Antimicrobial Peptides Mediate Apoptosis by Changing Mitochondrial Membrane Permeability
Abstract
:1. Introduction
2. AMPs and Apoptosis
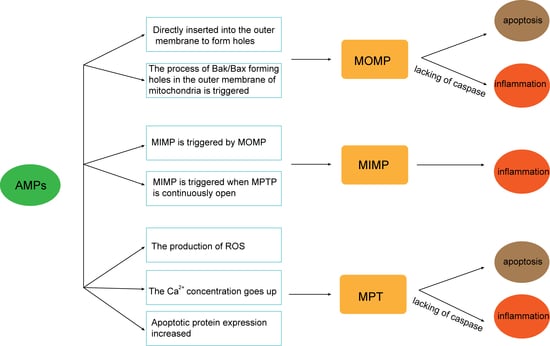
3. AMPs and MOMP
4. AMPs and MIMP
5. AMPs and MPTP
6. Challenges for Antimicrobial Peptides
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, M.; Zhang, Y.; Zhang, Q.; Li, J. Tumor Extracellular Matrix Modulating Strategies for Enhanced Antitumor Therapy of Nanomedicines. Mater. Today Bio 2022, 16, 100364. [Google Scholar] [CrossRef]
- Pacios, O.; Blasco, L.; Bleriot, I.; Fernandez-Garcia, L.; González Bardanca, M.; Ambroa, A.; López, M.; Bou, G.; Tomás, M. Strategies to Combat Multidrug-Resistant and Persistent Infectious Diseases. Antibiotics 2020, 9, 65. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, S.; Wang, Y.; Xue, Z.; Jia, Y.; Li, R.; He, C.; Chen, H. The Structure-Mechanism Relationship and Mode of Actions of Antimicrobial Peptides: A Review. Trends Food Sci. Technol. 2021, 109, 103–115. [Google Scholar] [CrossRef]
- Browne, K.; Chakraborty, S.; Chen, R.; Willcox, M.D.; Black, D.S.; Walsh, W.R.; Kumar, N. A New Era of Antibiotics: The Clinical Potential of Antimicrobial Peptides. Int. J. Mol. Sci. 2020, 21, 7047. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zuo, S.; Wang, B.; Zhang, K.; Wang, Y. Antimicrobial Mechanisms and Clinical Application Prospects of Antimicrobial Peptides. Molecules 2022, 27, 2675. [Google Scholar] [CrossRef]
- Luo, Y.; Song, Y. Mechanism of Antimicrobial Peptides: Antimicrobial, Anti-Inflammatory and Antibiofilm Activities. Int. J. Mol. Sci. 2021, 22, 11401. [Google Scholar] [CrossRef] [PubMed]
- Marchand, C.; Krajewski, K.; Lee, H.-F.; Antony, S.; Johnson, A.A.; Amin, R.; Roller, P.; Kvaratskhelia, M.; Pommier, Y. Covalent Binding of the Natural Antimicrobial Peptide Indolicidin to DNA Abasic Sites. Nucleic Acids Res. 2006, 34, 5157–5165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, X.; Wang, J. Development and progress of peptide antibiotics. J. Reg. Anat. Oper. 2013, 22, 659–662. [Google Scholar]
- Kumar, P.; Kizhakkedathu, J.N.; Straus, S.K. Antimicrobial Peptides: Diversity, Mechanism of Action and Strategies to Improve the Activity and Biocompatibility In Vivo. Biomolecules 2018, 8, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cochrane, S.A.; Findlay, B.; Bakhtiary, A.; Acedo, J.Z.; Rodriguez-Lopez, E.M.; Mercier, P.; Vederas, J.C. Antimicrobial Lipopeptide Tridecaptin A1 Selectively Binds to Gram-Negative Lipid II. Proc. Natl. Acad. Sci. USA 2016, 113, 11561–11566. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wadhwani, P.; Sekaran, S.; Strandberg, E.; Bürck, J.; Chugh, A.; Ulrich, A.S. Membrane Interactions of Latarcins: Antimicrobial Peptides from Spider Venom. Int. J. Mol. Sci. 2021, 22, 10156. [Google Scholar] [CrossRef]
- Vo, T.T.T.; Liu, J.-F.; Wu, C.-Z.; Lin, W.-N.; Chen, Y.-L.; Lee, I.-T. Surfactin from Bacillus Subtilis Induces Apoptosis in Human Oral Squamous Cell Carcinoma through ROS-Regulated Mitochondrial Pathway. J. Cancer 2020, 11, 7253–7263. [Google Scholar] [CrossRef]
