The Impact of Backbone Fluorination and Side-Chain Position in Thiophene-Benzothiadiazole-Based Hole-Transport Materials on the Performance and Stability of Perovskite Solar Cells
Abstract
:1. Introduction
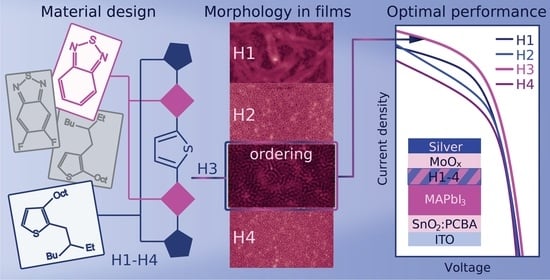
2. Results and Discussion
3. Materials and Methods
3.1. Materials and Instruments
3.2. Fabrication of the Solar Cells with H1-4 for the Efficiency Investigation
3.3. Fabrication of the Solar Cells with H1-4 for the Stability Evaluation
3.4. Fabrication of the Tri-layer Stacks for the AFM Measurements
3.5. Synthesis of Compound 2a
3.6. Synthesis of Compound 2b
3.7. Synthesis of Compound 2c
3.8. Synthesis of Compound 2d
3.9. Synthesis of H1
3.10. Synthesis of H2
3.11. Synthesis of H3
3.12. Synthesis of H4
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- NREL. Best Researcher Cell Efficiency Chart. 2022. Available online: https://www.nrel.gov/pv/cell-efficiency.html (accessed on 30 June 2022).
- Wang, P.; Wu, Y.; Cai, B.; Ma, Q.; Zheng, X.; Zhang, W.-H. Solution-Processable Perovskite Solar Cells toward Commercialization: Progress and Challenges. Adv. Funct. Mater. 2019, 29, 1807661. [Google Scholar] [CrossRef]
- Park, B.; Seok, S.I. Intrinsic Instability of Inorganic–Organic Hybrid Halide Perovskite Materials. Adv. Mater. 2019, 31, 1805337. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, Y.; Shi, L.; Li, Q.; Wang, J. Role of Water and Defects in Photo-Oxidative Degradation of Methylammonium Lead Iodide Perovskite. Small Methods 2019, 3, 1900154. [Google Scholar] [CrossRef]
- Duong, T.; Wu, Y.; Shen, H.; Peng, J.; Zhao, S.; Wu, N.; Lockrey, M.; White, T.; Weber, K.; Catchpole, K. Light and elevated temperature induced degradation (LeTID) in perovskite solar cells and development of stable semi-transparent cells. Sol. Energy Mater. Sol. Cells 2018, 188, 27–36. [Google Scholar] [CrossRef]
- Raja, S.N.; Bekenstein, Y.; Koc, M.A.; Fischer, S.; Zhang, D.; Lin, L.; Ritchie, R.O.; Yang, P.; Alivisatos, A.P. Encapsulation of Perovskite Nanocrystals into Macroscale Polymer Matrices: Enhanced Stability and Polarization. ACS Appl. Mater. Interfaces 2016, 8, 35523–35533. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Juarez-Perez, E.J.; Ono, L.K.; Maeda, M.; Jiang, Y.; Hawash, Z.; Qi, Y. Photodecomposition and thermal decomposition in methylammonium halide lead perovskites and inferred design principles to increase photovoltaic device stability. J. Mater. Chem. A 2018, 6, 9604–9612. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Han, Q.; Liu, Y.; Xie, C.; Yang, C.; Niu, D.; Li, Y.; Wang, H.; Xia, L.; Yuan, Y.; et al. ight-induced degradation and self-healing inside CH3NH3PbI3-based solar cells. Appl. Phys. Lett. 2020, 116, 253303. [Google Scholar] [CrossRef]
- Mazumdar, S.; Zhao, Y.; Zhang, X. Stability of Perovskite Solar Cells: Degradation Mechanisms and Remedies. Front. Electron. 2021, 2, 712785. [Google Scholar] [CrossRef]
- Li, R.; Wang, P.; Chen, B.; C ui, X.; Ding, Y.; Li, Y.; Zhang, D.; Zhao, Y.; Zhang, X. NiOx/Spiro Hole Transport Bilayers for Stable Perovskite Solar Cells with Efficiency Exceeding 21%. ACS Energy Lett. 2020, 5, 79–86. [Google Scholar] [CrossRef]
