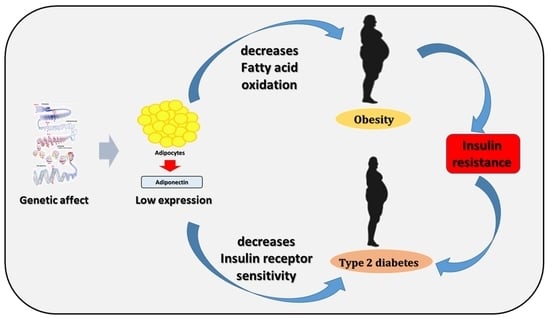
Genetic and Functional Effects of Adiponectin in Type 2 Diabetes Mellitus Development
Abstract
:1. Introduction
2. Results
2.1. Adiponectin Protein Expression in Hepatocytes from Leprdb Mice and Dock7m Mice
2.2. Adiponectin Expression in Liver Tissues from Leprdb Mice and Dock7m Mice
2.3. Adiponectin and T2DM Genetic Polymorphism Analysis
2.4. Serum Adiponectin Expression in the Human Control and T2DM Groups
2.5. Serum Adiponectin Protein Expression in the Control and T2DM Groups (<5 Years and >15 y Disease Course)
3. Discussion
4. Materials and Methods
4.1. Sample Collection
4.2. Animal Model
4.3. Western Blot Analysis
4.4. Immunohistochemistry (IHC) Analysis
4.5. Enzyme-Linked Immuno-Sorbent Assay (ELISA)
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| T2DM | Type 2 diabetes mellitus |
| IHC | Immunohistochemistry |
| SNPs | Immunohistochemistry |
| ELISA | Enzyme-linked immunosorbent assay |
| ADP | Adiponectin |
| PEPCK | phosphoenolpyruvate carboxykinase |
| G-6-Pase | glucose 6-phosphatase |
| CHB | Han Chinese in Beijing |
| JPT | Japanese in Tokyo |
| DAB | 3,3′-diaminobenzidine tetrahydrochloride |
References
- Saeedi, P.; Petersohn, I.; Salpea, P.; Malanda, B.; Karuranga, S.; Unwin, N.; Colagiuri, S.; Guariguata, L.; Motala, A.A.; Ogurtsova, K.; et al. Global and Regional Diabetes Prevalence Estimates for 2019 and Projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res. Clin. Pract. 2019, 157, 107843. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tao, Z.; Shi, A.; Zhao, J. Epidemiological Perspectives of Diabetes. Cell Biophys. 2015, 73, 181–185. [Google Scholar] [CrossRef] [PubMed]
- Oldroyd, J.; Banerjee, M.; Heald, A.; Cruickshank, K. Diabetes and Ethnic Minorities. Postgrad. J. 2005, 81, 486–490. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chamberlain, J.J.; Rhinehart, A.S.; Shaefer, C.F.; Neuman, A. Diagnosis and Management of Diabetes: Synopsis of the 2016 American Diabetes Association Standards of Medical Care in Diabetes. Ann. Intern. Med. 2016, 164, 542–552. [Google Scholar] [CrossRef] [PubMed]
- Olokoba, A.B.; Obateru, O.A.; Olokoba, L.B. Type 2 Diabetes Mellitus: A Review of Current Trends. Oman Med. J. 2012, 27, 269–273. [Google Scholar] [CrossRef]
- Astrup, A.; Finer, N. Redefining Type 2 Diabetes: ‘Diabesity’ or ‘Obesity Dependent Diabetes Mellitus’? Obes. Rev. 2000, 1, 57–59. [Google Scholar] [CrossRef]
- Maggio, C.A.; Pi-Sunyer, F.X. Obesity and Type 2 Diabetes. Endocrinol. Metab. Clin. N. Am. 2003, 32, 805–822. [Google Scholar] [CrossRef]
- Chen, S.-Y.; Hsu, Y.-M.; Lin, Y.-J.; Huang, Y.-C.; Chen, C.-J.; Lin, W.-D.; Liao, W.-L.; Chen, Y.-T.; Liu, Y.-H.; Yang, J.-S.; et al. Current Concepts Regarding Developmental Mechanisms in Diabetic Retinopathy in Taiwan. BioMedicine 2016, 6, 7. [Google Scholar] [CrossRef]
- Tattersall, R.; Pyke, D. Diabetes in Identical Twins. Lancet 1972, 300, 1120–1125. [Google Scholar] [CrossRef]
- Barroso, I. Genetics of Type 2 Diabetes. Diabet. Med. 2005, 22, 517–535. [Google Scholar] [CrossRef]
- Chen, C.-H.; Wang, Y.; Tsai, S.; Yu, T.; Chen, S.; Tsai, F. Antizyme Inhibitor 1 Genetic Polymorphisms Associated with Diabetic Patients Validated in the Livers of Diabetic Mice. Exp. Ther. Med. 2019, 18, 3139–3146. [Google Scholar] [CrossRef] [PubMed]
