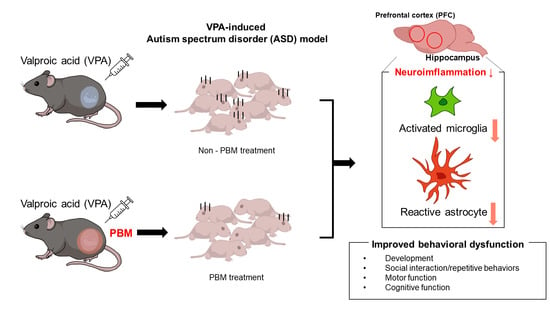
Photobiomodulation Attenuated Cognitive Dysfunction and Neuroinflammation in a Prenatal Valproic Acid-Induced Autism Spectrum Disorder Mouse Model
Abstract
:1. Introduction
2. Results
2.1. Effect of Laser Treatment on VPA-Induced Developmental Abnormalities
2.2. Laser Treatment Improved Motor Function in Mice Exposed to VPA
2.3. Laser Treatment Attenuated Social Cognitive Dysfunction in Mice Exposed to VPA
2.4. Laser Treatment Attenuated Impairment in Repetitive Behavior of Mice Exposed to VPA
2.5. Laser Treatment Attenuated Hyperactivity in Mice Exposed to VPA
2.6. Laser Treatment Improved Cognitive Function in Mice Exposed to VPA
2.7. Laser Treatment Decreased GFAP Expression in the PFC and Hippocampus of Mice Exposed to VPA
2.8. Laser Treatment Decreased Iba1 Expression in the Hippocampus of Mice Exposed to VPA
3. Discussion
4. Methods and Materials
4.1. Animals
4.2. Prenatal VPA Mouse Model for ASD
4.3. Behavioral Tests
4.3.1. Eye Opening Test
4.3.2. Righting Reflex Test
4.3.3. Bodyweight
4.3.4. Negative Geotaxis
4.3.5. Wire Maneuver Test
4.3.6. Three-Chamber Test
4.3.7. Y-Maze
4.3.8. Cylinder Test
4.3.9. Open Field Test
4.3.10. Novel Object Recognition Test
4.3.11. Morris Water Maze
4.4. Immunohistochemistry
4.5. Western Blot Analysis
4.6. Statistics
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Edition, F. Diagnostic and statistical manual of mental disorders. Am. Psychiatr. Assoc. 2013, 21, 591–643. [Google Scholar]
- Jęśko, H.; Cieślik, M.; Gromadzka, G.; Adamczyk, A. Dysfunctional proteins in neuropsychiatric disorders: From neurodegeneration to autism spectrum disorders. Neurochem. Int. 2020, 141, 104853. [Google Scholar] [CrossRef] [PubMed]
- Kern, J.K.; Geier, D.A.; Sykes, L.K.; Geier, M.R. Evidence of neurodegeneration in autism spectrum disorder. Transl. Neurodegener. 2013, 2, 17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nag, H.E.; Nordgren, A.; Anderlid, B.-M.; Nærland, T. Reversed gender ratio of autism spectrum disorder in Smith-Magenis syndrome. Mol. Autism 2018, 9, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Pallanti, S.; Di Ponzio, M.; Grassi, E.; Vannini, G.; Cauli, G. Transcranial Photobiomodulation for the Treatment of Children with Autism Spectrum Disorder (ASD): A Retrospective Study. Children 2022, 9, 755. [Google Scholar] [CrossRef]
- Depino, A.M. Peripheral and central inflammation in autism spectrum disorders. Mol. Cell. Neurosci. 2013, 53, 69–76. [Google Scholar] [CrossRef]
- Vargas, D.L.; Nascimbene, C.; Krishnan, C.; Zimmerman, A.W.; Pardo, C.A. Neuroglial activation and neuroinflammation in the brain of patients with autism. Ann. Neurol. 2004, 57, 67–81. [Google Scholar] [CrossRef]
- Sato, A.; Kotajima-Murakami, H.; Tanaka, M.; Katoh, Y.; Ikeda, K. Influence of Prenatal Drug Exposure, Maternal Inflammation, and Parental Aging on the Development of Autism Spectrum Disorder. Front. Psychiatry 2022, 13, 821455. [Google Scholar] [CrossRef]
- Nevitt, S.J.; Sudell, M.; Cividini, S.; Marson, A.G.; Smith, C.T. Antiepileptic drug monotherapy for epilepsy: A network meta-analysis of individual participant data. Cochrane Database Syst. Rev. 2022, 4, CD20114122. [Google Scholar] [CrossRef]
