Pioglitazone Attenuates the Effects of Peripheral Inflammation in a Human In Vitro Blood–Brain Barrier Model
Abstract
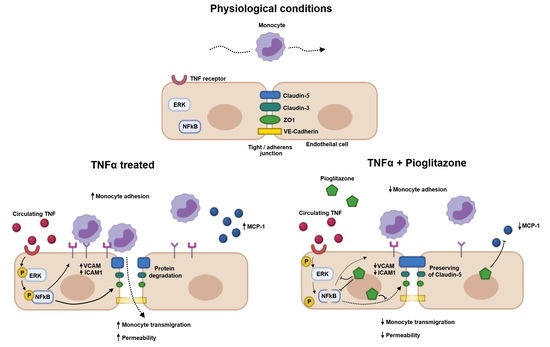
:1. Introduction
2. Results
2.1. The BBB Is Sensible to Peripheral Inflammatory Factors
2.2. Pioglitazone Improves BBB Permeability under TNFα Treatment Independently of Alterations in Junctional Protein Expression
2.3. TNFα-Induced Disarrangement of Claudin-5 Is Attenuated by Pioglitazone
2.4. Monocyte-Recruiting Alterations on the Inflamed BBB Are Halted by Pioglitazone
2.5. TNFα-Induced Phosphorylation of ERK and NF-kB Is Attenuated by Pioglitazone
2.6. Pioglitazone Reduces ICAM-1 Expression on Plasma-Treated BBB
3. Discussion
4. Materials and Methods
4.1. Human BBB Model Derived from Hematopoietic Stem Cells
4.1.1. Isolation and Culture of Human Endothelial Cells
4.1.2. Co-Culture and Brain-like Endothelial Cells
4.2. Collection of Blood from IBD Patients and Healthy Donors
4.3. Cell Treatments
4.4. Sodium Fluorescein (NaFlu) Permeability Coefficient (PeNaFlu)
4.5. Western Blotting
4.6. Conventional and Confocal Immunofluorescence Analysis
4.7. ELISA
4.8. Monocyte Culture and Transmigration Assay
4.9. Bioinformatics
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Huang, Y.; Wang, C.; Tian, X.; Mao, Y.; Hou, B.; Sun, Y.; Gu, X.; Ma, Z. Pioglitazone Attenuates Experimental Colitis-Associated Hyperalgesia through Improving the Intestinal Barrier Dysfunction. Inflammation 2020, 43, 568–578. [Google Scholar] [CrossRef] [PubMed]
- Kadry, H.; Noorani, B.; Cucullo, L. A blood–brain barrier overview on structure, function, impairment, and biomarkers of integrity. Fluids Barriers CNS 2020, 17, 69. [Google Scholar] [CrossRef]
- Sonar, S.A.; Lal, G. Blood-brain barrier and its function during inflammation and autoimmunity. J. Leukoc. Biol. 2018, 103, 839–853. [Google Scholar] [CrossRef]
- Honig, G.; Larkin, P.B.; Heller, C.; Hurtado-Lorenzo, A. Research-Based Product Innovation to Address Critical Unmet Needs of Patients with Inflammatory Bowel Diseases. Inflamm. Bowel Dis. 2021, 27, S1–S16. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.S.; Tan, W.R.; Low, Z.S.; Marvalim, C.; Lee, J.Y.H. Exploration and Development of PPAR Modulators in Health and Disease: An Update of Clinical Evidence. Int. J. Mol. Sci. 2019, 20, 5055. [Google Scholar] [CrossRef] [Green Version]
- Lewis, J.D.; Lichtenstein, G.R.; Deren, J.J.; Sands, B.E.; Hanauer, S.B.; Katz, J.A.; Lashner, B.; Present, D.H.; Chuai, S.; Ellenberg, J.H.; et al. Rosiglitazone for Active Ulcerative Colitis: A Randomized Placebo-Controlled Trial. Gastroenterology 2008, 134, 688–695. [Google Scholar] [CrossRef] [Green Version]
- Marder, W.; Khalatbari, S.; Myles, J.D.; Hench, R.; Lustig, S.; Yalavarthi, S.; Parameswaran, A.; Brook, R.D.; Kaplan, M.J. The Peroxisome Proliferator Activated Receptor-γ Pioglitazone Improves Vascular Function and Decreases Disease Activity in Patients With Rheumatoid Arthritis. J. Am. Heart. Assoc. 2013, 2, e000441. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shahin, D.; El Toraby, E.; Abdel-Malek, H.; Boshra, V.; Elsamanoudy, A.Z.; Shaheen, D. Effect of Peroxisome Proliferator-Activated Receptor Gamma Agonist (Pioglitazone) and Methotrexate on Disease Activity in Rheumatoid Arthritis (Experimental and Clinical Study). Clin. Med. Insights Arthritis Musculoskelet. Disord. 2011, 4, CMAMD-S5951. [Google Scholar] [CrossRef] [Green Version]