- Zhao, H.; Yan, L.; Xu, X.; Jiang, C.; Shi, J.; Zhang, Y.; Liu, L.; Lei, S.; Shao, D.; Huang, Q. Potential of Bacillus Subtilis Lipopeptides in Anti-Cancer I: Induction of Apoptosis and Paraptosis and Inhibition of Autophagy in K562 Cells. AMB Express 2018, 8, 78. [Google Scholar] [CrossRef]
- Jiang, H.; Ji, C.; Sui, J.; Sa, R.; Wang, X.; Liu, X.; Guo, T.L. Antibacterial and Antitumor Activity of Bogorol B-JX Isolated from Brevibacillus Laterosporus JX-5. World J. Microbiol. Biotechnol. 2017, 33, 177. [Google Scholar] [CrossRef]
- Bock, F.J.; Tait, S.W.G. Mitochondria as Multifaceted Regulators of Cell Death. Nat. Rev. Mol. Cell Biol. 2020, 21, 85–100. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Yin, F.; Xu, J.; Zhang, T.; Wang, G.; Mao, M.; Wang, Z.; Sun, W.; Han, J.; Yang, M. CYT997(Lexibulin) Induces Apoptosis and Autophagy through the Activation of Mutually Reinforced ER Stress and ROS in Osteosarcoma. J. Exp. Clin. Cancer Res. 2019, 38, 44. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, W.; Zhu, B.; Wang, X. Epithelial Mitochondrial Dysfunction in Lung Disease. In Mitochondrial DNA and Diseases; Sun, H., Wang, X., Eds.; Advances in Experimental Medicine and Biology; Springer: Singapore, 2017; pp. 201–217. ISBN 978-981-10-6674-0. [Google Scholar]
- Su, B.-C.; Pan, C.-Y.; Chen, J.-Y. Antimicrobial Peptide TP4 Induces ROS-Mediated Necrosis by Triggering Mitochondrial Dysfunction in Wild-Type and Mutant P53 Glioblastoma Cells. Cancers 2019, 11, 171. [Google Scholar] [CrossRef] [Green Version]
- Hou, D.; Hu, F.; Mao, Y.; Yan, L.; Zhang, Y.; Zheng, Z.; Wu, A.; Forouzanfar, T.; Pathak, J.L.; Wu, G. Cationic Antimicrobial Peptide NRC-03 Induces Oral Squamous Cell Carcinoma Cell Apoptosis via CypD-MPTP Axis-Mediated Mitochondrial Oxidative Stress. Redox Biol. 2022, 54, 102355. [Google Scholar] [CrossRef] [PubMed]
- Kroemer, G.; Reed, J.C. Mitochondrial Control of Cell Death. Nat. Med. 2000, 6, 513–519. [Google Scholar] [CrossRef] [PubMed]
- Aslam, M.; Kanthlal, S.K.; Panonummal, R. Peptides: A Supercilious Candidate for Activating Intrinsic Apoptosis by Targeting Mitochondrial Membrane Permeability for Cancer Therapy. Int. J. Pept. Res. Ther. 2021, 27, 2883–2893. [Google Scholar] [CrossRef]
- Zhao, Y.-H.; Shen, C.-F.; Kang, Y.; Qi, A.; Xu, W.-J.; Shi, W.-H.; Liu, J.-W. Curcumin Prevents Renal Cell Apoptosis in Acute Kidney Injury in a Rat Model of Dry-Heat Environment Heatstroke via Inhibition of the Mitochondrial Apoptotic Pathway. Exp. Ther. Med. 2021, 21, 126. [Google Scholar] [CrossRef]
- Gao, T.; Xu, H.; Jia, S.; Cai, Z.; Chen, B.; Fan, G.; Zhang, Z.; Chen, G. Magnolol Induces Human Ewing Sarcoma SK-ES-1 Cell Apoptosis via the Mitochondrial and Death Receptor Pathways. Am. J. Transl. Res. 2020, 12, 1672–1682. [Google Scholar] [PubMed]
- Moretta, A.; Scieuzo, C.; Petrone, A.M.; Salvia, R.; Manniello, M.D.; Franco, A.; Lucchetti, D.; Vassallo, A.; Vogel, H.; Sgambato, A.; et al. Antimicrobial Peptides: A New Hope in Biomedical and Pharmaceutical Fields. Front. Cell. Infect. Microbiol. 2021, 11, 453. [Google Scholar] [CrossRef] [PubMed]