- Clayton-Smith, J.; Bromley, R.; Dean, J.; Journel, H.; Odent, S.; Wood, A.; Williams, J.; Cuthbert, V.; Hackett, L.; Aslam, N.; et al. Diagnosis and management of individuals with Fetal Valproate Spectrum Disorder; a consensus statement from the European Reference Network for Congenital Malformations and Intellectual Disability. Orphanet J. Rare Dis. 2019, 14, 180. [Google Scholar] [CrossRef]
- Salehpour, F.; Mahmoudi, J.; Kamari, F.; Sadigh-Eteghad, S.; Rasta, S.H.; Hamblin, M.R. Brain Photobiomodulation Therapy: A Narrative Review. Mol. Neurobiol. 2018, 55, 6601–6636. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Wu, J.; Dong, X.; Yin, H.; Shi, X.; Su, S.; Che, B.; Li, Y.; Yang, J. Gut flora-targeted photobiomodulation therapy improves senile dementia in an Aß-induced Alzheimer’s disease animal model. J. Photochem. Photobiol. B Biol. 2021, 216, 112152. [Google Scholar] [CrossRef] [PubMed]
- De la Torre, J.C. Treating cognitive impairment with transcranial low level laser therapy. J. Photochem. Photobiol. B Biol. 2017, 168, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Montazeri, K.; Farhadi, M.; Fekrazad, R.; Akbarnejad, Z.; Chaibakhsh, S.; Mahmoudian, S. Transcranial photobiomodulation in the management of brain disorders. J. Photochem. Photobiol. B Biol. 2021, 221, 112207. [Google Scholar] [CrossRef]
- Hamblin, M.R. Could Photobiomodulation Treat Autism Spectrum Disorder? Photobiomodul. Photomed. Laser Surg. 2022, 40, 367–369. [Google Scholar] [CrossRef]
- Leisman, G.; Machado, C.; Machado, Y.; Chinchilla-Acosta, M. Effects of Low-Level Laser Therapy in Autism Spectrum Disorder. Adv. Exp. Med. Biol. 2018, 1116, 111–130. [Google Scholar] [CrossRef]
- Kwon, H.S.; Koh, S.H. Neuroinflammation in neurodegenerative disorders: The roles of microglia and astrocytes. Transl Neurodegener 2020, 9, 42. [Google Scholar] [CrossRef]
- Morgan, J.T.; Chana, G.; Pardo, C.A.; Achim, C.; Semendeferi, K.; Buckwalter, J.; Courchesne, E.; Everall, I.P. Microglial activation and increased microglial density observed in the dorsolateral prefrontal cortex in autism. Biol Psychiatry 2010, 68, 368–376. [Google Scholar] [CrossRef]
- Pardo, C.A.; Vargas, D.L.; Zimmerman, A.W. Immunity, neuroglia and neuroinflammation in autism. Int. Rev. Psychiatry 2005, 17, 485–495. [Google Scholar] [CrossRef] [Green Version]
- Cardoso, F.D.S.; Salehpour, F.; Coimbra, N.C.; Gonzalez-Lima, F.; Gomes da Silva, S. Photobiomodulation for the treatment of neuroinflammation: A systematic review of controlled laboratory animal studies. Front. Neurosci. 2022, 16, 1006031. [Google Scholar] [CrossRef]
- Mabunga, D.F.N.; Gonzales, E.L.T.; Kim, J.-W.; Kim, K.C.; Shin, K.C.K.A.C.Y. Exploring the Validity of Valproic Acid Animal Model of Autism. Exp. Neurobiol. 2015, 24, 285–300. [Google Scholar] [CrossRef] [PubMed]
- Sweatt, J.D. Hippocampal function in cognition. Psychopharmacology 2004, 174, 99–110. [Google Scholar] [CrossRef] [PubMed]
- Vertes, R.P. Differential projections of the infralimbic and prelimbic cortex in the rat. Synapse 2003, 51, 32–58. [Google Scholar] [CrossRef] [PubMed]
