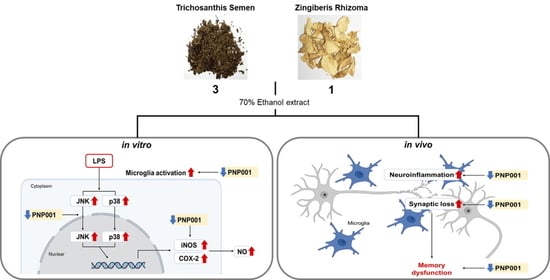
Trichosanthis Semen and Zingiberis Rhizoma Mixture Ameliorates Lipopolysaccharide-Induced Memory Dysfunction by Inhibiting Neuroinflammation
Abstract
:1. Introduction
2. Results
2.1. Effects of PNP001 on NO Production in LPS-Treated BV2 Microglial Cells
2.2. Effects of PNP001 on iNOS and COX-2 Expression in LPS-Treated BV2 Microglial Cells
2.3. Effects of PNP001 on Activation of MAPKs Pathway in LPS-Treated BV2 Microglial Cells
2.4. Effects of PNP001 on Microglial Activation in LPS-Injected Mice
2.5. Effects of PNP001 on Synapse Loss in LPS-Injected Mice
2.6. Effects of PNP001 on Long-Term Memory Impairment in LPS-Injected Mice
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Preparation of PNP001
4.3. Cell Culture and Treatment
4.4. Measurement of Cell Viability and Extracellular NO
4.5. Western Blot Analysis
4.6. Animals and Administration
4.7. Brain Tissue Preparation
4.8. Immunohistochemistry
4.9. Behavioral Test
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kaur, N.; Chugh, H.; Sakharkar, M.K.; Dhawan, U.; Chidambaram, S.B.; Chandra, R. Neuroinflammation Mechanisms and Phytotherapeutic Intervention: A Systematic Review. ACS Chem. Neurosci. 2020, 11, 3707–3731. [Google Scholar] [CrossRef] [PubMed]
- Kempuraj, D.; Thangavel, R.; Natteru, P.A.; Selvakumar, G.P.; Saeed, D.; Zahoor, H.; Zaheer, S.; Iyer, S.S.; Zaheer, A. Neuroinflammation Induces Neurodegeneration. J. Neurol. Neurosurg. Spine 2016, 1, 1003. [Google Scholar] [PubMed]
- Kwon, H.S.; Koh, S.H. Neuroinflammation in neurodegenerative disorders: The roles of microglia and astrocytes. Transl. Neurodegener. 2020, 9, 42. [Google Scholar] [CrossRef] [PubMed]
- Jha, M.K.; Jeon, S.; Suk, K. Glia as a Link between Neuroinflammation and Neuropathic Pain. Immune Netw. 2012, 12, 41–47. [Google Scholar] [CrossRef] [Green Version]
- Ahmad, M.A.; Kareem, O.; Khushtar, M.; Akbar, M.; Haque, M.R.; Iqubal, A.; Haider, M.F.; Pottoo, F.H.; Abdulla, F.S.; Al-Haidar, M.B.; et al. Neuroinflammation: A Potential Risk for Dementia. Int. J. Mol. Sci. 2022, 23, 616. [Google Scholar] [CrossRef]
- Da Re, C.; Souza, J.M.; Froes, F.; Taday, J.; Dos Santos, J.P.; Rodrigues, L.; Sesterheim, P.; Goncalves, C.A.; Leite, M.C. Neuroinflammation induced by lipopolysaccharide leads to memory impairment and alterations in hippocampal leptin signaling. Behav. Brain Res. 2020, 379, 112360. [Google Scholar] [CrossRef]
- Batista, C.R.A.; Gomes, G.F.; Candelario-Jalil, E.; Fiebich, B.L.; de Oliveira, A.C.P. Lipopolysaccharide-Induced Neuroinflammation as a Bridge to Understand Neurodegeneration. Int. J. Mol. Sci. 2019, 20, 2293. [Google Scholar] [CrossRef] [Green Version]
- Ju, I.G.; Huh, E.; Kim, N.; Lee, S.; Choi, J.G.; Hong, J.; Oh, M.S. Artemisiae Iwayomogii Herba inhibits lipopolysaccharide-induced neuroinflammation by regulating NF-kappaB and MAPK signaling pathways. Phytomed. Int. J. Phytother. Phytopharm. 2021, 84, 153501. [Google Scholar]
- Azevedo, E.P.; Ledo, J.H.; Barbosa, G.; Sobrinho, M.; Diniz, L.; Fonseca, A.C.; Gomes, F.; Romao, L.; Lima, F.R.; Palhano, F.L.; et al. Activated microglia mediate synapse loss and short-term memory deficits in a mouse model of transthyretin-related oculoleptomeningeal amyloidosis. Cell Death Dis. 2013, 4, e789. [Google Scholar] [CrossRef] [Green Version]
