Underestimated Properties of Nanosized Amorphous Titanium Dioxide
Abstract
:1. Introduction
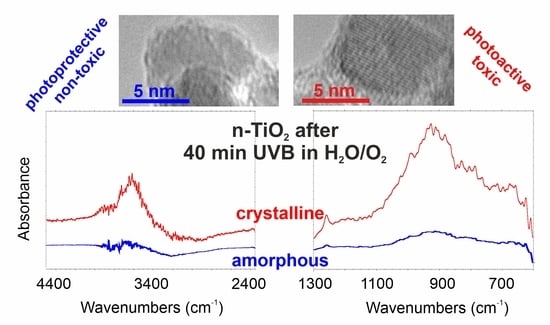
2. Results and Discussions
3. Materials and Methods
3.1. Synthesis of Nanostructural TiO2
3.2. The n-TiO2 Characterization
- High-resolution transmission electron microscopy (HRTEM): the images were taken using a transmission electron microscope F20X-TWIN (FEI-Tecnai) operated at 200 kV. The drop of sample solution was placed on a Cu grid coated with an ultrathin amorphous carbon film, and then dried under ambient condition.
- UV–vis absorption: the assay was carried out using Jasco 660 UV–vis spectrophotometer. The apparatus was also used for methylene blue (MB) photo-degradation study (co = 20 µmol/L, irradiation: 360 nm, 0.6 W·cm−2).
- The Fourier transform infrared (FTIR) measurements: spectra were accomplished by Bruker Vertex 70 infrared spectrophotometer using drift mode techniques in the frequency range 400–6000 cm−1.
- The Raman measurements: the nonpolarized spectra of carbon structures were investigated in the spectral range of 60−4500 cm−1. Raman spectra were recorded in the backscattering geometry using SENTERRA micro-Raman system. As an excitation light, we used the green laser operating at 532 nm. The laser beam was tightly focused on the sample surface through a 30× microscope objective. To prevent any damage of the sample, an excitation power was fixed at 20 mW. The resolution was 4 cm−1 and CCD temperature of 223 K, laser spot of about 10 μm, and total integration time of 100 s (50 × 2 s) were used. The position of the microscope objective with respect to the sample was piezoelectrically controlled (XY position).
- The bulk powder samples were characterized with XRD using a Philips XPERT Pro diffractometer with CuKα1 radiation.
- Nitrogen adsorption–desorption isotherms were measured using an ASAP 2010 volumetric adsorption analyzer from Micromeritics (Norcross, GA, USA) at liquid nitrogen temperature (77 K) in the relative pressure range from about 10–6 up to 0.999. Before the measurements, the samples were outgassed for at least 2 h at a temperature of 50 or 393 K. Low desorption temperature was used for amorphous n-TiO2 to avoid the crystalline phase formation.
3.3. In Vitro Cell Culture
3.4. Cytotoxicity Experiments
3.5. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Warheit, D.B.; Donner, E.M. Risk assessment strategies for nanoscale and fine-sized titanium dioxide particles: Recognizing hazard and exposure issues. Food Chem. Toxicol. 2015, 85, 138–147. [Google Scholar] [CrossRef] [PubMed]
- Noman, M.T.; Ashraf, M.A.; Ali, A. Synthesis and applications of nano-TiO2: A review. Environ. Sci. Pollut. Res. Int. 2019, 26, 3262–3291. [Google Scholar] [CrossRef]
- Krylova, G.; Na, C. Photoinduced Crystallization and Activation of Amorphous Titanium Dioxide. J. Phys. Chem. C 2015, 119, 12400–12407. [Google Scholar] [CrossRef]
- Guardia, L.; Villar-Rodil, S.; Paredes, J.I.; Rozada, R.; Martinez-Alonso, A.; Tascon, J.M.D. UV light exposure of aqueous graphene oxide suspensions to promote their direct reduction, formation of graphene–metal nanoparticle hybrids and dye degradation. Carbon 2012, 50, 1014–1024. [Google Scholar] [CrossRef]
- Shi, H.; Magaye, R.; Castranova, V.; Zhao, J. Titanium dioxide nanoparticles: A review of current toxicological data. Part. Fibre Toxicol. 2013, 10, 15. [Google Scholar] [CrossRef] [Green Version]
- Song, B.; Liu, J.; Feng, X.; Wei, L.; Shao, L. A review on potential neurotoxicity of titanium dioxide nanoparticles. Nanoscale Res. Lett. 2015, 10, 342. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Li, W.; Yang, Z. Toxicology of nanosized titanium dioxide: An update. Arch. Toxicol. 2015, 89, 2207–2217. [Google Scholar] [CrossRef]