- Liang, L.-Y.; Shewokis, P.A.; Getchell, N. Brain Activation in the Prefrontal Cortex during Motor and Cognitive Tasks in Adults. J. Behav. Brain Sci. 2016, 6, 463–474. [Google Scholar] [CrossRef] [Green Version]
- Haydon, P.G.; Nedergaard, M. How Do Astrocytes Participate in Neural Plasticity? Cold Spring Harb. Perspect. Biol. 2014, 7, a020438. [Google Scholar] [CrossRef] [Green Version]
- Casano, A.M.; Peri, F. Microglia: Multitasking Specialists of the Brain. Dev. Cell 2015, 32, 469–477. [Google Scholar] [CrossRef] [Green Version]
- Takano, T. Role of Microglia in Autism: Recent Advances. Dev. Neurosci. 2015, 37, 195–202. [Google Scholar] [CrossRef]
- Voineagu, I.; Wang, X.; Johnston, P.; Lowe, J.K.; Tian, Y.; Horvath, S.; Mill, J.; Cantor, R.M.; Blencowe, B.J.; Geschwind, D.H. Transcriptomic analysis of autistic brain reveals convergent molecular pathology. Nature 2011, 474, 380–384. [Google Scholar] [CrossRef] [Green Version]
- de Baumont, A.; Maschietto, M.; Lima, L.; Carraro, D.M.; Olivieri, E.H.; Fiorini, A.; Barreta, L.A.N.; Palha, J.A.; Belmonte-De-Abreu, P.; Filho, C.A.M.; et al. Innate immune response is differentially dysregulated between bipolar disease and schizophrenia. Schizophr. Res. 2015, 161, 215–221. [Google Scholar] [CrossRef] [Green Version]
- Schneider, T.; Roman, A.; Basta-Kaim, A.; Kubera, M.; Budziszewska, B.; Schneider, K.; Przewłocki, R. Gender-specific behavioral and immunological alterations in an animal model of autism induced by prenatal exposure to valproic acid. Psychoneuroendocrinology 2008, 33, 728–740. [Google Scholar] [CrossRef]
- Bond, A.M.; Berg, D.A.; Lee, S.; Garcia-Epelboim, A.S.; Adusumilli, V.S.; Ming, G.-L.; Song, H. Differential Timing and Coordination of Neurogenesis and Astrogenesis in Developing Mouse Hippocampal Subregions. Brain Sci. 2020, 10, 909. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Wang, Q.; Yan, T.; Zhang, Y.; Xu, H.-J.; Yu, H.-P.; Tu, Z.; Guo, X.; Jiang, Y.-H.; Li, X.-J.; et al. Maternal valproic acid exposure leads to neurogenesis defects and autism-like behaviors in non-human primates. Transl. Psychiatry 2019, 9, 267. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Campolongo, M.; Kazlauskas, N.; Falasco, G.; Urrutia, L.; Salgueiro, N.; Höcht, C.; Depino, A.M. Sociability deficits after prenatal exposure to valproic acid are rescued by early social enrichment. Mol. Autism 2018, 9, 36. [Google Scholar] [CrossRef] [PubMed]
- Yang, E.-J.; Ahn, S.; Lee, K.; Mahmood, U.; Kim, H.-S. Correction: Early Behavioral Abnormalities and Perinatal Alterations of PTEN/AKT Pathway in Valproic Acid Autism Model Mice. PLoS ONE 2016, 11, e0157202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schneider, T.; Przewłocki, R. Behavioral Alterations in Rats Prenatally Exposed to Valproic Acid: Animal Model of Autism. Neuropsychopharmacology 2004, 30, 80–89. [Google Scholar] [CrossRef]
- Chaliha, D.; Albrecht, M.; Vaccarezza, M.; Takechi, R.; Lam, V.; Al-Salami, H.; Mamo, J. A Systematic Review of the Valproic-Acid-Induced Rodent Model of Autism. Dev. Neurosci. 2020, 42, 12–48. [Google Scholar] [CrossRef]
