitochondrial membrane potential Release cytochrome c from mitochondria Increase Bax, Bcl-2, caspase-3, 9 Expressions | Human gastric cancer (MGC-803 and GES-1 cells) | 850 µM | In vitro | [55] | |
| Inhibit proliferation, migration and invasion of renal cancerous cells Induce cell-cycle arrest at G0/G1 and G2-phase Downregulate cyclin D1, CDK4, CDK6 and c-Myc expressions Increase E-cadherin level by decreasing N-cadherin and vimentin expressions | Renal carcinoma (786-O and SN12-PM6 cells) | 200 µg/mL | In vitro | [56] |
| Pharmacological Mechanism | Inhibition/Activation/ Downregulation/Upregulation | Model Used | Dosage | Application | Reference |
|---|---|---|---|---|---|
| Antioxidant | Increase phosphorylation of Nrf2 and NQO1 Activate ERK signaling pathways Show protective effect against H2O2-induced oxidative stress | H2O2-induced oxidative stress in C2C12 myoblasts cells | 5 µM | In vitro | [57] |
| Scavenge DPPH, hydroxyl and intracellular ROS Inhibit lipid peroxidation, protein carbonyl and DNA-damage induced by H2O2 | Chinese hamster lung fibroblast cells (V79-4 cells) | 10 µg/mL | In vitro | [58] | |
| Scavenge free radicals Inhibition of lipid peroxidation, AST, ALT and ALP in liver | CCl4-induced acute hepatotoxicity in male Sprague Dawley rats | 35 mg/kg | In vivo | [59] | |
| Activate Nrf2 Increase phosphorylation of ERK signaling and Akt signaling Increase glutathione levels | Amyloid protein-induced oxidative stress and neuronal death in SH-SY5Y cells | 20 µM | In vitro | [60] | |
| Inhibit DPPH, Xanthine oxidase, superoxide radicals Downregulate MMP-1 expression | Oxidative stress in human dermal fibroblasts cells (HDF-cells) | 0.6 µg/mL and 2.1 µg/mL | In vitro | [61] | |
| Inhibit phospho-MEK1, phospho-ERK1/2, phospho-SEK1 and phospho-JNK1/2 along with intracellular Ca2+ levels Inhibit MMP-1 expression | H2O2-induced oxidative stress in Human HaCaT keratinocytes cells | 5 µg/mL | In vitro | [62] | |
| Scavenge hydroxyl radicals and protect DNA from oxidative damage | Lipid-hydroperoxide-induced oxidative damage in human diploid fibroblast cells (TIG-7 cells) | 50 µL | In vitro | [63] |
| Pharmacological Mechanism | Inhibition/Activation/ Downregulation/Upregulation | Model Used | Dosage | Application | Reference |
|---|---|---|---|---|---|
| Downregulate inflammatory cytokines and chemokines (TNF-α, IL-1β, IL-6, CCL2 and iNOS) Inhibit NF-κB, STAT1 and STAT3 expression in macrophage Attenuate IKKα/β, IKBα phosphorylation and p65 levels in LPS-stimulated macrophage Inhibit translocation of p65 from cytoplasm to nucleus in LPS-stimulated macrophage Downregulate phosphorylation of ERK1/2, JNK and p38 levels in macrophage Suppress STAT1 and STAT3 activation in LPS-induced macrophage and sepsis mice | E. coli-induced mice sepsis mice and LPS-stimulated macrophage of lung injury (RAW 264.7 cells) | 20, 40 and 60 mg/kg | In vitro and in vivo | [67] | |
| Decrease iNOS and COX-2 level Inhibition of NO and PGE2 production Inhibit TNF-α, IL-1β expression Inhibit LPS-mediated nuclear translocation of NF-κBp65 by suppressing IKβ-α degradation Inhibit ROS generation | LPS-induced inflammation in RAW 264.7 cells | 12 µg/mL | In vitro | [68] | |
