Chitosan/Lactic Acid Systems: Liquid Crystalline Behavior, Rheological Properties, and Riboflavin Release In Vitro
Abstract
:1. Introduction
2. Results and Discussion
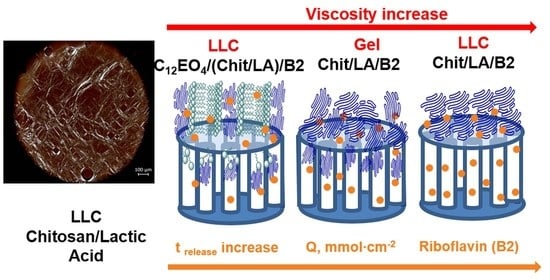
2.1. Liquid Crystal Properties of Chit/LA Binary Systems
2.2. Liquid Crystal Properties of Ternary C12EO4/(Chit:LA) Systems
2.3. FTIR Spectroscopy Studies
2.4. Rheological Properties
2.5. The Study of Riboflavin Release from Gels and LLC Systems
2.6. Model of Riboflavin Penetration through a Hydrophobic Membrane
3. Materials and Methods
3.1. Materials
3.2. Preparation of Gels and Llcs
3.3. Polarized Optical Microscopy (POM)
3.4. Determination of Viscosity
3.5. FTIR Spectroscopy
3.6. In Vitro Riboflavin Release
3.7. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- De, R.; Mahata, M.K.; Kim, K.-T. Structure-Based Varieties of Polymeric Nanocarriers and Influences of Their Physicochemical Properties on Drug Delivery Profiles. Adv. Sci. 2022, 9, 2105373. [Google Scholar] [CrossRef] [PubMed]
- Malmstena, M. Soft drug delivery systems. Soft Matter 2006, 2, 760–769. [Google Scholar] [CrossRef] [PubMed]
- Waheed, A.; Aqil, M. Lyotropic liquid crystalline nanoparticles: Scaffolds for delivery of myriad therapeutics and diagnostics. J. Mol. Liq. 2021, 338, 116919. [Google Scholar] [CrossRef]
- Rajabalaya, R.; Musa, M.N.; Kifli, N.; David, S.R. Oral and transdermal drug delivery systems: Role of lipid-based lyotropic liquid crystals. Drug Des. Dev. Ther. 2017, 11, 393–406. [Google Scholar] [CrossRef] [Green Version]
- Zhai, J.; Fong, C.; Tran, N.; Drummond, C.J. Non-Lamellar Lyotropic Liquid Crystalline Lipid Nanoparticles for the Next Generation of Nanomedicine. ACS Nano 2019, 13, 6178–6206. [Google Scholar] [CrossRef]
- Rapalli, V.K.; Waghule, T.; Hans, N.; Mahmood, A.; Gorantla, S.; Dubey, S.K.; Singhvi, G. Insights of lyotropic liquid crystals in topical drug delivery for targeting various skin disorders. J. Mol. Liq. 2020, 3, 113771. [Google Scholar] [CrossRef]
- Guo, C.Y.; Wang, J.; Cao, F.; Lee, R.J.; Zhai, G.X. Lyotropic liquid crystal systems in drug delivery. Drug Discov. Today 2010, 15, 1032–1040. [Google Scholar] [CrossRef] [PubMed]
- Silvestrini, A.V.P.; Caron, A.L.; Viegas, J.; Praga, F.G.; Bentley, M.V.L.B. Advances in lyotropic liquid crystal systems for skin drug delivery. Expert Opin Drug Deliv. 2020, 17, 1781–1805. [Google Scholar] [CrossRef]
- Selivanova, N.M.; Gubaidullin, A.T.; Galyametdinov, Y.G. Incorporating a Tetrapeptide into Lyotropic Direct Hexagonal Mesophase. J. Phys. Chem. B 2020, 124, 2715–2722. [Google Scholar] [CrossRef]
- Selivanova, N.M.; Gubaidullin, A.T.; Galyametdinov, Y.G. Characterization of hexagonal lyotropic liquid crystal microstructure: Effects of vitamin E molecules. Colloids Surf. A Physicochem. Eng. Asp. 2021, 620, 126570. [Google Scholar] [CrossRef]
- Selivanova, N.; Gubaidullin, A.; Padnya, P.; Stoikov, I.; Galyametdinov, Y. Phase behaviour, structural properties and intermolecular interactions of systems based on substituted thiacalix[4]arene and nonionic surfactants. Liq. Cryst. 2019, 46, 415–421. [Google Scholar] [CrossRef]