- Al Sagheer, T.; Haida, O.; Balbous, A.; Francheteau, M.; Matas, E.; Fernagut, P.-O.; Jaber, M. Motor Impairments Correlate with Social Deficits and Restricted Neuronal Loss in an Environmental Model of Autism. Int. J. Neuropsychopharmacol. 2018, 21, 871–882. [Google Scholar] [CrossRef] [Green Version]
- Yang, L.; Wu, C.; Parker, E.; Li, Y.; Dong, Y.; Tucker, L.; Brann, D.W.; Lin, H.W.; Zhang, Q. Non-invasive photobiomodulation treatment in an Alzheimer Disease-like transgenic rat model. Theranostics 2022, 12, 2205–2231. [Google Scholar] [CrossRef]
- Vogel, D.D.S.; Ortiz-Villatoro, N.N.; Araújo, N.S.; Marques, M.J.G.; Aimbire, F.; Scorza, F.A.; Scorza, C.A.; Albertini, R. Transcranial low-level laser therapy in an in vivo model of stroke: Relevance to the brain infarct, microglia activation and neuroinflammation. J. Biophotonics 2021, 14, e202000500. [Google Scholar] [CrossRef]
- Foo, A.S.C.; Soong, T.W.; Yeo, T.T.; Lim, K.L. Mitochondrial Dysfunction and Parkinson’s Disease-Near-Infrared Photobiomodulation as a Potential Therapeutic Strategy. Front. Aging Neurosci. 2020, 12, 89. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.W.; Pang, Y. Dysregulation of neurogenesis by neuroinflammation: Key differences in neurodevelopmental and neurological disorders. Neural Regen. Res. 2017, 12, 366–371. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Tucker, D.; Dong, Y.; Wu, C.; Lu, Y.; Li, Y.; Zhang, J.; Liu, T.C.; Zhang, Q. Photobiomodulation therapy promotes neurogenesis by improving post-stroke local microenvironment and stimulating neuroprogenitor cells. Exp. Neurol. 2018, 299, 86–96. [Google Scholar] [CrossRef]
- Hong, N.; Kang, G.W.; Park, J.O.; Chung, P.S.; Lee, M.Y.; Ahn, J.C. Photobiomodulation regulates adult neurogenesis in the hippocampus in a status epilepticus animal model. Sci. Rep. 2022, 12, 15246. [Google Scholar] [CrossRef] [PubMed]
- Chung, H.; Dai, T.; Sharma, S.K.; Huang, Y.Y.; Carroll, J.D.; Hamblin, M.R. The nuts and bolts of low-level laser (light) therapy. Ann. Biomed. Eng. 2012, 40, 516–533. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsai, C.M.; Chang, S.F.; Li, C.C.; Chang, H. Transcranial photobiomodulation (808 nm) attenuates pentylenetetrazole-induced seizures by suppressing hippocampal neuroinflammation, astrogliosis, and microgliosis in peripubertal rats. Neurophotonics 2022, 9, 015006. [Google Scholar] [CrossRef] [PubMed]
- Tucker, L.D.; Lu, Y.; Dong, Y.; Yang, L.; Li, Y.; Zhao, N.; Zhang, Q. Photobiomodulation Therapy Attenuates Hypoxic-Ischemic Injury in a Neonatal Rat Model. J. Mol. Neurosci. 2018, 65, 514–526. [Google Scholar] [CrossRef] [PubMed]
- Maliske, L.; Kanske, P. The Social Connectome—Moving Toward Complexity in the Study of Brain Networks and Their Interactions in Social Cognitive and Affective Neuroscience. Front. Psychiatry 2022, 13, 845492. [Google Scholar] [CrossRef]
- Vig, S.; Jedrysek, E. Autistic features in young children with significant cognitive impairment: Autism or mental retardation? J. Autism Dev. Disord. 1999, 29, 235–248. [Google Scholar] [CrossRef]