- Di Somma, A.; Recupido, F.; Cirillo, A.; Romano, A.; Romanelli, A.; Caserta, S.; Guido, S.; Duilio, A. Antibiofilm Properties of Temporin-L on Pseudomonas Fluorescens in Static and In-Flow Conditions. Int. J. Mol. Sci. 2020, 21, E8526. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Zhang, H.; Gao, C.; Chen, R.; Li, C. Antimicrobial Mechanism of PBD2 against Staphylococcus Aureus. Molecules 2020, 25, 3513. [Google Scholar] [CrossRef]
- Li, J.; Hui, L.; Zhan, S.; Wang, X. Inhibition of Antimicrobial Peptide 17BIPHE2 on Lung Adenocarcinoma A549 Cells and Related Mechanism. Cancer Res. Prev. Treat. 2017, 44, 659–664. [Google Scholar]
- Hazafa, A.; Batool, A.; Ahmad, S.; Amjad, M.; Chaudhry, S.N.; Asad, J.; Ghuman, H.F.; Khan, H.M.; Naeem, M.; Ghani, U. Humanin: A Mitochondrial-Derived Peptide in the Treatment of Apoptosis-Related Diseases. Life Sci. 2021, 264, 118679. [Google Scholar] [CrossRef] [PubMed]
- Morris, D.L.; Kastner, D.W.; Johnson, S.; Strub, M.-P.; He, Y.; Bleck, C.K.E.; Lee, D.-Y.; Tjandra, N. Humanin Induces Conformational Changes in the Apoptosis Regulator BAX and Sequesters It into Fibers, Preventing Mitochondrial Outer-Membrane Permeabilization. J. Biol. Chem. 2019, 294, 19055–19065. [Google Scholar] [CrossRef]
- Bankell, E.; Liu, X.; Lundqvist, M.; Svensson, D.; Swärd, K.; Sparr, E.; Nilsson, B.-O. The Antimicrobial Peptide LL-37 Triggers Release of Apoptosis-Inducing Factor and Shows Direct Effects on Mitochondria. Biochem. Biophys. Rep. 2022, 29, 101192. [Google Scholar] [CrossRef]
- Zhou, H.; Forveille, S.; Sauvat, A.; Sica, V.; Izzo, V.; Durand, S.; Müller, K.; Liu, P.; Zitvogel, L.; Rekdal, Ø.; et al. The Oncolytic Peptide LTX-315 Kills Cancer Cells through Bax/Bak-Regulated Mitochondrial Membrane Permeabilization. Oncotarget 2015, 6, 26599–26614. [Google Scholar] [CrossRef] [Green Version]
- Niklison Chirou, M.V.; Minahk, C.J.; Morero, R.D. Antimitochondrial Activity Displayed by the Antimicrobial Peptide Microcin J25. Biochem. Biophys. Res. Commun. 2004, 317, 882–886. [Google Scholar] [CrossRef] [PubMed]
- Ugarte-Alvarez, O.; Muñoz-López, P.; Moreno-Vargas, L.M.; Prada-Gracia, D.; Mateos-Chávez, A.A.; Becerra-Báez, E.I.; Luria-Pérez, R. Cell-Permeable Bak BH3 Peptide Induces Chemosensitization of Hematologic Malignant Cells. J. Oncol. 2020, 2020, e2679046. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Achirica, P.; Prieto, S.; Ubach, J.; Andreu, D.; Rial, E.; Rivas, L. Permeabilization of the Mitochondrial Inner Membrane by Short Cecropin-A–Melittin Hybrid Peptides. Eur. J. Biochem. 1994, 224, 257–263. [Google Scholar] [CrossRef] [PubMed]
- Lemeshko, V.V. Potential-Dependent Membrane Permeabilization and Mitochondrial Aggregation Caused by Anticancer Polyarginine-KLA Peptides. Arch. Biochem. Biophys. 2010, 493, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Kerkhofs, M.; La Rovere, R.; Welkenhuysen, K.; Janssens, A.; Vandenberghe, P.; Madesh, M.; Parys, J.B.; Bultynck, G. BIRD-2, a BH4-Domain-Targeting Peptide of Bcl-2, Provokes Bax/Bak-Independent Cell Death in B-Cell Cancers through Mitochondrial Ca2+-Dependent MPTP Opening. Cell Calcium 2021, 94, 102333. [Google Scholar] [CrossRef]