- Hu, Z.; Zhou, H.; Zhao, J.; Sun, J.; Li, M.; Sun, X. Microwave-assisted extraction, characterization and immunomodulatory activity on RAW264.7 cells of polysaccharides from Trichosanthes kirilowii Maxim seeds. Int. J. Biol. Macromol. 2020, 164, 2861–2872. [Google Scholar] [CrossRef]
- Lee, S.; Ju, I.G.; Choi, Y.; Park, S.; Oh, M.S. Trichosanthis Semen Suppresses Lipopolysaccharide-Induced Neuroinflammation by Regulating the NF-kappaB Signaling Pathway and HO-1 Expression in Microglia. Toxins 2021, 13, 898. [Google Scholar] [CrossRef] [PubMed]
- Ozaki, Y.; Xing, L.; Satake, M. Antiinflammatory effect of Trichosanthes kirilowii Maxim, and its effective parts. Biol. Pharm. Bull. 1996, 19, 1046–1048. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, S.M.; Jeon, S.K.; Kim, O.H.; Ahn, J.Y.; Kim, C.H.; Park, S.D.; Lee, J.H. Anti-tumor effects of the ethanolic extract of Trichosanthes kirilowii seeds in colorectal cancer. Chin. Med. 2019, 14, 43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akihisa, T.; Yasukawa, K.; Kimura, Y.; Takido, M.; Kokke, W.C.; Tamura, T. Five D:C-friedo-oleanane triterpenes from the seeds of Trichosanthes kirilowii Maxim. and their anti-inflammatory effects. Chem. Pharm. Bull. 1994, 42, 1101–1105. [Google Scholar] [CrossRef] [Green Version]
- Haniadka, R.; Saldanha, E.; Sunita, V.; Palatty, P.L.; Fayad, R.; Baliga, M.S. A review of the gastroprotective effects of ginger (Zingiber officinale Roscoe). Food Funct. 2013, 4, 845–855. [Google Scholar] [CrossRef]
- Van Breemen, R.B.; Tao, Y.; Li, W. Cyclooxygenase-2 inhibitors in ginger (Zingiber officinale). Fitoterapia 2011, 82, 38–43. [Google Scholar] [CrossRef] [Green Version]
- Hussein, U.K.; Hassan, N.E.Y.; Elhalwagy, M.E.A.; Zaki, A.R.; Abubakr, H.O.; Venkata, K.C.N.; Jang, K.Y.; Bishayee, A. Ginger and Propolis Exert Neuroprotective Effects against Monosodium Glutamate-Induced Neurotoxicity in Rats. Molecules 2017, 22, 1928. [Google Scholar] [CrossRef] [Green Version]
- Schepici, G.; Contestabile, V.; Valeri, A.; Mazzon, E. Ginger, a Possible Candidate for the Treatment of Dementias? Molecules 2021, 26, 5700. [Google Scholar] [CrossRef]
- Choi, J.G.; Kim, S.Y.; Jeong, M.; Oh, M.S. Pharmacotherapeutic potential of ginger and its compounds in age-related neurological disorders. Pharmacol. Ther. 2018, 182, 56–69. [Google Scholar] [CrossRef]
- Banati, R.B.; Gehrmann, J.; Schubert, P.; Kreutzberg, G.W. Cytotoxicity of microglia. Glia 1993, 7, 111–118. [Google Scholar] [CrossRef]
- Moita, E.; Gil-Izquierdo, A.; Sousa, C.; Ferreres, F.; Silva, L.R.; Valentao, P.; Dominguez-Perles, R.; Baenas, N.; Andrade, P.B. Integrated analysis of COX-2 and iNOS derived inflammatory mediators in LPS-stimulated RAW macrophages pre-exposed to Echium plantagineum L. bee pollen extract. PLoS ONE 2013, 8, e59131. [Google Scholar] [CrossRef] [PubMed]
- Kaminska, B.; Gozdz, A.; Zawadzka, M.; Ellert-Miklaszewska, A.; Lipko, M. MAPK signal transduction underlying brain inflammation and gliosis as therapeutic target. Anat. Rec. 2009, 292, 1902–1913. [Google Scholar] [CrossRef] [PubMed]