- Gąssowska-Dobrowolska, M.; Cieślik, M.; Czapski, G.A.; Jęśko, H.; Frontczak-Baniewicz, M.; Gewartowska, M.; Dominiak, A.; Polowy, R.; Filipkowski, R.K.; Babiec, L.; et al. Prenatal Exposure to Valproic Acid Affects Microglia and Synaptic Ultrastructure in a Brain-Region-Specific Manner in Young-Adult Male Rats: Relevance to Autism Spectrum Disorders. Int. J. Mol. Sci. 2020, 21, 3576. [Google Scholar] [CrossRef]
- Carson, M.J.; Bilousova, T.V.; Puntambekar, S.S.; Melchior, B.; Doose, J.M.; Ethell, I.M. A rose by any other name? The potential consequences of microglial heterogeneity during CNS health and disease. Neurotherapeutics 2007, 4, 571–579. [Google Scholar] [CrossRef] [Green Version]
- Edmonson, C.; Ziats, M.N.; Rennert, O.M. Altered glial marker expression in autistic post-mortem prefrontal cortex and cerebellum. Mol. Autism 2014, 5, 3. [Google Scholar] [CrossRef] [PubMed]
- Eng, L.F. Glial fibrillary acidic protein (GFAP): The major protein of glial intermediate filaments in differentiated astrocytes. J. Neuroimmunol. 1985, 8, 203–214. [Google Scholar] [CrossRef]
- Ohsawa, K.; Imai, Y.; Sasaki, Y.; Kohsaka, S. Microglia/macrophage-specific protein Iba1 binds to fimbrin and enhances its actin-bundling activity. J. Neurochem. 2004, 88, 844–856. [Google Scholar] [CrossRef] [PubMed]
- Czlonkowska, A.; Kurkowska-Jastrzebska, I. Inflammation and gliosis in neurological diseases--clinical implications. J. Neuroimmunol. 2011, 231, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Behringer, R.; Gertsenstein, M.; Nagy, K.V.; Nagy, A. Selecting Female Mice in Estrus and Checking Plugs. Cold Spring Harb. Protoc. 2016, 8, pdb-prot092387. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Dong, Y.; Wu, C.; Youngblood, H.; Li, Y.; Zong, X.; Li, L.; Xu, T.; Zhang, Q. Effects of prenatal photobiomodulation treatment on neonatal hypoxic ischemia in rat offspring. Theranostics 2021, 11, 1269–1294. [Google Scholar] [CrossRef]
- Soria-Ortiz, M.B.; Reyes-Ortega, P.; Martínez-Torres, A.; Reyes-Haro, D. A Functional Signature in the Developing Cerebellum: Evidence From a Preclinical Model of Autism. Front. Cell Dev. Biol. 2021, 9, 727079. [Google Scholar] [CrossRef]
- Wang, R.; Tan, J.; Guo, J.; Zheng, Y.; Han, Q.; So, K.-F.; Yu, J.; Zhang, L. Aberrant Development and Synaptic Transmission of Cerebellar Cortex in a VPA Induced Mouse Autism Model. Front. Cell. Neurosci. 2018, 12, 500. [Google Scholar] [CrossRef] [Green Version]
- Dorchies, O.M.; Reutenauer-Patte, J.; Dahmane, E.; Ismail, H.M.; Petermann, O.; Patthey-Vuadens, O.; Comyn, S.A.; Gayi, E.; Piacenza, T.; Handa, R.J.; et al. The Anticancer Drug Tamoxifen Counteracts the Pathology in a Mouse Model of Duchenne Muscular Dystrophy. Am. J. Pathol. 2013, 182, 485–504. [Google Scholar] [CrossRef] [Green Version]
- Nygaard, K.R.; Maloney, S.E.; Dougherty, J.D. Erroneous inference based on a lack of preference within one group: Autism, mice, and the social approach task. Autism Res. 2019, 12, 1171–1183. [Google Scholar] [CrossRef]
- Markram, K.; Rinaldi, T.; La Mendola, D.; Sandi, C.; Markram, H. Abnormal Fear Conditioning and Amygdala Processing in an Animal Model of Autism. Neuropsychopharmacology 2007, 33, 901–912. [Google Scholar] [CrossRef]