- Risso, A.; Braidot, E.; Sordano, M.C.; Vianello, A.; Macrì, F.; Skerlavaj, B.; Zanetti, M.; Gennaro, R.; Bernardi, P. BMAP-28, an Antibiotic Peptide of Innate Immunity, Induces Cell Death through Opening of the Mitochondrial Permeability Transition Pore. Mol. Cell. Biol. 2002, 22, 1926–1935. [Google Scholar] [CrossRef] [Green Version]
- Li, C. Preliminary Study on the Mechanism of Mitochondrial Membrane Potential by Antimicrobial Peptide CGA-N12. Master’s Thesis, Henan University of Technology, Zhengzhou, China, 2017. [Google Scholar]
- Hua, X.; Wang, T.; Bi, Y.; Tao, Y.; Kong, D.; Tang, R.; Wu, J. Activity and mechanism of the antibacterial peptide Mt6-21DLeu derived from a Musca domestica antifungal peptide against Candida albicans. J. Pathog. Biol. 2020, 15, 761–767. [Google Scholar] [CrossRef]
- Liu, S.; Aweya, J.J.; Zheng, L.; Wang, F.; Zheng, Z.; Zhong, M.; Lun, J.; Zhang, Y. A Litopenaeus Vannamei Hemocyanin-Derived Antimicrobial Peptide (Peptide B11) Attenuates Cancer Cells’ Proliferation. Molecules 2018, 23, 3202. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.; Hwang, J.-S.; Lee, D.G. Scolopendin, an Antimicrobial Peptide from Centipede, Attenuates Mitochondrial Functions and Triggers Apoptosis in Candida Albicans. Biochem. J. 2017, 474, 635–645. [Google Scholar] [CrossRef]
- Yang, Y.; Chen, H.-Y.; Hao, H.; Wang, K.-J. The Anticancer Activity Conferred by the Mud Crab Antimicrobial Peptide Scyreprocin through Apoptosis and Membrane Disruption. Int. J. Mol. Sci. 2022, 23, 5500. [Google Scholar] [CrossRef]
- Yuan, C.-H.; Ma, Y.-L.; Shih, P.-C.; Chen, C.-T.; Cheng, S.-Y.; Pan, C.-Y.; Jean, Y.-H.; Chu, Y.-M.; Lin, S.-C.; Lai, Y.-C.; et al. The Antimicrobial Peptide Tilapia Piscidin 3 Induces Mitochondria-Modulated Intrinsic Apoptosis of Osteosarcoma Cells. Biochem. Pharmacol. 2020, 178, 114064. [Google Scholar] [CrossRef]
- Su, B.-C.; Liu, Y.-C.; Ting, C.-H.; Lyu, P.-C.; Chen, J.-Y. Antimicrobial Peptide TP4 Targets Mitochondrial Adenine Nucleotide Translocator 2. Mar. Drugs 2020, 18, 417. [Google Scholar] [CrossRef]
- Candé, C.; Cohen, I.; Daugas, E.; Ravagnan, L.; Larochette, N.; Zamzami, N.; Kroemer, G. Apoptosis-Inducing Factor (AIF): A Novel Caspase-Independent Death Effector Released from Mitochondria. Biochimie 2002, 84, 215–222. [Google Scholar] [CrossRef]
- Hüttemann, M.; Pecina, P.; Rainbolt, M.; Sanderson, T.H.; Kagan, V.E.; Samavati, L.; Doan, J.W.; Lee, I. The Multiple Functions of Cytochrome c and Their Regulation in Life and Death Decisions of the Mammalian Cell: From Respiration to Apoptosis. Mitochondrion 2011, 11, 369–381. [Google Scholar] [CrossRef] [Green Version]
- Waterhouse, N.J.; Goldstein, J.C.; von Ahsen, O.; Schuler, M.; Newmeyer, D.D.; Green, D.R. Cytochrome C Maintains Mitochondrial Transmembrane Potential and Atp Generation after Outer Mitochondrial Membrane Permeabilization during the Apoptotic Process. J. Cell Biol. 2001, 153, 319–328. [Google Scholar] [CrossRef]