- Ito, D.; Imai, Y.; Ohsawa, K.; Nakajima, K.; Fukuuchi, Y.; Kohsaka, S. Microglia-specific localisation of a novel calcium binding protein, Iba1. Brain Res. Mol. Brain Res. 1998, 57, 1–9. [Google Scholar] [CrossRef]
- Deng, X.H.; Ai, W.M.; Lei, D.L.; Luo, X.G.; Yan, X.X.; Li, Z. Lipopolysaccharide induces paired immunoglobulin-like receptor B (PirB) expression, synaptic alteration, and learning-memory deficit in rats. Neuroscience 2012, 209, 161–170. [Google Scholar] [CrossRef]
- Zhao, W.; Xu, Z.; Cao, J.; Fu, Q.; Wu, Y.; Zhang, X.; Long, Y.; Zhang, X.; Yang, Y.; Li, Y.; et al. Elamipretide (SS-31) improves mitochondrial dysfunction, synaptic and memory impairment induced by lipopolysaccharide in mice. J. Neuroinflamm. 2019, 16, 230. [Google Scholar] [CrossRef]
- Li, T.; Zhang, S. Microgliosis in the Injured Brain: Infiltrating Cells and Reactive Microglia Both Play a Role. Neurosci. A Rev. J. Bringing Neurobiol. Neurol. Psychiatry 2016, 22, 165–170. [Google Scholar] [CrossRef]
- Zhao, J.; Bi, W.; Xiao, S.; Lan, X.; Cheng, X.; Zhang, J.; Lu, D.; Wei, W.; Wang, Y.; Li, H.; et al. Neuroinflammation induced by lipopolysaccharide causes cognitive impairment in mice. Sci. Rep. 2019, 9, 5790. [Google Scholar] [CrossRef] [Green Version]
- Cornell, J.; Salinas, S.; Huang, H.Y.; Zhou, M. Microglia regulation of synaptic plasticity and learning and memory. Neural Regen. Res. 2022, 17, 705–716. [Google Scholar]
- Pintado, C.; Gavilan, M.P.; Gavilan, E.; Garcia-Cuervo, L.; Gutierrez, A.; Vitorica, J.; Castano, A.; Rios, R.M.; Ruano, D. Lipopolysaccharide-induced neuroinflammation leads to the accumulation of ubiquitinated proteins and increases susceptibility to neurodegeneration induced by proteasome inhibition in rat hippocampus. J. Neuroinflamm. 2012, 9, 87. [Google Scholar] [CrossRef] [Green Version]
- Lim, S.; Moon, M.; Oh, H.; Kim, H.G.; Kim, S.Y.; Oh, M.S. Ginger improves cognitive function via NGF-induced ERK/CREB activation in the hippocampus of the mouse. J. Nutr. Biochem. 2014, 25, 1058–1065. [Google Scholar] [CrossRef]
- Picon-Pages, P.; Garcia-Buendia, J.; Munoz, F.J. Functions and dysfunctions of nitric oxide in brain. Biochim. Biophys. Acta Mol. Basis Dis. 2019, 1865, 1949–1967. [Google Scholar] [CrossRef] [PubMed]
- Togo, T.; Katsuse, O.; Iseki, E. Nitric oxide pathways in Alzheimer’s disease and other neurodegenerative dementias. Neurol. Res. 2004, 26, 563–566. [Google Scholar] [CrossRef] [PubMed]
- Brown, G.C. Mechanisms of inflammatory neurodegeneration: iNOS and NADPH oxidase. Biochem. Soc. Trans. 2007, 35 Pt 5, 1119–1121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Udayabanu, M.; Kumaran, D.; Nair, R.U.; Srinivas, P.; Bhagat, N.; Aneja, R.; Katyal, A. Nitric oxide associated with iNOS expression inhibits acetylcholinesterase activity and induces memory impairment during acute hypobaric hypoxia. Brain Res. 2008, 1230, 138–149. [Google Scholar] [CrossRef] [PubMed]
- Chabrier, P.E.; Demerle-Pallardy, C.; Auguet, M. Nitric oxide synthases: Targets for therapeutic strategies in neurological diseases. Cell. Mol. Life Sci. CMLS 1999, 55, 1029–1035. [Google Scholar] [CrossRef] [PubMed]