- Quan, Q.; Qian, Y.; Li, X.; Li, M. Pioglitazone Reduces beta Amyloid Levels via Inhibition of PPARγ Phosphorylation in a Neuronal Model of Alzheimer’s Disease. Front. Aging Neurosci. 2019, 11, 178. [Google Scholar] [CrossRef] [Green Version]
- Graham, D.J.; Ouellet-Hellstrom, R.; MaCurdy, T.E.; Ali, F.; Sholley, C.; Worrall, C.; Kelman, J.A. Risk of Acute Myocardial Infarction, Stroke, Heart Failure, and Death in Elderly Medicare Patients Treated With Rosiglitazone or Pioglitazone. JAMA 2010, 304, 411–418. [Google Scholar] [CrossRef] [PubMed]
- Nissen, S.E.; Wolski, K. Rosiglitazone Revisited. Arch. Intern. Med. 2010, 170, 1191–1201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Villumsen, M.; Aznar, S.; Pakkenberg, B.; Jess, T.; Brudek, T. Inflammatory bowel disease increases the risk of Parkinson’s disease: A Danish nationwide cohort study 1977–2014. Gut 2019, 68, 18–24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, B.; Wang, H.E.; Bai, Y.-M.; Tsai, S.-J.; Su, T.-P.; Chen, T.-J.; Wang, Y.-P.; Chen, M.-H. Inflammatory bowel disease is associated with higher dementia risk: A nationwide longitudinal study. Gut 2021, 70, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Hanscom, M.; Loane, D.J.; Aubretch, T.; Leser, J.; Molesworth, K.; Hedgekar, N.; Ritzel, R.M.; Abulwerdi, G.; Shea-Donohue, T.; Faden, A.I. Acute colitis during chronic experimental traumatic brain injury in mice induces dysautonomia and persistent extraintestinal, systemic, and CNS inflammation with exacerbated neurological deficits. J. Neuroinflammation 2021, 18, 1–31. [Google Scholar] [CrossRef] [PubMed]
- Da Rocha, G.H.O.; De Paula-Silva, M.; Broering, M.F.; Scharf, P.R.d.S.; Matsuyama, L.S.A.S.; Maria-Engler, S.S.; Farsky, S.H.P. Pioglitazone-Mediated Attenuation of Experimental Colitis Relies on Cleaving of Annexin A1 Released by Macrophages. Front. Pharmacol. 2020, 11, 591561. [Google Scholar] [CrossRef] [PubMed]
- Prasad Byrav, D.S.; Medhi, B.; Prakash, A.; Chakrabarti, A.; Vaiphei, K.; Khanduja, K.L. Comparative evaluation of different doses of PPAR-γ agonist alone and in combination with sulfasalazine in experimentally induced inflammatory bowel disease in rats. Pharmacol. Rep. 2013, 65, 951–959. [Google Scholar] [CrossRef]
- Takagi, T.; Naito, Y.; Tomatsuri, N.; Handa, O.; Ichikawa, H.; Yoshida, N.; Yoshikawa, T. Pioglitazone, a PPAR-γ ligand, provides protection from dextran sulfate sodium-induced colitis in mice in association with inhibition of the NF-κB-cytokine cascade. Redox Rep. 2022, 17, 47. [Google Scholar] [CrossRef]
- Liang, H.-L.; Ouyang, Q. A clinical trial of combined use of rosiglitazone and 5-aminosalicylate for ulcerative colitis. World J. Gastroenterol. 2008, 14, 114–119. [Google Scholar] [CrossRef]
- Akhter, N.; Wilson, A.; Thomas, R.; Al-Rashed, F.; Kochumon, S.; Al-Roub, A.; Arefanian, H.; Al-Madhoun, A.; Al-Mulla, F.; Ahmad, R.; et al. ROS/TNF-α Crosstalk Triggers the Expression of IL-8 and MCP-1 in Human Monocytic THP-1 Cells via the NF-κB and ERK1/2 Mediated Signaling. Int. J. Mol. Sci. 2021, 22, 10519. [Google Scholar] [CrossRef]