- Sougata, J.; Subrata, J. Functional Chitosan Drug Delivery and Biomedical Applications; Chapter XII; Springer Nature: Singapore, 2019; 489p. [Google Scholar] [CrossRef]
- Hamedi, H.; Moradi, S.; Hudson, S.M.; Tonelli, A.E.; King, M.W. Chitosan based bioadhesives for biomedical applications. Carbohydr. Polym. 2022, 282, 119100. [Google Scholar] [CrossRef] [PubMed]
- Iacob, A.T.; Lupascu, F.G.; Apotrosoaei, M.; Vasincu, I.M.; Tauser, R.G.; Lupascu, D.; Giusca, S.E.; Caruntu, I.D.; Profire, L. Recent Biomedical Approaches for Chitosan Based Materials as Drug Delivery Nanocarriers. Pharmaceutics 2021, 13, 587. [Google Scholar] [CrossRef]
- Hu, L.M.; Sun, Y.; Wu, Y. Advances in chitosan-based drug delivery vehicles. Nanoscale 2013, 5, 3103–3111. [Google Scholar] [CrossRef] [PubMed]
- Parhi, R. Drug delivery applications of chitin and chitosan. Environ. Chem. Lett. 2020, 18, 577–594. [Google Scholar] [CrossRef]
- Dubey, S.K.; Bhatt, T.; Agrawal, M.; Saha, R.N.; Saraf, S.; Saraf, S.; Alexander, A. Application of chitosan modified nanocarriers in breast cancer Dubey. Int. J. Biol. Macromol. 2022, 194, 521–538. [Google Scholar] [CrossRef]
- Peers, S.; Montembault, A.; Ladavière, C. Chitosan hydrogels incorporating colloids for sustained drug delivery. Carbohydr. Polym. 2022, 275, 118689. [Google Scholar] [CrossRef]
- Prabaharan, M.; Borges, J.P.; Godinho, M.H.; Mano, J.F. Liquid Crystalline Behaviour of Chitosan in Formic, Acetic, and Monochloroacetic Acid Solutions. Mater. Sci. Forum 2006, 514–516, 1010–1014. [Google Scholar] [CrossRef]
- Chang, J.; Chang, K.; Tsai, M. Liquid-Crystalline Behavior of chitisan in malic acid. J. Appl. Polym. Sci. 2007, 105, 2670–2675. [Google Scholar] [CrossRef]
- Kuse, Y.; Asahina, D.; Nishio, Y. Molecular Structure and Liquid-Crystalline Characteristics of Chitosan Phenylcarbamate. Biomacromolecules 2009, 10, 166–173. [Google Scholar] [CrossRef]
- Salmazi, R.; Calixto, G.; Bernegossi, J.; dos Santos Ramos, M.A.; Bauab, T.M.; Chorilli, M. A curcumin-loaded liquid crystal precursor mucoadhesive system for the treatment of vaginal candidiasis. Int. J. Nanomed. 2015, 10, 4815–4824. [Google Scholar] [CrossRef] [Green Version]
- Selivanova, N.M.; Galyametdinov, Y.G.; Gubaidullin, A.T. Lyotropic mesophases based on chitosane biopolymer, acetic acid and non-ionic surfactants, as delivery systems of bioactive substances. Liq. Cryst. Appl. 2018, 18, 6–13. [Google Scholar] [CrossRef]
- Selivanova, N.M.; Zimina, M.V.; Galyametdinov, Y.G. Phase behavior of chitosan in organic acids. Liq. Cryst. Appl. 2019, 19, 76–82. [Google Scholar] [CrossRef]
- Suwannasom, N.; Kao, I.; Pruß, A.; Georgieva, R.; Bäumler, H. Riboflavin: The Health Benefits of a Forgotten Natural Vitamin. Int. J. Mol. Sci. 2020, 21, 950. [Google Scholar] [CrossRef] [Green Version]
- Szczuko, M.; Ziętek, M.; Kulpa, D.; Seidler, T. Riboflavin—Properties, occurrence and its use in medicine. Pteridines 2019, 30, 33–47. [Google Scholar] [CrossRef]
- Darguzyte, M.; Drude, N.; Lammers, T.; Kiessling, F. Riboflavin-Targeted Drug Delivery. Cancers 2020, 12, 295. [Google Scholar] [CrossRef] [Green Version]
- Sato, T.; Takeuchi, H.; Sakurai, T.; Tanaka, K.; Matsuki, K.; Higashi, K.; Moribe, K.; Yamamoto, K. Characterization of a Riboflavin Non-aqueous Nanosuspension Prepared by Bead Milling for Cutaneous Application. Chem. Pharm. Bull. 2015, 63, 88–94. [Google Scholar] [CrossRef] [Green Version]