- Green, D.R.; Kroemer, G. The Pathophysiology of Mitochondrial Cell Death. Science 2004, 305, 626–629. [Google Scholar] [CrossRef]
- Czabotar, P.E.; Westphal, D.; Dewson, G.; Ma, S.; Hockings, C.; Fairlie, W.D.; Lee, E.F.; Yao, S.; Robin, A.Y.; Smith, B.J.; et al. Bax Crystal Structures Reveal How BH3 Domains Activate Bax and Nucleate Its Oligomerization to Induce Apoptosis. Cell 2013, 152, 519–531. [Google Scholar] [CrossRef] [Green Version]
- Salvador-Gallego, R.; Mund, M.; Cosentino, K.; Schneider, J.; Unsay, J.; Schraermeyer, U.; Engelhardt, J.; Ries, J.; García-Sáez, A.J. Bax Assembly into Rings and Arcs in Apoptotic Mitochondria Is Linked to Membrane Pores. EMBO J. 2016, 35, 389–401. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qi, H.; Jiang, Y.; Yin, Z.; Jiang, K.; Li, L.; Shuai, J. Optimal Pathways for the Assembly of the Apaf-1·cytochrome c Complex into Apoptosome. Phys. Chem. Chem. Phys. 2018, 20, 1964–1973. [Google Scholar] [CrossRef] [PubMed]
- Giampazolias, E.; Zunino, B.; Dhayade, S.; Bock, F.; Cloix, C.; Cao, K.; Roca, A.; Lopez, J.; Ichim, G.; Proïcs, E.; et al. Mitochondrial Permeabilization Engages NF-ΚB-Dependent Anti-Tumour Activity under Caspase Deficiency. Nat. Cell Biol. 2017, 19, 1116–1129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qi, H.; Li, Z.; Shi, Z. The roles of mitochondrial membrane integrity in the regulation of cell fate. Prog. Biochem. Biophys. 2021, 1–11. [Google Scholar] [CrossRef]
- McArthur, K.; Whitehead, L.W.; Heddleston, J.M.; Li, L.; Padman, B.S.; Oorschot, V.; Geoghegan, N.D.; Chappaz, S.; Davidson, S.; San Chin, H.; et al. BAK/BAX Macropores Facilitate Mitochondrial Herniation and MtDNA Efflux during Apoptosis. Science 2018, 359, eaao6047. [Google Scholar] [CrossRef] [PubMed]
- White, M.J.; McArthur, K.; Metcalf, D.; Lane, R.M.; Cambier, J.C.; Herold, M.J.; van Delft, M.F.; Bedoui, S.; Lessene, G.; Ritchie, M.E.; et al. Apoptotic Caspases Suppress MtDNA-Induced STING-Mediated Type I IFN Production. Cell 2014, 159, 1549–1562. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Cui, S.; Meng, W.; Hu, H.; Wang, C. Mitochondrial DNA and cGAS-STING Innate Immune Signaling Pathway: Latest Research Progress. J. Sichuan Univ. Med. Sci. Ed. 2021, 52, 387–395. [Google Scholar] [CrossRef]
- Riley, J.S.; Quarato, G.; Cloix, C.; Lopez, J.; O’Prey, J.; Pearson, M.; Chapman, J.; Sesaki, H.; Carlin, L.M.; Passos, J.F.; et al. Mitochondrial Inner Membrane Permeabilisation Enables MtDNA Release during Apoptosis. EMBO J. 2018, 37, e99238. [Google Scholar] [CrossRef] [PubMed]
- Martin, S.J.; Henry, C.M.; Cullen, S.P. A Perspective on Mammalian Caspases as Positive and Negative Regulators of Inflammation. Mol. Cell 2012, 46, 387–397. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Wang, Y.; Zhang, M. Mitochondria-mediated apoptosis:a review of recent studies. J. Environ. Health 2013, 30, 182–185. [Google Scholar] [CrossRef]