- Wang, S.; Peng, L.; Gai, Z.; Zhang, L.; Jong, A.; Cao, H.; Huang, S.-H. Pathogenic Triad in Bacterial Meningitis: Pathogen Invasion, NF-κB Activation, and Leukocyte Transmigration that Occur at the Blood-Brain Barrier. Front. Microbiol. 2016, 7, 148. [Google Scholar] [CrossRef]
- Sagar, D.; Lamontagne, A.; Foss, C.A.; Khan, Z.K.; Pomper, M.G.; Jain, P. Dendritic cell CNS recruitment correlates with disease severity in EAE via CCL2 chemotaxis at the blood–brain barrier through paracellular transmigration and ERK activation. J. Neuroinflammation 2012, 9, 245. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wosik, K.; Biernacki, K.; Khouzam, M.-P.; Prat, A. Death receptor expression and function at the human blood brain barrier. J. Neurol. Sci. 2007, 259, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Rochfort, K.D.; Cummins, P.M. The blood–brain barrier endothelium: A target for pro-inflammatory cytokines. Biochem. Soc. Trans. 2015, 43, 702–706. [Google Scholar] [CrossRef]
- Low, Y.L.; Jin, L.; Morris, E.R.; Pan, Y.; Nicolazzo, J.A. Pioglitazone Increases Blood–Brain Barrier Expression of Fatty Acid-Binding Protein 5 and Docosahexaenoic Acid Trafficking into the Brain. Mol. Pharm. 2020, 17, 873–884. [Google Scholar] [CrossRef] [PubMed]
- Gust, J.; Hay, K.A.; Hanafi, L.-A.; Li, D.; Myerson, D.; Gonzalez-Cuyar, L.F.; Yeung, C.; Liles, W.C.; Wurfel, M.; Lopez, J.A.; et al. Endothelial Activation and Blood–Brain Barrier Disruption in Neurotoxicity after Adoptive Immunotherapy with CD19 CAR-T Cells. Cancer Discov. 2017, 7, 1404–1419. [Google Scholar] [CrossRef] [Green Version]
- Borg-Bartolo, S.P.; Boyapati, R.K.; Satsangi, J.; Kalla, R. Precision medicine in inflammatory bowel disease: Concept, progress and challenges. F1000Research 2020, 9, 54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Korolkova, O.Y.; Myers, J.N.; Pellom, S.T.; Wang, L.; M’Koma, A.E. Characterization of Serum Cytokine Profile in Predominantly Colonic Inflammatory Bowel Disease to Delineate Ulcerative and Crohn’s Colitides. Clin. Med. Insights: Gastroenterol. 2015, 8, 29–44. [Google Scholar] [CrossRef] [Green Version]
- Morris, G.; Fernandes, B.S.; Puri, B.K.; Walker, A.; Carvalho, A.F.; Berk, M. Leaky brain in neurological and psychiatric disorders: Drivers and consequences. Aust. New Zealand J. Psychiatry 2018, 52, 924–948. [Google Scholar] [CrossRef]
- Dias, M.C.; Coisne, C.; Lazarevic, I.; Baden, P.; Hata, M.; Iwamoto, N.; Francisco, D.M.F.; Vanlandewijck, M.; He, L.; Baier, F.A.; et al. Claudin-3-deficient C57BL/6J mice display intact brain barriers. Sci. Rep. 2019, 9, 203. [Google Scholar] [CrossRef] [Green Version]
- Greene, C.; Kealy, J.; Humphries, M.M.; Gong, Y.; Hou, J.; Hudson, N.; Cassidy, L.M.; Martiniano, R.; Shashi, V.; Hooper, S.R.; et al. Dose-dependent expression of claudin-5 is a modifying factor in schizophrenia. Mol. Psychiatry 2018, 23, 2156–2166. [Google Scholar] [CrossRef]
- Saitou, M.; Furuse, M.; Sasaki, H.; Schulzke, J.D.; Fromm, M.; Takano, H.; Tsukita, S. Complex Phenotype of Mice Lacking Occludin, a Component of Tight Junction Strands. Mol. Biol. Cell 2000, 11, 4131–4142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fisher, D.; Mentor, S. Are claudin-5 tight-junction proteins in the blood-brain barrier porous? Neural Regen. Res. 2020, 15, 1838–1839. [Google Scholar] [CrossRef] [PubMed]