- Fleming, S.M.; Ekhator, O.R.; Ghisays, V. Assessment of Sensorimotor Function in Mouse Models of Parkinson’s Disease. J. Vis. Exp. 2013, 17, e50303. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.-W.; Seung, H.; Kwon, K.J.; Ko, M.J.; Lee, E.J.; Oh, H.A.; Choi, C.S.; Kim, K.C.; Gonzales, E.L.; You, J.S.; et al. Subchronic Treatment of Donepezil Rescues Impaired Social, Hyperactive, and Stereotypic Behavior in Valproic Acid-Induced Animal Model of Autism. PLoS ONE 2014, 9, e104927. [Google Scholar] [CrossRef] [Green Version]
- Takuma, K.; Hara, Y.; Kataoka, S.; Kawanai, T.; Maeda, Y.; Watanabe, R.; Takano, E.; Hayata-Takano, A.; Hashimoto, H.; Ago, Y.; et al. Chronic treatment with valproic acid or sodium butyrate attenuates novel object recognition deficits and hippocampal dendritic spine loss in a mouse model of autism. Pharmacol. Biochem. Behav. 2014, 126, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Zou, M.; Liu, Y.; Xie, S.; Wang, L.; Li, D.; Li, L.; Wang, F.; Zhang, Y.; Xia, W.; Sun, C.; et al. Alterations of the endocannabinoid system and its therapeutic potential in autism spectrum disorder. Open Biol. 2021, 11, 200306. [Google Scholar] [CrossRef] [PubMed]
- Bromley-Brits, K.; Deng, Y.; Song, W. Morris Water Maze Test for Learning and Memory Deficits in Alzheimer’s Disease Model Mice. J. Vis. Exp. 2011, 53, e2920. [Google Scholar] [CrossRef] [PubMed]










| Parameter | Value |
|---|---|
| Wavelength | 830 nm |
| Operating mode | Continuous |
| Distance | 5 cm |
| Beam shape | Circular |
| Spot size | Diameter = 7.07 cm2 |
| Power density (mW/cm2) | 70 mW/cm2 |
| Exposure duration (min) | 10 min |
| Energy density (J/cm2) | 42 J/cm2 |
| Application technique | Without skin contact on the abdomen |
| Number and frequency of treatment sessions | 3 sessions, once for 2 days |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, U.-J.; Hong, N.; Ahn, J.-C. Photobiomodulation Attenuated Cognitive Dysfunction and Neuroinflammation in a Prenatal Valproic Acid-Induced Autism Spectrum Disorder Mouse Model. Int. J. Mol. Sci. 2022, 23, 16099. https://doi.org/10.3390/ijms232416099
Kim U-J, Hong N, Ahn J-C. Photobiomodulation Attenuated Cognitive Dysfunction and Neuroinflammation in a Prenatal Valproic Acid-Induced Autism Spectrum Disorder Mouse Model. International Journal of Molecular Sciences. 2022; 23(24):16099. https://doi.org/10.3390/ijms232416099
Chicago/Turabian StyleKim, Ui-Jin, Namgue Hong, and Jin-Chul Ahn. 2022. "Photobiomodulation Attenuated Cognitive Dysfunction and Neuroinflammation in a Prenatal Valproic Acid-Induced Autism Spectrum Disorder Mouse Model" International Journal of Molecular Sciences 23, no. 24: 16099. https://doi.org/10.3390/ijms232416099
APA StyleKim, U.-J., Hong, N., & Ahn, J.-C. (2022). Photobiomodulation Attenuated Cognitive Dysfunction and Neuroinflammation in a Prenatal Valproic Acid-Induced Autism Spectrum Disorder Mouse Model. International Journal of Molecular Sciences, 23(24), 16099. https://doi.org/10.3390/ijms232416099