| Reverse LTA-induced IkB degradation Reverse NF-κBp65 phosphorylation Increase Nrf2 activity and scavenge DPPH radicals Inhibit NF-κBp65 translocation to nucleus | RAW 264.7 cells | 20 µM | In vitro | [69] | |
| Reduce IL-1β, IL-6, TNF-α in serum and hippocampus Downregulate iNOS and COX-2 in hippocampus Inhibit LPS-induced pIKK-α, pIKK-β, pIKB-α and p-NF-kB65 activation Upregulate p-TrKB protein expression in hippocampus due to activation of BDNF/TrKB signaling pathway, thus exhibit neuroprotective activity | LPS-induced neuro-inflammation in mice and hippocampus protein extract | 20, 40 mg/kg | In vivo | [72] | |
| Increase endocytic activity and augmented NO and iNOS levels in LPS-treated macrophage | LPS-induced inflammation in RAW 264.7 cells and BALB/c mice | 80 and 120 µM | In vitro and in vivo | [73] | |
| Reduce MMP-1 in cartilage Reduce NO and PGE2 in synovial fluid | Knee OA model of rabbit | 100 and 200 mg/kg | In vivo | [74] | |
| Decrease NO, TNF-α and MCP-1 expression Inhibit PPARϒ and CCAAT/enhancer binding protein-α in adipocyte Inhibit iNOS level in macrophage Increase silencing of heme oxygenase | Adipose tissue inflammation model (RAW264.7 cells and 3T3-L1 adipocyte cells) | 100 µM | In vitro | [75] | |
| Inhibit pro-inflammatory cytokines (IL-2, IL-1β, TNF-α, INF-ϒ) in colon Inhibit ROS generation Inhibit MPO and ALP Decrease GSH depletion | TNBS-induced colitis in male Wistar rats and RAW 264.7 cells | 5 mg/kg, 100 µM | In vitro and in vivo | [77] | |
| Increase GSH and serotonin (5-HT) level in brain tissue Decrease TBARS, TNF-α, IL-1β levels in brain tissue | Reserpine-induced fibromyalgia in female Swiss albino mice | 100 mg/kg | In vivo | [78] | |
| Suppress histamine-induced expressions and secretion of IL-6, IL-8, MUC5AC by inhibiting NF-kB signaling pathway Suppress histamine-induced p-p65 expression and p-IKBα degradation | Allergic rhinitis model (Human nasal epithelial cells) | 10, 20 and 40 µmol/L | In vivo | [79] | |
| Anti-inflammatory | Reduction in ear swelling Decrease DFE/DNCB-induced scratching Decrease epidermal and dermal thickness Decrease accumulation of mast cells Decrease TNF-α, INF-ϒ, IL-4, IL-13, IL-31, IL-17A-induced phosphorylation of STAT1 and NF-κB (p65) translocation by degrading IKBα | DNCB/DFE—induced atopic skin inflammation model (Female BALB/c mice and Human HaCaT keratinocytes cells) | 2, 10, 50 mg/kg and 10 µM | In vitro and in vivo | [80] |
| Decrease attenuation of LPS-induced phosphorylation of ERK1/2 and NF-κB expression Protect cells from LPS-induced apoptosis and necrosis Decrease LPS-induced TRAIL, IL-1β, TNFR expression Inhibit LPS-induced MnSOD and GPx Downregulate IL-6, IL-12, VEGF expressions | LPS-induced inflammation in Human retinal pigment epithelial cells (ARPE-19 cells) | 5 µM | In vitro | [81] | |
| Decrease MPO, IL-6, TNF-α, IL-1β expression Inhibit neutrophils infiltration Inhibit LPS-induced RhoA/Rho kinase pathway Block NF-κB activation | LPS-induced acute lung injury (lung epithelial A549 cells and BALB/c mice) | 20, 40 mg/kg and 0.1, 1 and 10 µM | In vitro and in vivo | [82] | |