- Cai, X.; Chen, G.; Chen, Z.; Wang, Z. Mitochondrial Transmembrane Potential and Cell Apoptosis. Prog. Biochem. Biophys. 2001, 12, 3–6. [Google Scholar]
- Rasheed, M.Z.; Tabassum, H.; Parvez, S. Mitochondrial Permeability Transition Pore: A Promising Target for the Treatment of Parkinson’s Disease. Protoplasma 2017, 254, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.; Wei, J.; Han, Q.; Liu, R.; Duan, X.; Fu, Y.; Huang, X.; Wang, X.; Kang, Z. PsANT, the Adenine Nucleotide Translocase of Puccinia Striiformis, Promotes Cell Death and Fungal Growth. Sci. Rep. 2015, 5, 11241. [Google Scholar] [CrossRef] [Green Version]
- Reina, S.; Checchetto, V.; Saletti, R.; Gupta, A.; Chaturvedi, D.; Guardiani, C.; Guarino, F.; Scorciapino, M.A.; Magrì, A.; Foti, S.; et al. VDAC3 as a Sensor of Oxidative State of the Intermembrane Space of Mitochondria: The Putative Role of Cysteine Residue Modifications. Oncotarget 2016, 7, 2249–2268. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Halestrap, A.P.; Richardson, A.P. The Mitochondrial Permeability Transition: A Current Perspective on Its Identity and Role in Ischaemia/Reperfusion Injury. J. Mol. Cell. Cardiol. 2015, 78, 129–141. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Li, D.; Tao, M.; Shi, W.; Li, R. Antimicrobial Peptide CGA-N12-induced Reactive Oxygen Species Opern the Mitochondrial Permeability Transition Pores of Candida tropicalis. Chin. J. Biochem. Mol. 2020, 36, 1090–1098. [Google Scholar] [CrossRef]
- Hurst, S.; Hoek, J.; Sheu, S.-S. Mitochondrial Ca2+ and Regulation of the Permeability Transition Pore. J. Bioenerg. Biomembr. 2017, 49, 27–47. [Google Scholar] [CrossRef] [Green Version]
- Pistritto, G.; Trisciuoglio, D.; Ceci, C.; Garufi, A.; D’Orazi, G. Apoptosis as Anticancer Mechanism: Function and Dysfunction of Its Modulators and Targeted Therapeutic Strategies. Aging 2016, 8, 603–619. [Google Scholar] [CrossRef] [Green Version]
- Zong, W.-X.; Rabinowitz, J.D.; White, E. Mitochondria and Cancer. Mol. Cell 2016, 61, 667–676. [Google Scholar] [CrossRef] [Green Version]
- Rima, M.; Rima, M.; Fajloun, Z.; Sabatier, J.-M.; Bechinger, B.; Naas, T. Antimicrobial Peptides: A Potent Alternative to Antibiotics. Antibiotics 2021, 10, 1095. [Google Scholar] [CrossRef]
- Li, D.; Yang, Y.; Li, R.; Huang, L.; Wang, Z.; Deng, Q.; Dong, S. N-Terminal Acetylation of Antimicrobial Peptide L163 Improves Its Stability against Protease Degradation. J. Pept. Sci. 2021, 27, e3337. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Zhang, W.; Gou, S.; Huang, H.; Yao, J.; Yang, Z.; Liu, H.; Zhong, C.; Liu, B.; Ni, J.; et al. Intramolecular Cyclization of the Antimicrobial Peptide Polybia-MPI with Triazole Stapling: Influence on Stability and Bioactivity. J. Pept. Sci. 2017, 23, 824–832. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.-Q.; Zhu, L.-J.; Xi, T.-K.; Zhu, H.-Y.; Chen, X.-X.; Wu, M.; Sun, C.; Xu, C.; Fang, G.-M.; Meng, X. Delivery of Cell Membrane Impermeable Peptides into Living Cells by Using Head-to-Tail Cyclized Mitochondria-Penetrating Peptides. Org. Biomol. Chem. 2019, 17, 9693–9697. [Google Scholar] [CrossRef]