- Desai, P.; Patlolla, R.R.; Singh, M. Interaction of nanoparticles and cell-penetrating peptides with skin for transdermal drug delivery. Mol. Membr. Biol. 2010, 27, 247–259. [Google Scholar] [CrossRef] [Green Version]
- Pedroni, V.; Schulz, P.C.; Gschaider de Ferreira, M.E.; Morini, M.A. A chitosan monolithic siliceaous mesoporous–macroporous material. Colloid Polym. Sci. 2000, 278, 964–971. [Google Scholar] [CrossRef]
- Selivanova, N.M.; Galeeva, A.I.; Gubaydullin, A.T.; Lobkov, V.S.; Galyametdinov, Y.G. Mesogenic and luminescent properties of lyotropic liquid crystals containing Eu(III) and Tb(III) ions. J. Phys. Chem. B 2012, 116, 735–742. [Google Scholar] [CrossRef]
- Selivanova, N.M.; Konov, A.B.; Romanova, K.A.; Gubaidullin, A.T.; Galyametdinov, Y.G. Lyotropic La-containing lamellar liquid crystals: Phase behavior, thermal and structural properties. Soft Matter 2015, 11, 7809–7816. [Google Scholar] [CrossRef] [PubMed]
- Sinha, N.; Singh, B.K.; Dutta, P.K. Research on Antibacterial Screening and Drug Delivery using Chitosan-Stearic Acid Derivative. J. Polym. Mater. 2017, 34, 11–20. [Google Scholar]
- Tien, C.Le.; Lacroix, M.; Ispas-Szabo, P.; Mateescu, M.A. N-acylated chitosan: Hydrophobic matrices for controlled drug release. J. Control. Release 2003, 93, 1–13. [Google Scholar] [CrossRef]
- Bellamy, L.J. The Infra-Red Spectra of Complex Molecules, 3rd ed.; Chapter XIX; John Wiley Sons: New York, NY, USA, 1975; 433p. [Google Scholar] [CrossRef]
- Milan, E.P.; Bertolo, M.R.V.; Martins, V.C.A.; Sobrero, C.E.; Plepis, A.M.G.; Fuhrmann-Lieker, T.; Horn, M.M. Effects of Mangosteen Peel Phenolic Compounds on Tilapia Skin Collagen-Based Mineralized Scaffold Properties. ACS Omega 2022, 7, 34022–34033. [Google Scholar] [CrossRef]
- Selivanova, N.M.; Galeeva, A.I.; Galyametdinov, Y.G. Biocompatible delivery systems based on κ-carrageenan and nonionic surfactants. Liq. Cryst. Appl. 2020, 20, 23–34. [Google Scholar] [CrossRef]
- Malkin, A.Y.; Isayev, A.I. Rheology: Conceptions, Methods, Applications, 2nd ed.; ChemTec: Toronto, ON, Canada, 2012; 474p. [Google Scholar]
- Shouche, S.V.; Chokappa, D.K.; Khakhar, D.V.; Naik, V.M. Effect of Particulate Solids on the Rheology of a Lyotropic Gel Medium. J. Rheol. 1994, 38, 1871–1884. [Google Scholar] [CrossRef]
- Matveenko, V.N.; Kirsanov, E.A. Structural Rationale of a Non-Newtonian Flow. Moscow Univ. Chem. Bull. 2017, 72, 69–91. [Google Scholar] [CrossRef]
- Mezzenga, R.; Cedric, M.; Collin, S.; Romoscanu, A.I.; Sagalowicz, L.; Hayward, R.C. Shear Rheology of Lyotropic Liquid Crystals: A Case Study. Langmuir 2005, 21, 3322–3333. [Google Scholar] [CrossRef]
- Silva-Weiss, A.; Bifani, V.; Ihl, M.; Sobral, P.J.A.; Gómez-Guillén, M.C. Structural properties of films and rheology of film-forming solutions based on chitosan and chitosan-starch blend enriched with murta leaf extract. Food Hydrocoll. 2013, 31, 458–466. [Google Scholar] [CrossRef]
- Vigilato, M.A.; Horn, M.M.; Martins, V.C.; Plepis, A.M. Rheological study of gels based on chitosan and carbon nanotubes. Braz. J. Therm. Anal. 2015, 4, 35–38. [Google Scholar] [CrossRef] [Green Version]