- Haruwaka, K.; Ikegami, A.; Tachibana, Y.; Ohno, N.; Konishi, H.; Hashimoto, A.; Matsumoto, M.; Kato, D.; Ono, R.; Kiyama, H.; et al. Dual microglia effects on blood brain barrier permeability induced by systemic inflammation. Nat. Commun. 2019, 10, 5816. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Campbell, H.K.; Maiers, J.L.; DeMali, K.A. Interplay between tight junctions & adherens junctions. Exp. Cell Res. 2017, 358, 39–44. [Google Scholar] [CrossRef]
- Sladojevic, N.; Stamatovic, S.M.; Johnson, A.M.; Choi, J.; Hu, A.; Dithmer, S.; Blasig, I.E.; Keep, R.F.; Andjelkovic, A.V. Claudin-1-Dependent Destabilization of the Blood–Brain Barrier in Chronic Stroke. J. Neurosci. 2019, 39, 743–757. [Google Scholar] [CrossRef] [Green Version]
- Ali, D.-E.; Shah, M.; Ali, A.; Malik, M.O.; Rehman, F.; Badshah, H.; Ehtesham, E.; Vitale, S.G. Treatment with Metformin and Combination of Metformin Plus Pioglitazone on Serum Levels of IL-6 and IL-8 in Polycystic Ovary Syndrome: A Randomized Clinical Trial. Horm. Metab. Res. 2019, 51, 714–722. [Google Scholar] [CrossRef]
- DeRosa, G.; Mereu, R.; D’Angelo, A.; Salvadeo, S.A.; Ferrari, I.; Fogari, E.; Gravina, A.; Palumbo, I.; Maffioli, P.; Randazzo, S.; et al. ORIGINAL ARTICLE: Effect of pioglitazone and acarbose on endothelial inflammation biomarkers during oral glucose tolerance test in diabetic patients treated with sulphonylureas and metformin. J. Clin. Pharm. Ther. 2010, 35, 565–579. [Google Scholar] [CrossRef]
- El-Gowilly, S.M.; Helmy, M.M.; El-Gowelli, H. Pioglitazone ameliorates methotrexate-induced renal endothelial dysfunction via amending detrimental changes in some antioxidant parameters, systemic cytokines and Fas production. Vasc. Pharmacol. 2015, 74, 139–150. [Google Scholar] [CrossRef]
- Palacios-Ramírez, R.; Hernanz, R.; Martín, A.; Pérez-Girón, J.V.; Barrús, M.T.; González-Carnicero, Z.; Aguado, A.; Jaisser, F.; Briones, A.M.; Salaices, M.; et al. Pioglitazone Modulates the Vascular Contractility in Hypertension by Interference with ET-1 Pathway. Sci. Rep. 2019, 9, 16461. [Google Scholar] [CrossRef] [Green Version]
- Dohgu, S.; Takata, F.; Matsumoto, J.; Kimura, I.; Yamauchi, A.; Kataoka, Y. Monomeric α-synuclein induces blood–brain barrier dysfunction through activated brain pericytes releasing inflammatory mediators in vitro. Microvasc. Res. 2019, 124, 61–66. [Google Scholar] [CrossRef]
- Rossi, B.; Santos-Lima, B.; Terrabuio, E.; Zenaro, E.; Constantin, G. Common Peripheral Immunity Mechanisms in Multiple Sclerosis and Alzheimer’s Disease. Front. Immunol. 2021, 12, 639369. [Google Scholar] [CrossRef] [PubMed]
- Saresella, M.; Marventano, I.; Calabrese, E.; Piancone, F.; Rainone, V.; Gatti, A.; Alberoni, M.; Nemni, R.; Clerici, M. A Complex Proinflammatory Role for Peripheral Monocytes in Alzheimer’s Disease. J. Alzheimer’s Dis. 2014, 38, 403–413. [Google Scholar] [CrossRef] [PubMed]