- Sweiss, N.; Sharma, K. Adiponectin Effects on the Kidney. Best Pr. Res. Clin. Endocrinol. Metab. 2013, 28, 71–79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kershaw, E.E.; Flier, J.S. Adipose Tissue as an Endocrine Organ. J. Clin. Endocrinol. Metab. 2004, 89, 2548–2556. [Google Scholar] [CrossRef] [PubMed]
- Karbowska, J.; Kochan, Z. Role of Adiponectin in the Regulation of Carbohydrate and Lipid Metabolism. J. Physiol. Pharmacol. 2006, 57, 103–113. [Google Scholar] [PubMed]
- Salmenniemi, U.; Zacharova, J.; Ruotsalainen, E.; Vauhkonen, I.; Pihlajamäki, J.; Kainulainen, S.; Punnonen, K.; Laakso, M. Association of Adiponectin Level and Variants in the Adiponectin Gene with Glucose Metabolism, Energy Expenditure, and Cytokines in Offspring of Type 2 Diabetic Patients. J. Clin. Endocrinol. Metab. 2005, 90, 4216–4223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hotta, K.; Funahashi, T.; Arita, Y.; Takahashi, M.; Matsuda, M.; Okamoto, Y.; Iwahashi, H.; Kuriyama, H.; Ouchi, N.; Maeda, K.; et al. Plasma Concentrations of a Novel, Adipose-Specific Protein, Adiponectin, in Type 2 Diabetic Patients. Arter. Thromb. Vasc. Biol. 2000, 20, 1595–1599. [Google Scholar] [CrossRef] [Green Version]
- Matsuzawa, Y.; Funahashi, T.; Kihara, S.; Shimomura, I. Adiponectin and Metabolic Syndrome. Arter. Thromb. Vasc. Biol. 2004, 24, 29–33. [Google Scholar] [CrossRef] [Green Version]
- Bauer, S.; Weigert, J.; Neumeier, M.; Wanninger, J.; Schäffler, A.; Luchner, A.; Schnitzbauer, A.A.; Aslanidis, C.; Buechler, C. Low-Abundant Adiponectin Receptors in Visceral Adipose Tissue of Humans and Rats Are Further Reduced in Diabetic Animals. Arch. Med. Res. 2010, 41, 75–82. [Google Scholar] [CrossRef]
- Hara, K.; Yamauchi, T.; Kadowaki, T. Adiponectin: An adipokine Linking Adipocytes and Type 2 Diabetes in Humans. Curr. Diabetes Rep. 2005, 5, 136–140. [Google Scholar] [CrossRef]
- Choe, E.Y.; Wang, H.J.; Kwon, O.; Kim, K.J.; Kim, B.S.; Lee, B.-W.; Ahn, C.W.; Cha, B.S.; Lee, H.C.; Kang, E.S.; et al. Variants of the Adiponectin Gene and Diabetic Microvascular Complications in Patients with Type 2 Diabetes. Metabolism 2012, 62, 677–685. [Google Scholar] [CrossRef]
- Abuhendi, N.; Qush, A.; Naji, F.; Abunada, H.; Al Buainain, R.; Shi, Z.; Zayed, H. Genetic Polymorphisms Associated with Type 2 Diabetes in the Arab World: A Systematic Review and Meta-Analysis. Diabetes Res. Clin. Pr. 2019, 151, 198–208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsochatzis, E.; Papatheodoridis, G.V.; Archimandritis, A.J. The Evolving Role of Leptin and Adiponectin in Chronic Liver Diseases. Am. J. Gastroenterol. 2006, 101, 2629–2640. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fang, H.; Judd, R.L. Adiponectin Regulation and Function. Compr. Physiol. 2018, 8, 1031–1063. [Google Scholar] [PubMed]
- Ghoshal, K.; Bhattacharyya, M. Adiponectin: Probe of the Molecular Paradigm Associating Diabetes and Obesity. World J. Diabetes 2015, 6, 151–166. [Google Scholar] [CrossRef]