| Decrease MPO, COX-2, iNOS levels Activate HIF-1in HCT116 cells and increase HIF-1α protein expression Increase secretion of VEGF in HCT116 cells Inhibit HIF-prolyl hydroxylase-2 Enzyme | TNBC-induced colitis (Human colon carcinoma HCT116 cells and Sprague Dawley colitic rats) | 100 and 200 µM | In vitro and in vivo | [83] | |
| Ameliorate skin lesion of psoriatic mice Inhibit CD3+ and CD8+ T-cell infiltration in psoriatic mice skin Decrease Ki67 and K10 mRNA expression Lower effector CD8+ T-cells in lymph nodes and spleen Inhibit NF-κB signaling by suppressing phosphor-IKKα and phosphor-p65 expression Increase CD4+ FOXp3+ Treg frequency in lymph node and spleen Downregulate IL-6, TNF-α, IFN-ϒ, IL-17A, IL-22, IL-23 | Imiquimod-induced psoriasis in BALB/c mice | 50 and 100 mg/kg | In vivo | [84] |
| Pharmacological Mechanism | Inhibition/Activation/ Downregulation/Upregulation | Model Used | Dosage | Application | Reference |
|---|---|---|---|---|---|
| Anti-arthritic | Inhibit IL-1α-induced release of proteoglycan in cartilage Downregulate proMMP-1 and MMP-3 expression in cartilage Inhibit matrix degradation in rabbit joints | OA rabbit model | 10–100 µM | In vivo | [95] |
| Suppress proteoglycan depletion in chondrocyte Inhibit MMP production Downregulate pro-MMP 1 and pro-MMP3 in rabbit chondrocyte | Rabbit articular chondrocyte | 100 µM | In vivo | [96] | |
| Decrease leukotriene B4 level in plasma | AIA in male Lewis rats | 10 mg/kg | In vivo | [97] | |
| Decline paw volume Prevent swelling, bone and cartilage destruction Downregulate Cat-D, ACP, ALP and TRAP bone degrading enzymes Inhibit endogenous generation of ROS and TNF-α, IL-1β, IL-6, COX-2 and PGE2 level Increase ALT and AST level Inhibit NF-κB and Akt signaling pathway Restore SOD, CAT and GST enzyme | AIA in adult Wistar rats | 50 mg/kg | In vivo | [98] | |
| Inhibition of proteoglycan and collagen resorption Inhibit IL-1α + oncostatin M stimulated resorption and decreased the MMP-1 level Reduce IL-1α + oncostatin M induced expressions of MMP-1, MMP-3 and MMP-13 | Transformed human chondrocyte cells (T/C28a4 cells) | 66 µM, 100 µM and 50 µmol/L | In vitro | [99] |
| Pharmacological Mechanism | Inhibition/Activation/ Downregulation/Upregulation | Model Used | Dosage | Application | Reference |
|---|---|---|---|---|---|
| Anti-diabetic and its complications | Restore level of antioxidant enzymes (GST, COD, CAT, GPx) Increase plasma insulin in diabetic rats Decrease blood glucose in diabetic rats Decrease TBARS, lipid hydroperoxides and conjugated dienes in liver and kidney Increase vitamin C, tocopherol and reduce glutathione in kidney tissues of diabetic rats | STZ-induced diabetes in male albino rats | 40 mg/kg | In vivo | [103] |
| Prevent increase in angiotensin II type I receptor and angiotensin II type 2 receptor expression Improve insulin sensitivity and reduce systolic blood pressure Attenuate vascular hyper-responsiveness to Angiotensin II and impair acetylcholine-mediated relaxation Decrease TGF-β and KEAP-1 expression | HFD + STZ-induced hyperinsulinemia and hyperglycemia in male Wistar rats | 50 and 100 mg/kg | In vivo | [104] | |
| Decrease blood glucose, urea nitrogen, plasma creatinine Increase plasma albumin level Attenuate downregulation of PPARϒ in diabetic kidney and blocks TGF-β1-mediated fibronectin expression Attenuate decrease in mono-methylation (k4) and acetylation of histone H3 in diabetic kidney Decrease Bmp6 expression and increase Mmp13 expression in diabetic kidney | STZ-induced diabetic nephropathy in Sprague Dawley rats | 50 and 100 mg/kg | In vivo | [105] | |