- Haarmann, A.; Nowak, E.; Deiß, A.; van der Pol, S.; Monoranu, C.-M.; Kooij, G.; Müller, N.; van der Valk, P.; Stoll, G.; de Vries, H.E.; et al. Soluble VCAM-1 impairs human brain endothelial barrier integrity via integrin α-4-transduced outside-in signalling. Acta Neuropathol. 2015, 129, 639–652. [Google Scholar] [CrossRef] [Green Version]
- Stamatovic, S.M.; Shakui, P.; Keep, R.; Moore, B.; Kunkel, S.L.; Van Rooijen, N.; Andjelkovic, A.V. Monocyte Chemoattractant Protein-1 Regulation of Blood–Brain Barrier Permeability. J. Cereb. Blood Flow Metab. 2005, 25, 593–606. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Pu, J.; Tang, C.; Yang, W.S.; Kim, J.J.; Han, N.J.; Lee, E.K.; Liang, H.-B.; Cao, Y.; Ma, Q.; et al. Tanshinone II A Attenuates TNF-α-Induced Expression of VCAM-1 and ICAM-1 in Endothelial Progenitor Cells by Blocking Activation of NF-κB. Cell. Physiol. Biochem. 2017, 41, 2132. [Google Scholar] [CrossRef] [Green Version]
- Hou, Y.; Moreau, F.; Chadee, K. PPARγ is an E3 ligase that induces the degradation of NFκB/p65. Nat. Commun. 2012, 3, 1300. [Google Scholar] [CrossRef] [Green Version]
- Rahimi, V.B.; Momeni-Moghaddam, M.A.; Chini, M.G.; Saviano, A.; Maione, F.; Bifulco, G.; Rahmanian-Devin, P.; Jebalbarezy, A.; Askari, V.R. Carnosol Attenuates LPS-Induced Inflammation of Cardiomyoblasts by Inhibiting NF-κB: A Mechanistic in Vitro and in Silico Study. Evidence-Based Complement. Altern. Med. 2022, 2022, 7969422. [Google Scholar] [CrossRef]
- Rahimi, V.B.; Mousavi, S.H.; Haghighi, S.; Soheili-Far, S.; Askari, V.R. Cytotoxicity and apoptogenic properties of the standardized extract of Portulaca oleracea on glioblastoma multiforme cancer cell line (U-87): A mechanistic study. EXCLI J. 2019, 18, 165–186. [Google Scholar] [CrossRef]
- Hofer, S.; Bopp, C.; Hoerner, C.; Plaschke, K.; Faden, R.M.; Martin, E.; Bardenheuer, H.J.; Weigand, M.A. Injury of the Blood Brain Barrier and Up-Regulation of ICAM-1 in Polymicrobial Sepsis. J. Surg. Res. 2008, 146, 276–281. [Google Scholar] [CrossRef] [PubMed]
- Huber, J.D.; Campos, C.R.; Mark, K.S.; Davis, T.P. Alterations in blood-brain barrier ICAM-1 expression and brain microglial activation after λ-carrageenan-induced inflammatory pain. Am. J. Physiol. Circ. Physiol. 2006, 290, H732–H740. [Google Scholar] [CrossRef]
- Reyes, R.; Wu, Y.; Lai, Q.; Mrizek, M.; Berger, J.; Jimenez, D.F.; Barone, C.M.; Ding, Y. Early inflammatory response in rat brain after peripheral thermal injury. Neurosci. Lett. 2006, 407, 11–15. [Google Scholar] [CrossRef] [PubMed]
- Kinouchi, T.; Kitazato, K.T.; Shimada, K.; Yagi, K.; Tada, Y.; Matsushita, N.; Kurashiki, Y.; Satomi, J.; Sata, M.; Nagahiro, S. Treatment with the PPARγ Agonist Pioglitazone in the Early Post-ischemia Phase Inhibits Pro-inflammatory Responses and Promotes Neurogenesis Via the Activation of Innate- and Bone Marrow-Derived Stem Cells in Rats. Transl. Stroke Res. 2018, 9, 306–316. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Swanson, C.R.; Joers, V.; Bondarenko, V.; Brunner, K.; Simmons, H.A.; Ziegler, T.E.; Kemnitz, J.W.; Johnson, J.A.; Emborg, M.E. The PPAR-γ agonist pioglitazone modulates inflammation and induces neuroprotection in parkinsonian monkeys. J. Neuroinflammation 2011, 8, 91. [Google Scholar] [CrossRef] [Green Version]