- Liu, S.; Wang, B.; Sheng, Y.; Dong, S.; Liu, G. Rational Design of Self-Assembled Mitochondria-Targeting Lytic Peptide Conjugates with Enhanced Tumor Selectivity. Chem.–Eur. J. 2022, 28, e202103517. [Google Scholar] [CrossRef]
- Mao, Y. Study on the Structure-Activity Relationship of a-Helix Peptide. Master’s Thesis, China University of Petroleum (East China), Qingdao, China, 2018. [Google Scholar]
- Wang, C.; Hong, T.; Cui, P.; Wang, J.; Xia, J. Antimicrobial Peptides towards Clinical Application: Delivery and Formulation. Adv. Drug Deliv. Rev. 2021, 175, 113818. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.-Y.; Yan, Z.-B.; Meng, Y.-M.; Hong, X.-Y.; Shao, G.; Ma, J.-J.; Cheng, X.-R.; Liu, J.; Kang, J.; Fu, C.-Y. Antimicrobial Peptides: Mechanism of Action, Activity and Clinical Potential. Mil. Med. Res. 2021, 8, 48. [Google Scholar] [CrossRef] [PubMed]
- Oberst, A.; Bender, C.; Green, D.R. Living with Death: The Evolution of the Mitochondrial Pathway of Apoptosis in Animals. Cell Death Differ. 2008, 15, 1139–1146. [Google Scholar] [CrossRef] [Green Version]
- Scovassi, A.I.; Soldani, C.; Veneroni, P.; Bottone, M.G.; Pellicciari, C. Changes of Mitochondria and Relocation of the Apoptosis-Inducing Factor during Apoptosis. Ann. N. Y. Acad. Sci. 2009, 1171, 12–17. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Zhang, Y.-F.; Yang, F.-H.; Mao, H.-Q.; Chen, Z.; Zhang, L. Mitochondrial DNA Leakage Induces Odontoblast Inflammation via the CGAS-STING Pathway. Cell Commun. Signal. 2021, 19, 58. [Google Scholar] [CrossRef] [PubMed]



| Membrane Targets | Peptide | Source | Structure | Object | Mechanism | References |
|---|---|---|---|---|---|---|
| Outer mitochondrial membrane (OMM) | 17BIPHE2 | LL-37-derived peptide | α-helices | Lung adenocarcinoma A549 Cells | MOMP was changed by upregulating the expression of Bax and downregulating the expression of Bcl-2 | [27] |
| Humanin | Mitochondrial-derived peptides | α-helices; a loop structure | In vitro experiment | Combined with Bak and prevented MOMP | [28,29] | |
| LL-37 | White blood cells; epithelial cells | α-helices | Human osteoblast-like MG63 cells | Released Cyt c and promoted MOMP | [30] | |
| LTX-315 | Lactoferricin derivative | α-helices | U2OS cells | Released Cyt c and promoted MOMP | [31] | |
| MccJ25 | Escherichia coli | a loop structure | Rat heart mitochondria | Inserted in the mitochondrial membrane and changed membrane permeability | [32] | |
| PETK | Cell-permeable Bak BH3 peptide | α-helices; a loop structure | Human T acute lymphoblastic leukemia CCRF-CEM cells | Combined with Bak and promoted MOMP | [33] | |
| Inner mitochondrial membrane (IMM) | Cecropin A–melittin Hybrid Peptides | Artificial peptide | —— | Rat liver mitochondria | MIMP was changed, and the IMM was destructed | [34] |
| KLA | Artificial peptide | rich in Arg | Red blood cell (RBC) of Sprague Dawley rats | MIMP was changed | [35] | |