- Pawate, S.; Bhat, N.R. C-Jun N-terminal kinase (JNK) regulation of iNOS expression in glial cells: Predominant role of JNK1 isoform. Antioxid. Redox Signal. 2006, 8, 903–909. [Google Scholar] [CrossRef]
- Jablonska, E.; Ratajczak, W.; Jablonski, J. Role of the p38 MAPK pathway in induction of iNOS expression in human leukocytes. Folia Biol. 2008, 56, 83–89. [Google Scholar] [CrossRef]
- Pasqualetti, G.; Brooks, D.J.; Edison, P. The role of neuroinflammation in dementias. Curr. Neurol. Neurosci. Rep. 2015, 15, 17. [Google Scholar] [CrossRef]
- Krukowski, K.; Chou, A.; Feng, X.; Tiret, B.; Paladini, M.S.; Riparip, L.K.; Chaumeil, M.M.; Lemere, C.; Rosi, S. Traumatic Brain Injury in Aged Mice Induces Chronic Microglia Activation, Synapse Loss, and Complement-Dependent Memory Deficits. Int. J. Mol. Sci. 2018, 19, 3753. [Google Scholar] [CrossRef] [Green Version]
- Qin, L.; Wu, X.; Block, M.L.; Liu, Y.; Breese, G.R.; Hong, J.S.; Knapp, D.J.; Crews, F.T. Systemic LPS causes chronic neuroinflammation and progressive neurodegeneration. Glia 2007, 55, 453–462. [Google Scholar] [CrossRef] [Green Version]
- Xin, Y.R.; Jiang, J.X.; Hu, Y.; Pan, J.P.; Mi, X.N.; Gao, Q.; Xiao, F.; Zhang, W.; Luo, H.M. The Immune System Drives Synapse Loss During Lipopolysaccharide-Induced Learning and Memory Impairment in Mice. Front. Aging Neurosci. 2019, 11, 279. [Google Scholar] [CrossRef] [PubMed]
- Sheppard, O.; Coleman, M.P.; Durrant, C.S. Lipopolysaccharide-induced neuroinflammation induces presynaptic disruption through a direct action on brain tissue involving microglia-derived interleukin 1 beta. J. Neuroinflamm. 2019, 16, 106. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Ali, T.; He, K.; Liu, Z.; Shah, F.A.; Ren, Q.; Liu, Y.; Jiang, A.; Li, S. Ibrutinib alleviates LPS-induced neuroinflammation and synaptic defects in a mouse model of depression. Brain Behav. Immun. 2021, 92, 10–24. [Google Scholar] [CrossRef] [PubMed]
- Mottahedin, A.; Ardalan, M.; Chumak, T.; Riebe, I.; Ek, J.; Mallard, C. Effect of Neuroinflammation on Synaptic Organization and Function in the Developing Brain: Implications for Neurodevelopmental and Neurodegenerative Disorders. Front. Cell Neurosci. 2017, 11, 190. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manabe, T.; Racz, I.; Schwartz, S.; Oberle, L.; Santarelli, F.; Emmrich, J.V.; Neher, J.J.; Heneka, M.T. Systemic inflammation induced the delayed reduction of excitatory synapses in the CA3 during ageing. J. Neurochem. 2021, 159, 525–542. [Google Scholar] [CrossRef]
- Fu, W.Y.; Wang, X.; Ip, N.Y. Targeting Neuroinflammation as a Therapeutic Strategy for Alzheimer’s Disease: Mechanisms, Drug Candidates, and New Opportunities. ACS Chem. Neurosci. 2019, 10, 872–879. [Google Scholar] [CrossRef] [PubMed]
- Impellizzeri, D.; Siracusa, R.; Cordaro, M.; Crupi, R.; Peritore, A.F.; Gugliandolo, E.; D’Amico, R.; Petrosino, S.; Evangelista, M.; Di Paola, R.; et al. N-Palmitoylethanolamine-oxazoline (PEA-OXA): A new therapeutic strategy to reduce neuroinflammation, oxidative stress associated to vascular dementia in an experimental model of repeated bilateral common carotid arteries occlusion. Neurobiol. Dis. 2019, 125, 77–91. [Google Scholar] [CrossRef]