- Da Silva, O.; Probst, N.; Landry, C.; Hanak, A.-S.; Warnault, P.; Coisne, C.; Calas, A.-G.; Gosselet, F.; Courageux, C.; Gastellier, A.-J.; et al. A New Class of Bi- and Trifunctional Sugar Oximes as Antidotes against Organophosphorus Poisoning. J. Med. Chem. 2022, 65, 4649–4666. [Google Scholar] [CrossRef]
- Deligne, C.; Hachani, J.; Duban-Deweer, S.; Meignan, S.; Leblond, P.; Carcaboso, A.M.; Sano, Y.; Shimizu, F.; Kanda, T.; Gosselet, F.; et al. Development of a human in vitro blood–brain tumor barrier model of diffuse intrinsic pontine glioma to better understand the chemoresistance. Fluids Barriers CNS 2020, 17, 37. [Google Scholar] [CrossRef]
- Loiola, R.A.; García-Gabilondo, M.; Grayston, A.; Bugno, P.; Kowalska, A.; Duban-Deweer, S.; Rizzi, E.; Hachani, J.; Sano, Y.; Shimizu, F.; et al. Secretome of endothelial progenitor cells from stroke patients promotes endothelial barrier tightness and protects against hypoxia-induced vascular leakage. Stem Cell Res. Ther. 2021, 12, 552. [Google Scholar] [CrossRef]
- Cecchelli, R.; Aday, S.; Sevin, E.; Almeida, C.; Culot, M.; Dehouck, L.; Coisne, C.; Engelhardt, B.; Dehouck, M.-P.; Ferreira, L. A Stable and Reproducible Human Blood-Brain Barrier Model Derived from Hematopoietic Stem Cells. PLoS ONE 2014, 9, e99733. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pedroso, D.C.; Tellechea, A.; Moura, L.; Fidalgo-Carvalho, I.; Duarte, J.; Carvalho, E.; Ferreira, L. Improved Survival, Vascular Differentiation and Wound Healing Potential of Stem Cells Co-Cultured with Endothelial Cells. PLoS ONE 2011, 6, e16114. [Google Scholar] [CrossRef] [Green Version]
- Shimizu, F.; Sano, Y.; Abe, M.-A.; Maeda, T.; Ohtsuki, S.; Terasaki, T.; Kanda, T. Peripheral nerve pericytes modify the blood-nerve barrier function and tight junctional molecules through the secretion of various soluble factors. J. Cell. Physiol. 2011, 226, 255–266. [Google Scholar] [CrossRef]
- Heymans, M.; Figueiredo, R.; Dehouck, L.; Francisco, D.; Sano, Y.; Shimizu, F.; Kanda, T.; Bruggmann, R.; Engelhardt, B.; Winter, P.; et al. Contribution of brain pericytes in blood–brain barrier formation and maintenance: A transcriptomic study of cocultured human endothelial cells derived from hematopoietic stem cells. Fluids Barriers CNS 2020, 17, 1–28. [Google Scholar] [CrossRef] [PubMed]
- Rizzi, E.; Deligne, C.; Dehouck, L.; Bilardo, R.; Sano, Y.; Shimizu, F.; Kanda, T.; Resmini, M.; Gosselet, F.; Dehouck, M.-P.; et al. A Triple Culture Cell System Modeling the Human Blood-Brain Barrier. J. Vis. Exp. 2021, 177, e63134. [Google Scholar] [CrossRef] [PubMed]
- Vandenhaute, E.; Drolez, A.; Sevin, E.; Gosselet, F.; Mysiorek, C.; Dehouck, M.-P. Adapting coculture in vitro models of the blood–brain barrier for use in cancer research: Maintaining an appropriate endothelial monolayer for the assessment of transendothelial migration. Lab. Investig. 2016, 96, 588–598. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jennings, P.; Koppelstaetter, C.; Aydin, S.; Abberger, T.; Wolf, A.M.; Mayer, G.; Pfaller, W. Cyclosporine A induces senescence in renal tubular epithelial cells. Am. J. Physiol. Physiol. 2007, 293, F831–F838. [Google Scholar] [CrossRef] [Green Version]