- Yang, D.; Zhang, X.; Wang, K.; Wu, C.; Yang, R.; Hou, Y.; Jiang, Y.; Liu, S.; Priya, S. Stable Efficiency Exceeding 20.6% for Inverted Perovskite Solar Cells through Polymer-Optimized PCBM Electron-Transport Layers. Nano Lett. 2019, 19, 3313–3320. [Google Scholar] [CrossRef]
- Schloemer, T.H.; Raiford, J.A.; Gehan, T.S.; Moot, T.; Nanayakkara, S.; Harvey, S.P.; Bramante, R.C.; Dunfield, S.; Louks, A.E.; Maughan, A.E.; et al. The Molybdenum Oxide Interface Limits the High-Temperature Operational Stability of Unencapsulated Perovskite Solar Cells. ACS Energy Lett. 2020, 5, 2349–2360. [Google Scholar] [CrossRef]
- Tepliakova, M.M.; Mikheeva, A.N.; Frolova, L.A.; Boldyreva, A.G.; Elakshar, A.; Novikov, A.V.; Tsarev, S.A.; Ustinova, M.I.; Yamilova, O.R.; Nasibulin, A.G.; et al. Incorporation of Vanadium(V) Oxide in Hybrid Hole Transport Layer Enables Long-term Operational Stability of Perovskite Solar Cells. J. Phys. Chem. 2020, 11, 5563–5568. [Google Scholar] [CrossRef]
- Tepliakova, M.M.; Mikheeva, A.N.; Somov, P.A.; Statnik, E.S.; Korsunsky, A.M.; Stevenson, K.J. Combination of Metal Oxide and Polytriarylamine: A Design Principle to Improve the Stability of Perovskite Solar Cells. Energies 2021, 14, 5115. [Google Scholar] [CrossRef]
- Desoky, M.M.H.; Bonomo, M.; Buscaino, R.; Fin, A.; Viscardi, G.; Barolo, C.; Quagliotto, P. Dopant-Free All-Organic Small-Molecule HTMs for Perovskite Solar Cells: Concepts and Structure–Property Relationships. Energies 2021, 14, 2279. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, M. Organic hole-transporting materials for efficient perovskite solar cells Mater. Today Energy 2018, 7, 208–220. [Google Scholar] [CrossRef]
- Li, S.; Cao, Y.-L.; Li, W.-H.; Bo, Z.-S. A brief review of hole transporting materials commonly used in perovskite solar cells. Rare Met. 2021, 40, 2712–2729. [Google Scholar] [CrossRef]
- Peng, Y.; Kong, F.; Chen, S.; Zhao, C.; Zhang, J.; Zhang, X.; Ghadari, R.; Liu, W.; Hu, L. Constructing hole transporting highway for high-efficiency perovskite solar cells. Synth. Met. 2022, 291, 117174. [Google Scholar] [CrossRef]
- Meng, F.; Wang, Y.; Wen, Y.; Lai, X.; Li, W.; Kyaw, A.K.K.; Zhang, R.; Fan, D.; Li, Y.; Du, M.; et al. Dopant-Free and Green-Solvent-Processable Hole-Transporting Materials for Highly Efficient Inverted Planar Perovskite Solar Cells. Sol. RRL 2020, 4, 2000327. [Google Scholar] [CrossRef]
- Lai, X.; Meng, F.; Zhang, Q.-Q.; Wang, K.; Li, G.; Wen, Y.; Ma, H.; Li, W.; Li, X.; Kyaw, A.K.K.; et al. A Bifunctional Saddle-Shaped Small Molecule as a Dopant-Free Hole Transporting Material and Interfacial Layer for Efficient and Stable Perovskite Solar Cells. Sol. RRL 2019, 3, 1900011. [Google Scholar] [CrossRef]
- Ding, X.; Chen, C.; Sun, L.; Li, H.; Chen, H.; Su, J.; Li, H.; Li, H.; Xu, L.; Cheng, M. Highly efficient phenothiazine 5,5-dioxide-based hole transport materials for planar perovskite solar cells with a PCE exceeding 20%. J. Mater. Chem. A 2019, 7, 9510–9516. [Google Scholar] [CrossRef]
- Sharma, A.; Singh, R.; Kini, G.P.; Hyeon Kim, J.; Parashar, M.; Kim, M.; Kumar, M.; Kim, J.S.; Lee, J.-J. Side-Chain Engineering of Diketopyrrolopyrrole-Based Hole-Transport Materials to Realize High-Efficiency Perovskite Solar Cells. ACS Appl. Mater. Interfaces 2021, 13, 7405–7415. [Google Scholar] [CrossRef] [PubMed]
- Bronstein, H.; Nielsen, C.B.; Schroeder, B.C.; McCulloch, I. The role of chemical design in the performance of organic semiconductors. Nat. Rev. Chem. 2020, 4, 66–77. [Google Scholar] [CrossRef]