| Attenuate alteration in RAS, KEAP-1 and cell proliferation (Ki67) Decrease systolic blood pressure, plasma glucose, triacylglycerol and total cholesterol in insulin resistance and type 2 diabetic rats Prevent cardiac hypertrophy and cardiac fibrosis in diabetic rats Reduce AT1R, A2TR, KEAP, Ki67 and increase ACE2 expression in insulin resistance and type 2 diabetic rats Attenuate H2AK119Ub and H2BK12OUb level in heart tissue of insulin resistance and type 2 diabetic rats | STZ-induced type diabetes and diabetic cardiomyopathy | 50 and 100 mg/kg | In vivo | [106] | |
|
Improve insulin sensitivity, hyperglycemia, and renal dysfunction Increase SOD1, GSH level and decrease TBARS levels in diabetic rats Increase angiotensin I converting enzyme 2 (ACE2) Decrease angiotensin II receptor type I and angiotensin II converting enzyme Decrease MCP-1 and TGF-β expressions in diabetic kidney Decrease H2AK119Ub expression in diabetic kidney | HFD + STZ-induced diabetic nephropathy | 50 and 100 mg/kg | In vivo | [107] |
| Pharmacological Mechanism | Inhibition/Activation/ Downregulation/Upregulation | Model Used | Dosage | Application | Reference |
|---|---|---|---|---|---|
| Anti-hepatic | Decrease plasma triglyceride, cholesterol, and insulin levels Increase AST, and ALT and prevent hepatic fibrosis Inhibit lipid peroxidation and increase GSH level in HFD-fed rats Increase FOXO1 phosphorylation in liver tissue of HFD-fed rats Prevent accumulation of extracellular matrix protein in the liver by reducing TGF-β expression | HFD-induced fatty liver in male Wistar rats | 50 and 100 mg/kg | In vivo | [108] |
| Downregulate lipid synthesis genes (Fasn, Dgat2, pap) and inflammatory genes (TLR4, Myd88, NF-kB, TNF-α, IL-6, and MCP-1) Increase SOD level and inhibit lipid peroxidation | HFD-induced non-alcoholic fatty liver in diabetes in C57BL/6N mice | 0.01% w/w | In vivo | [109] | |
| Increase phosphorylation of AMPK-α (Thr172) and ACC (Ser79) Decrease SREBP1c and FAS Activate AMPK signaling pathway | Free fatty acid-induced lipid accumulation in Human HepG2 cells | 25, 50 and 100 µM | In vitro | [110] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garg, S.S.; Gupta, J.; Sahu, D.; Liu, C.-J. Pharmacological and Therapeutic Applications of Esculetin. Int. J. Mol. Sci. 2022, 23, 12643. https://doi.org/10.3390/ijms232012643
Garg SS, Gupta J, Sahu D, Liu C-J. Pharmacological and Therapeutic Applications of Esculetin. International Journal of Molecular Sciences. 2022; 23(20):12643. https://doi.org/10.3390/ijms232012643
Chicago/Turabian StyleGarg, Sourbh Suren, Jeena Gupta, Debasis Sahu, and Chuan-Ju Liu. 2022. "Pharmacological and Therapeutic Applications of Esculetin" International Journal of Molecular Sciences 23, no. 20: 12643. https://doi.org/10.3390/ijms232012643
APA StyleGarg, S. S., Gupta, J., Sahu, D., & Liu, C. -J. (2022). Pharmacological and Therapeutic Applications of Esculetin. International Journal of Molecular Sciences, 23(20), 12643. https://doi.org/10.3390/ijms232012643