- Casson, N. Reology of Disperse Systems; Mill, C.C., Ed.; Pergamon Press: London, UK, 1959; pp. 84–104. [Google Scholar]
- Lee, K.W.; Nguyen, T.H.; Hanley, T.; Boyd, B.J. Nanostructure of liquid crystalline matrix determines in vitro sustained release and in vivo oral absorption kinetics for hydrophilic model drugs. Int. J. Pharm. 2009, 365, 190–199. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y. Factors affecting the structure of lyotropic liquid crystals and the correlation between structure and drug diffusion. RSC Adv. 2018, 8, 6978–6987. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, Y.; Hu, Y.; Shen, J.; Guo, S. Optimized microporous structure of ePTFE membranes by controlling the particle size of PTFE fine powders for achieving high oil-water separation performances. J. Membr. Sci. 2021, 629, 119294. [Google Scholar] [CrossRef]
- Dillon, M.E. Microporous polytetrafluoroethylene and polydimethylsiloxane IPN membrane. Polym. Mater. Sci. Eng. 1990, 62, 814–818. [Google Scholar]
- Olejnik, A.; Nowak, I. Atomic force microscopy analysis of synthetic membranes applied in release studies. Appl. Surf. Sci. 2015, 355, 686–697. [Google Scholar] [CrossRef]
- Zadymova, N.M.; Poteshnova, M.V. Microemulsions and microheterogeneous microemulsion-based polymeric matrices for transdermal delivery of lipophilic drug (Felodipine). Colloid Polym. Sci. 2019, 297, 453–468. [Google Scholar] [CrossRef]







| System | = 0.07–7.5 s−1 | = 7.5–90 s−1 | ||||
|---|---|---|---|---|---|---|
| μ, mPa·s | τ0, Pa | R, % | μ, mPa·s | τ0, Pa | R, % | |
| C12EO4/(Chit:LA) LCC | 556.0 | 1.38 | 97.0 | 181.6 | 6.24 | 97.9 |
| Chit/LA Gel | 754.7 | 0.02 | 96.6 | 252.4 | 1.80 | 96.9 |
| Chit/LA LCC | 5190.0 | 18.17 | 98.1 | 298.7 | 1.25 | 95.9 |
| System | Induction Period | t, min | dQ/dt |
|---|---|---|---|
| C12EO4/(Chit: LA) LLC | - | 47 | 3.4 (0 ≤ t ≤ 23) 1 (25 ≤ t ≤ 45) |
| Chit/LA gel | + | 210 | 1.7 (51 ≤ t ≤ 80) 0.5 (167 ≤ t ≤ 206) |
| Chit/LA LLC | + | 540 | 1 (7 ≤ t ≤ 543) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Selivanova, N.M.; Galeeva, A.I.; Galyametdinov, Y.G. Chitosan/Lactic Acid Systems: Liquid Crystalline Behavior, Rheological Properties, and Riboflavin Release In Vitro. Int. J. Mol. Sci. 2022, 23, 13207. https://doi.org/10.3390/ijms232113207
Selivanova NM, Galeeva AI, Galyametdinov YG. Chitosan/Lactic Acid Systems: Liquid Crystalline Behavior, Rheological Properties, and Riboflavin Release In Vitro. International Journal of Molecular Sciences. 2022; 23(21):13207. https://doi.org/10.3390/ijms232113207
Chicago/Turabian StyleSelivanova, Natalia M., Aliya I. Galeeva, and Yuriy G. Galyametdinov. 2022. "Chitosan/Lactic Acid Systems: Liquid Crystalline Behavior, Rheological Properties, and Riboflavin Release In Vitro" International Journal of Molecular Sciences 23, no. 21: 13207. https://doi.org/10.3390/ijms232113207
APA StyleSelivanova, N. M., Galeeva, A. I., & Galyametdinov, Y. G. (2022). Chitosan/Lactic Acid Systems: Liquid Crystalline Behavior, Rheological Properties, and Riboflavin Release In Vitro. International Journal of Molecular Sciences, 23(21), 13207. https://doi.org/10.3390/ijms232113207