- Chakraborty, B.B.; Anwar, S.; Das, S.; Paul, S.B.; Mohiuddin, G.; De, J.; Choudhury, S. Aggregation dependent fluorescence switching in benzothiazole derivative based H-bonded mesogen. Liq. Cryst. 2018, 45, 1644–1653. [Google Scholar] [CrossRef]
- Lin, Y.-H.; Ezhumalai, Y.; Yang, Y.-L.; Liao, C.-T.; Hsu, H.-F.; Wu, C. Influence of Mesogenic Properties of Cruciform-Shaped Liquid Crystals by Incorporating Side-Arms with a Laterally-Substituted-Fluorine. Crystals 2013, 3, 339–349. [Google Scholar] [CrossRef] [Green Version]
- Kuznetsov, I.E.; Anokhin, D.V.; Piryazev, A.A.; Sideltsev, M.E.; Akhkiamova, A.F.; Novikov, A.V.; Kurbatov, V.G.; Ivanov, D.A.; Akkuratov, A.V. Tailoring the charge transport characteristics in ordered small-molecule organic semiconductors by side-chain engineering and fluorine substitution. Phys. Chem. Chem. Phys. 2022, 24, 16041–16049. [Google Scholar] [CrossRef]
- Makuła, P.; Pacia, M.; Macyk, W. How To Correctly Determine the Band Gap Energy of Modified Semiconductor Photocatalysts Based on UV–Vis Spectra. J. Phys. Chem. 2018, 9, 6814–6817. [Google Scholar] [CrossRef] [Green Version]
- Tepliakova, M.M.; Kuznetsov, I.E.; Avilova, I.A.; Stevenson, K.J.; Akkuratov, A.V. Impact of Synthetic Route on Photovoltaic Properties of Isoindigo-Containing Conjugated Polymers Macromol. Chem. Phys. 2021, 222, 2100136. [Google Scholar]
- Hummelen, J.C.; Knight, B.W.; LePeq, F.; Wudl, F.; Yao, J.; Wilkins, C.L. Preparation and Characterization of Fulleroid and Methanofullerene Derivatives. J. Org. Chem. 1995, 60, 532–538. [Google Scholar] [CrossRef]
- Kim, H.-S.; Lee, C.-R.; Im, J.-H.; Lee, K.-B.; Moehl, T.; Marchioro, A.; Moon, S.-J.; Humphry-Baker, R.; Yum, J.-H.; Moser, J.E.; et al. Lead Iodide Perovskite Sensitized All-Solid-State Submicron Thin Film Mesoscopic Solar Cell with Efficiency Exceeding 9%. Sci. Rep. 2012, 2, 591. [Google Scholar] [CrossRef] [Green Version]
- Yue, Y.; Salim, N.; Wu, Y.; Yang, X.; Islam, A.; Chen, W.; Liu, J.; Bi, E.; Xie, F.; Cai, M.; et al. Enhanced Stability of Perovskite Solar Cells through Corrosion-Free Pyridine Derivatives in Hole-Transporting Materials. Adv. Mater. 2016, 28, 10738–10743. [Google Scholar] [CrossRef]
- Luo, J.; Jia, C.; Wan, Z.; Han, F.; Zhao, B.; Wang, R. The novel dopant for hole-transporting material opens a new processing route to efficiently reduce hysteresis and improve stability of planar perovskite solar cells. J. Power Sources 2017, 342, 886–895. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, F.; Liu, T.; Zhang, W.; Li, Y.; Cai, B.; He, L.; Guo, Y.; Yang, X.; Xu, B.; et al. A crosslinked polymer as dopant-free hole-transport material for efficient n-i-p type perovskite solar cells. J. Energy Chem. 2021, 55, 211–218. [Google Scholar] [CrossRef]
- Liu, X.; Rezaee, E.; Shan, H.; Xu, J.; Zhang, Y.; Feng, Y.; Dai, J.; Chen, Z.-K.; Huang, W.; Xu, Z.-X. Dopant-free hole transport materials based on alkyl-substituted indacenodithiophene for planar perovskite solar cells. J. Mater. Chem. C 2018, 6, 4706–4713. [Google Scholar] [CrossRef]
- Wang, H.; Xu, Y.; Yu, X.; Xing, R.; Liu, J.; Han, Y. Structure and Morphology Control in Thin Films of Conjugated Polymers for an Improved Charge Transport. Polymers 2013, 5, 1272–1324. [Google Scholar] [CrossRef] [Green Version]