- Heneka, M.T.; Carson, M.J.; El Khoury, J.; Landreth, G.E.; Brosseron, F.; Feinstein, D.L.; Jacobs, A.H.; Wyss-Coray, T.; Vitorica, J.; Ransohoff, R.M.; et al. Neuroinflammation in Alzheimer’s disease. Lancet Neurol. 2015, 14, 388–405. [Google Scholar] [CrossRef] [Green Version]
- Tian, Z.; Ji, X.; Liu, J. Neuroinflammation in Vascular Cognitive Impairment and Dementia: Current Evidence, Advances, and Prospects. Int. J. Mol. Sci. 2022, 23, 6224. [Google Scholar] [CrossRef]
- Zhang, L.Y.; Pan, J.; Mamtilahun, M.; Zhu, Y.; Wang, L.; Venkatesh, A.; Shi, R.; Tu, X.; Jin, K.; Wang, Y.; et al. Microglia exacerbate white matter injury via complement C3/C3aR pathway after hypoperfusion. Theranostics 2020, 10, 74–90. [Google Scholar] [CrossRef]
- Miyanohara, J.; Kakae, M.; Nagayasu, K.; Nakagawa, T.; Mori, Y.; Arai, K.; Shirakawa, H.; Kaneko, S. TRPM2 Channel Aggravates CNS Inflammation and Cognitive Impairment via Activation of Microglia in Chronic Cerebral Hypoperfusion. J. Neurosci. Off. J. Soc. Neurosci. 2018, 38, 3520–3533. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taragano, F.E.; Allegri, R.F.; Lyketsos, C. Mild behavioral impairment: A prodromal stage of dementia. Dement. Neuropsychol. 2008, 2, 256–260. [Google Scholar] [CrossRef] [PubMed]
- Bossu, P.; Salani, F.; Ciaramella, A.; Sacchinelli, E.; Mosca, A.; Banaj, N.; Assogna, F.; Orfei, M.D.; Caltagirone, C.; Gianni, W.; et al. Anti-inflammatory Effects of Homotaurine in Patients With Amnestic Mild Cognitive Impairment. Front. Aging Neurosci. 2018, 10, 285. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.; Yeom, M.; Shim, I.; Lee, H.; Hahm, D.H. Inhibitory effect of carvacrol on lipopolysaccharide-induced memory impairment in rats. Korean J. Physiol. Pharmacol. 2020, 24, 27–37. [Google Scholar] [CrossRef] [PubMed]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Im, H.; Ju, I.G.; Kim, J.H.; Lee, S.; Oh, M.S. Trichosanthis Semen and Zingiberis Rhizoma Mixture Ameliorates Lipopolysaccharide-Induced Memory Dysfunction by Inhibiting Neuroinflammation. Int. J. Mol. Sci. 2022, 23, 14015. https://doi.org/10.3390/ijms232214015
Im H, Ju IG, Kim JH, Lee S, Oh MS. Trichosanthis Semen and Zingiberis Rhizoma Mixture Ameliorates Lipopolysaccharide-Induced Memory Dysfunction by Inhibiting Neuroinflammation. International Journal of Molecular Sciences. 2022; 23(22):14015. https://doi.org/10.3390/ijms232214015
Chicago/Turabian StyleIm, Hyeri, In Gyoung Ju, Jin Hee Kim, Seungmin Lee, and Myung Sook Oh. 2022. "Trichosanthis Semen and Zingiberis Rhizoma Mixture Ameliorates Lipopolysaccharide-Induced Memory Dysfunction by Inhibiting Neuroinflammation" International Journal of Molecular Sciences 23, no. 22: 14015. https://doi.org/10.3390/ijms232214015
APA StyleIm, H., Ju, I. G., Kim, J. H., Lee, S., & Oh, M. S. (2022). Trichosanthis Semen and Zingiberis Rhizoma Mixture Ameliorates Lipopolysaccharide-Induced Memory Dysfunction by Inhibiting Neuroinflammation. International Journal of Molecular Sciences, 23(22), 14015. https://doi.org/10.3390/ijms232214015