- Tsai, M.K.; Wang, H.M.; Shiang, J.C.; Chen, I.H.; Wang, C.C.; Shiao, Y.F.; Liu, W.S.; Lin, T.J.; Chen, T.M.; Chen, Y.H. Sequence Variants of ADIPOQ and Association with Type 2 Diabetes Mellitus in Taiwan Chinese Han population. Sci. World J. 2014, 2014, 650393. [Google Scholar] [CrossRef] [Green Version]
- Vasseur, F.; Helbecque, N.; Dina, C.; Lobbens, S.; Delannoy, V.; Gaget, S.; Boutin, P.; Vaxillaire, M.; Lepretre, F.; Dupont, S.; et al. Single-Nucleotide Polymorphism Haplotypes in the Both Proximal Promoter and Exon 3 of the APM1 Gene Modulate Adipocyte-Secreted Adiponectin Hormone Levels and Contribute to the Genetic Risk for Type 2 Diabetes in French Caucasians. Hum. Mol. Genet. 2002, 11, 2607–2614. [Google Scholar] [CrossRef] [Green Version]
- Kawano, J.; Arora, R. The role of adiponectin in obesity, diabetes, and cardiovascular disease. J. Cardiometab. Syndr. 2009, 4, 44–49. [Google Scholar] [CrossRef]
- Fasshauer, M.; Paschke, R.; Stumvoll, M. Adiponectin, Obesity, and Cardiovascular Disease. Biochimie 2004, 86, 779–784. [Google Scholar] [CrossRef]
- Yang, W.S.; Jeng, C.Y.; Wu, T.J.; Tanaka, S.; Funahashi, T.; Matsuzawa, Y.; Wang, J.P.; Chen, C.L.; Tai, T.Y.; Chuang, L.M. Synthetic Peroxisome Proliferator-Activated Receptor-Gamma Agonist, Rosiglitazone, Increases Plasma Levels of Adiponectin in Type 2 Diabetic Patients. Diabetes Care 2002, 25, 376–380. [Google Scholar] [CrossRef] [Green Version]
- Inoue, M.; Maehata, E.; Yano, M.; Taniyama, M.; Suzuki, S. Correlation between the Adiponectin-Leptin Ratio and Parameters of Insulin Resistance in Patients with Type 2 Diabetes. Metabolism 2005, 54, 281–286. [Google Scholar] [CrossRef]
- American Diabetes Association. Diagnosis and Classification of Diabetes Mellitus. Diabetes Care 2010, 33, S62–S69. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, C.; Chen, Y.; Chen, S.; Hsu, Y.; Lin, C.; Tsao, J.; Juan, Y.; Yang, J.; Tsai, F. Hematopoietically Expressed Homeobox Gene is Associated with Type 2 Diabetes in KK Cg-Ay/J Mice and a Taiwanese Han Chinese Population. Exp. Ther. Med. 2018, 16, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, D.M. Reduce, Refine, Replace: The Failure of the Three R’s and the Future of Animal Experimentation. Univ. Chic. Leg. Forum. 2006, 1, 7. [Google Scholar]
- Mandel, A.M.; Mahmoud, A.A. Impairment of Cell-Mediated Immunity in Mutation Diabetic Mice (db/db). J. Immunol. 1978, 120, 1375–1377. [Google Scholar] [PubMed]
- Didovyk, A.; Tonooka, T.; Tsimring, L.; Hasty, J. Rapid and Scalable Preparation of Bacterial Lysates for Cell-Free Gene Expression. ACS Synth. Biol. 2017, 6, 2198–2208. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.-M.; Hsu, Y.-M.; Ying, M.-C.; Tsai, F.-J.; Tsai, C.-H.; Chung, J.-G.; Yang, J.-S.; Tang, C.-H.; Cheng, L.-Y.; Su, P.-H.; et al. High-Density Lipoprotein Ameliorates Palmitic Acid-Induced Lipotoxicity and Oxidative Dysfunction in H9c2 Cardiomyoblast Cells via ROS Suppression. Nutr. Metab. 2019, 16, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Huang, C.-Y.; Chen, S.-Y.; Tsai, H.-C.; Hsu, H.-C.; Tang, C.-H. Thrombin Induces Epidermal Growth Factor Receptor Transactivation and CCL2 Expression in Human Osteoblasts. Arthritis Care Res. 2012, 64, 3344–3354. [Google Scholar] [CrossRef]