- Vladimirov, I.; Kühn, M.; Geßner, T.; May, F.; Weitz, R.T. Energy barriers at grain boundaries dominate charge carrier transport in an electron-conductive organic semiconductor. Sci. Rep. 2018, 8, 14868. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dhouib, A.; Filali, S. Operating Temperatures of Photovoltaic Panels. In Energy and the Environment; Sayigh, A.A.M., Ed.; Pergamon: Oxford, UK, 1990; pp. 494–498. [Google Scholar]
- Brunetti, B.; Cavallo, C.; Ciccioli, A.; Gigli, G.; Latini, A. On the Thermal and Thermodynamic (In) Stability of Methylammonium Lead Halide Perovskites. Sci. Rep. 2016, 6, 31896. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Z.; Shi, Z.; Li, T.; Chen, Y.; Huang, W. Stability of Perovskite Solar Cells: A Prospective on the Substitution of the A Cation and X Anion Angew. Chem. Int. Ed. 2017, 56, 1190–1212. [Google Scholar] [CrossRef]





| HTM | VOC, mV | JSC, mA cm−2 | FF, % | PCE, % |
|---|---|---|---|---|
| H1 | 1020 * (970 ± 40) | 22.3 (20.6 ± 0.7) | 56 (48 ± 4) | 12.0 (9.8 ± 1.2) |
| H2 | 960 (940 ± 10) | 20.3 (19.7 ± 0.5) | 53 (49 ± 1) | 10.0 (9.3 ± 0.3) |
| H3 | 1020 (1020 ± 10) | 22.9 (20.9 ± 0.8) | 55 (50 ± 3) | 12.6 (10.7 ± 0.9) |
| H4 | 1000 (980 ± 10) | 21.6 (20.9 ± 0.8) | 52 (46 ± 2) | 10.8 (9.4 ± 0.8) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tepliakova, M.M.; Kuznetsov, I.E.; Mikheeva, A.N.; Sideltsev, M.E.; Novikov, A.V.; Furasova, A.D.; Kapaev, R.R.; Piryazev, A.A.; Kapasharov, A.T.; Pugacheva, T.A.; et al. The Impact of Backbone Fluorination and Side-Chain Position in Thiophene-Benzothiadiazole-Based Hole-Transport Materials on the Performance and Stability of Perovskite Solar Cells. Int. J. Mol. Sci. 2022, 23, 13375. https://doi.org/10.3390/ijms232113375
Tepliakova MM, Kuznetsov IE, Mikheeva AN, Sideltsev ME, Novikov AV, Furasova AD, Kapaev RR, Piryazev AA, Kapasharov AT, Pugacheva TA, et al. The Impact of Backbone Fluorination and Side-Chain Position in Thiophene-Benzothiadiazole-Based Hole-Transport Materials on the Performance and Stability of Perovskite Solar Cells. International Journal of Molecular Sciences. 2022; 23(21):13375. https://doi.org/10.3390/ijms232113375
Chicago/Turabian StyleTepliakova, Marina M., Ilya E. Kuznetsov, Aleksandra N. Mikheeva, Maxim E. Sideltsev, Artyom V. Novikov, Aleksandra D. Furasova, Roman R. Kapaev, Alexey A. Piryazev, Artur T. Kapasharov, Tatiana A. Pugacheva, and et al. 2022. "The Impact of Backbone Fluorination and Side-Chain Position in Thiophene-Benzothiadiazole-Based Hole-Transport Materials on the Performance and Stability of Perovskite Solar Cells" International Journal of Molecular Sciences 23, no. 21: 13375. https://doi.org/10.3390/ijms232113375
APA StyleTepliakova, M. M., Kuznetsov, I. E., Mikheeva, A. N., Sideltsev, M. E., Novikov, A. V., Furasova, A. D., Kapaev, R. R., Piryazev, A. A., Kapasharov, A. T., Pugacheva, T. A., Makarov, S. V., Stevenson, K. J., & Akkuratov, A. V. (2022). The Impact of Backbone Fluorination and Side-Chain Position in Thiophene-Benzothiadiazole-Based Hole-Transport Materials on the Performance and Stability of Perovskite Solar Cells. International Journal of Molecular Sciences, 23(21), 13375. https://doi.org/10.3390/ijms232113375




.jpeg)