- Dehouck, M.-P.; Jolliet-Riant, P.; Brée, F.; Fruchart, J.-C.; Cecchelli, R.; Tillement, J.-P. Drug Transfer Across the Blood-Brain Barrier: Correlation Between In Vitro and In Vivo Models. J. Neurochem. 1992, 58, 1790–1797. [Google Scholar] [CrossRef] [PubMed]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 Years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Brezovjakova, H.; Tomlinson, C.; Naim, N.M.; Swiatlowska, P.; Erasmus, J.E.; Huveneers, S.; Gorelik, J.; Bruche, S.; Braga, V.M. Junction Mapper is a novel computer vision tool to decipher cell–cell contact phenotypes. eLife 2019, 8, e45413. [Google Scholar] [CrossRef]
- Abdi, H.; Williams, L.J. Principal component analysis. Wiley Interdiscip. Rev. Comput. Stat. 2010, 2, 433–459. [Google Scholar] [CrossRef]







| Parameters | Healthy Donors | CD/Remission | CD/Fail | UC |
|---|---|---|---|---|
| Average age (min. − max.) | 39 (21 − 60) | 36.2 (19 − 53) | 38 (19 − 63) | 48 |
| Standard deviation | 12.2 | 12.0 | 13.9 | 7.2 |
| Females | 17 | 6 | 8 | 3 |
| Males | 13 | 4 | 7 | 2 |
| Total | 30 | 10 | 15 | 5 |
| Medication | ||||
| Infliximab | 0 | 10 (100%) | 15 (100%) | 5 (100%) |
| Corticosteroids | 0 | 2 (20%) | 7 (47%) | 1 (20%) |
| Mesalazine | 0 | 7 (70%) | 9 (60%) | 2 (40%) |
| Azathioprine | 0 | 5 (50%) | 7 (47%) | 1 (20%) |
| Anti-diarrheic | 0 | 0 | 2 (13%) | 0 |
| Anti-depressants | 3 (10%) | 1 (10%) | 1 (7%) | 0 |
| Antibiotics | 0 | 0 | 2 (13%) | 1 (20%) |
| Others | 6 (20%) | 4 (40%) | 11 (73%) | 0 |
| Cytokine | Healthy Donors | IBD Patients | p Value |
|---|---|---|---|
| TNFα (pg/mL) | 6.12 (4.61; 8.25) | 4.37 (3.83; 5.46) | 0.0009 |
| TGFß (ng/mL) | 7.35 (4.12; 9.92) | 5.71 (2.15; 12.62) | 0.64 |
| IFNg (pg/mL) | 17.35 (14.01; 24.18) | 18.03 (16.51; 21.53) | 0.70 |
| MCP-1 (pg/mL) | 90.57 (70.28; 117.4) | 83.51 (68.44; 107.8) | 0.31 |
| MMP-9 (ng/mL) | 39.67 (32.95; 59.15) | 26.88 (17.56; 46.83) | 0.0093 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
da Rocha, G.H.O.; Loiola, R.A.; de Paula-Silva, M.; Shimizu, F.; Kanda, T.; Vieira, A.; Gosselet, F.; Farsky, S.H.P. Pioglitazone Attenuates the Effects of Peripheral Inflammation in a Human In Vitro Blood–Brain Barrier Model. Int. J. Mol. Sci. 2022, 23, 12781. https://doi.org/10.3390/ijms232112781
da Rocha GHO, Loiola RA, de Paula-Silva M, Shimizu F, Kanda T, Vieira A, Gosselet F, Farsky SHP. Pioglitazone Attenuates the Effects of Peripheral Inflammation in a Human In Vitro Blood–Brain Barrier Model. International Journal of Molecular Sciences. 2022; 23(21):12781. https://doi.org/10.3390/ijms232112781
Chicago/Turabian Styleda Rocha, Gustavo Henrique Oliveira, Rodrigo Azevedo Loiola, Marina de Paula-Silva, Fumitaka Shimizu, Takashi Kanda, Andrea Vieira, Fabien Gosselet, and Sandra Helena Poliselli Farsky. 2022. "Pioglitazone Attenuates the Effects of Peripheral Inflammation in a Human In Vitro Blood–Brain Barrier Model" International Journal of Molecular Sciences 23, no. 21: 12781. https://doi.org/10.3390/ijms232112781
APA Styleda Rocha, G. H. O., Loiola, R. A., de Paula-Silva, M., Shimizu, F., Kanda, T., Vieira, A., Gosselet, F., & Farsky, S. H. P. (2022). Pioglitazone Attenuates the Effects of Peripheral Inflammation in a Human In Vitro Blood–Brain Barrier Model. International Journal of Molecular Sciences, 23(21), 12781. https://doi.org/10.3390/ijms232112781