| Mitochondrial permeability transition pore (MPTP) | BBJX | Brevibacillus laterosporus JX-5 | —— | Human U-937, mouse spleen cells | Opened the MPTP and stimulated the production of ROS | [14] |
| BIRD-2 | BH4 domain-targeting peptide | —— | The SU-DHL-4 and KARPAS-422 DLBCL cell lines | Provoked mitochondrial Ca2+ overload followed by sustained MPTP opening | [36] | |
| BMAP-28 | Bovine | α-helices | The U937 and K562 cell lines | Caused depolarization of the IMM and released Cyt c | [37] | |
| CGA-N12 | Chromogranin-derived peptide | α-helices | Candida tropicalis | The accumulation of ROS in mitochondria causes MPTP to remain open | [38] | |
| Iturin | Bacillus subtilis | a loop structure | Myelogenous leukemia cells K562 | Caused ROS burst and upregulated expression of Cyt c, Bax, and Bad, together with downregulated expression of Bcl-2 | [13] | |
| Mt6-21DLeu | Musca domestica antifungal peptide MAF-1A derivative | α-helices | Candida albicans | Excess ROS was produced, followed by opening of the MPTP | [39] | |
| Peptide B11 | Litopenaeus vannamei hemocyanin-derived peptide | α-helices | Human cervical cancer cells (HeLa), human hepatocellular carcinoma (HepG2) | Mitochondrial membrane potential was lost | [40] | |
| Scolopendin | Scolopendra subspinipes mutilans | α-helices | Candida albicans | Ca2+ homeostasis, membrane potential, and Cyt c levels were disrupted in mitochondria | [41] | |
| Scyreprocin | Mud crab Scylla paramamosain | rich in Lys | Human non-small-cell lung cancer NCI-H460 cells | Induced the generation of ROS and led to Ca2+ release | [42] | |
| Surfactin | Bacillus subtilis | a loop structure | Human OSCC cell linesSCC4/SCC25 | Induced mitochondrial depolarization and mitochondrial-derived ROS production, Cyt c release | [12] | |
| TP3 | Oreochromis niloticus | α-helices | OS MG63 cells | Excess ROS was produced, followed by sustained MPTP opening | [43] | |
| TP4 | Oreochromis niloticus | α-helices | MCF-7 and A549 cell lines | Colocalized with ANT2 and regulated MPTP | [44] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.; Zhang, C.; Li, M.; Liu, C.; Wang, J.; Ou, X.; Han, Y. Antimicrobial Peptides Mediate Apoptosis by Changing Mitochondrial Membrane Permeability. Int. J. Mol. Sci. 2022, 23, 12732. https://doi.org/10.3390/ijms232112732
Wang H, Zhang C, Li M, Liu C, Wang J, Ou X, Han Y. Antimicrobial Peptides Mediate Apoptosis by Changing Mitochondrial Membrane Permeability. International Journal of Molecular Sciences. 2022; 23(21):12732. https://doi.org/10.3390/ijms232112732
Chicago/Turabian StyleWang, Hongji, Chaowen Zhang, Mengnan Li, Chaoran Liu, Jingyi Wang, Xuan Ou, and Yuzhu Han. 2022. "Antimicrobial Peptides Mediate Apoptosis by Changing Mitochondrial Membrane Permeability" International Journal of Molecular Sciences 23, no. 21: 12732. https://doi.org/10.3390/ijms232112732
APA StyleWang, H., Zhang, C., Li, M., Liu, C., Wang, J., Ou, X., & Han, Y. (2022). Antimicrobial Peptides Mediate Apoptosis by Changing Mitochondrial Membrane Permeability. International Journal of Molecular Sciences, 23(21), 12732. https://doi.org/10.3390/ijms232112732