- Chen, S.-Y.; Lin, J.-R.V.; Darbha, R.; Lin, P.; Liu, T.-Y.; Chen, Y.-M.A. Glycine N -Methyltransferase Tumor Susceptibility Gene in the Benzo(a)Pyrene-Detoxification Pathway. Cancer Res. 2004, 64, 3617–3623. [Google Scholar] [CrossRef] [Green Version]
- Yang, S.-Y.; Fang, C.-J.; Chen, Y.-W.; Chen, W.-P.; Lee, L.-Y.; Chen, C.-C.; Lin, Y.-Y.; Liu, S.-C.; Tsai, C.-H.; Huang, W.-C.; et al. Hericium erinaceus Mycelium Ameliorates In Vivo Progression of Osteoarthritis. Nutrients 2022, 14, 2605. [Google Scholar] [CrossRef]
- Steneberg, P.; Bernardo, L.; Edfalk, S.; Lundberg, L.; Backlund, F.; Ostenson, C.G.; Edlund, H. The Type 2 Diabetes-Associated Gene Ide is Required for Insulin Secretion and Suppression of α-Synuclein Levels in β-Cells. Diabetes 2013, 62, 2004–2014. [Google Scholar] [CrossRef] [Green Version]
- Lee, K.; Su, C.; Liu, S.; Chen, B.; Chang, J.; Tsai, C.; Huang, W.; Chen, W.; Wu, Y.; Tang, C. Cordycerebroside A Inhibits ICAM-1-Dependent M1 Monocyte Adhesion to Osteoarthritis Synovial Fibroblasts. J. Food Biochem. 2022, 46, 14108. [Google Scholar] [CrossRef] [PubMed]
- Cotsapas, C.; Prokunina-Olsson, L.; Welch, C.; Saxena, R.; Weaver, C.; Usher, N.; Guiducci, C.; Bonakdar, S.; Turner, N.; LaCroix, B.; et al. Expression Analysis of Loci Associated with Type 2 Diabetes in Human Tissues. Diabetologia 2010, 53, 2334–2339. [Google Scholar] [CrossRef] [PubMed]





| dbSNP ID | Patient with T2D | Control | OR (95% CI) | p-Value | |
|---|---|---|---|---|---|
| (N = 570) | (N = 1700) | ||||
| rs1063538 | |||||
| Genotype | (N = 570) | (N = 1696) | |||
| CC | 102 (17.9) | 285(16.8) | 1.13 (0.86–1.50) | 0.637 | |
| CT | 287 (50.4) | 838 (49.4) | 1.08 (0.88–1.34) | ||
| TT | 181 (31.8) | 573(33.8) | Ref | ||
| Allele frequency | |||||
| C | 491 (43.1) | 1408 (41.5) | 1.06 (0.93–1.22) | 0.355 | |
| T | 649 (56.9) | 1984 (58.5) | Ref | ||
| rs2241766 | |||||
| Genotype | (N = 566) | (N = 1695) | |||
| GG | 39 (6.9) | 173 (10.2) | 0.61 (0.42–0.89) | 0.025 * | |
| GT | 230 (40.6) | 717 (42.3) | 0.87 (0.71–1.06) | ||
| TT | 297 (52.5) | 805 (47.5) | Ref | ||
| Allele frequency | |||||
| G | 308 (27.2) | 1063 (31.4) | 0.82 (0.70–0.95) | 0.009 * | |
| T | 824 (72.8) | 2327 (68.6) | Ref |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, Y.-H.; Wang, Y.-H.; Chen, C.-C.; Chan, C.-J.; Tsai, F.-J.; Chen, S.-Y. Genetic and Functional Effects of Adiponectin in Type 2 Diabetes Mellitus Development. Int. J. Mol. Sci. 2022, 23, 13544. https://doi.org/10.3390/ijms232113544
Tang Y-H, Wang Y-H, Chen C-C, Chan C-J, Tsai F-J, Chen S-Y. Genetic and Functional Effects of Adiponectin in Type 2 Diabetes Mellitus Development. International Journal of Molecular Sciences. 2022; 23(21):13544. https://doi.org/10.3390/ijms232113544
Chicago/Turabian StyleTang, Yu-Hui, Yeh-Han Wang, Chin-Chang Chen, Chia-Jung Chan, Fuu-Jen Tsai, and Shih-Yin Chen. 2022. "Genetic and Functional Effects of Adiponectin in Type 2 Diabetes Mellitus Development" International Journal of Molecular Sciences 23, no. 21: 13544. https://doi.org/10.3390/ijms232113544
APA StyleTang, Y. -H., Wang, Y. -H., Chen, C. -C., Chan, C. -J., Tsai, F. -J., & Chen, S. -Y. (2022). Genetic and Functional Effects of Adiponectin in Type 2 Diabetes Mellitus Development. International Journal of Molecular Sciences, 23(21), 13544. https://doi.org/10.3390/ijms232113544