- Ferraris, C.; Rimicci, C.; Garelli, S.; Ugazio, E.; Battaglia, L. Nanosystems in Cosmetic Products: A Brief Overview of Functional, Market, Regulatory and Safety Concerns. Pharmaceutics 2021, 13, 1408. [Google Scholar] [CrossRef]
- Marquez-Ramirez, S.G.; Delgado-Buenrostro, N.L.; Chirino, Y.I.; Gutiérrez Iglesias, G.; López-Marure, R. Titanium dioxide nanoparticles inhibit proliferation and induce morphological changes and apoptosis in glial cells. Toxicology 2012, 302, 146–156. [Google Scholar] [CrossRef]
- Park, E.J.; Yi, J.; Chung, K.H.; Ryu, D.Y.; Choi, J.; Park, K. Oxidative stress and apoptosis induced by titanium dioxide nanoparticles in cultured BEAS-2B cells. Toxicol. Lett. 2008, 180, 222–229. [Google Scholar] [CrossRef]
- Ursini, C.L.; Cavallo, D.; Fresegna, A.M.; Ciervo, A.; Maiello, R.; Tassone, P.; Buresti, G.; Casciardi, S.; Iavicoli, S. Evaluation of cytotoxic, genotoxic and inflammatory response in human alveolar and bronchial epithelial cells exposed to titanium dioxide nanoparticles. J. Appl. Toxicol. 2014, 34, 1209–1219. [Google Scholar] [CrossRef] [PubMed]
- Setyawati, M.I.; Tay, C.Y.; Leong, D. Mechanistic Investigation of the Biological Effects of SiO2, TiO2, and ZnO Nanoparticles on Intestinal Cells. Small 2015, 11, 3458–3468. [Google Scholar] [CrossRef] [PubMed]
- Niska, K.; Pyszka, K.; Tukaj, C.; Wozniak, M.; Radomski, M.W.; Inkielewicz-Stepniak, I. Titanium dioxide nanoparticles enhance production of superoxide anion and alter the antioxidant system in human osteoblast cells. Int. J. Nanomed. 2015, 10, 1095–1107. [Google Scholar]
- Khataee, A.; Mansoori, G.A. Nanostructured Titanium Dioxide Materials: Properties, Preparation and Applications; World Scientific Publishing: Singapore, 2011. [Google Scholar]
- Singh, P.; Nanda, A. Enhanced sun protection of nano-sized metal oxide particles over conventional metal oxide particles: An in vitro comparative study. Int. J. Cosmet. Sci. 2014, 36, 273–283. [Google Scholar] [CrossRef]
- Jiang, J.; Oberdörster, G.; Elder, A.; Gelei, R.; Mercer, P.; Biswas, P. Does Nanoparticle Activity Depend upon Size and Crystal Phase? Nanotoxicology 2008, 2, 33–42. [Google Scholar] [CrossRef] [Green Version]
- Regulation (EC) No 1223/2009 of the European Parliament and of the Council of 30 November 2009 on Cosmetic Products (Text with EEA Relevance). Available online: www.eumonitor.eu/9353000/1/j9vvik7m1c3gyxp/vibn2mp7slr0 (accessed on 1 January 2022).
- Li, Y.; Yang, D.; Lu, S.; Qiu, X.; Qian, Y.; Li, P.W. Encapsulating TiO2 in Lignin-Based Colloidal Spheres for High Sunscreen Performance and Weak Photocatalytic Activity. ACS Sustain. Chem. Eng. 2019, 7, 6234–6242. [Google Scholar] [CrossRef]
- Smijs, T.G.; Pavel, S. Titanium dioxide and zinc oxide nanoparticles in sunscreens: Focus on their safety and effectiveness. Nanotechnol. Sci. Appl. 2011, 4, 95–112. [Google Scholar] [CrossRef] [Green Version]
- Mudiyanselage, K.; Idriss, H. Characterization of peroxo species on TiOx/Rh(111) single crystal. Surf. Sci. 2019, 680, 61–67. [Google Scholar] [CrossRef]
- Ohno, T.; Masaki, Y.; Hirayama, S.; Matsumura, M. TiO2-Photocatalyzed epoxidation of 1-decene by H2O2 under visible light. J. Catal. 2001, 204, 163–168. [Google Scholar] [CrossRef]
- Munuera, G.; Gonzalez-Elipe, A.R.; Fernandez, A.; Malet, P.; Espinos, J.P. Spectroscopic characterisation and photochemical behaviour of a titanium hydroxyperoxo compound. J. Chem. Soc. Farad. Trans. 1989, 85, 1279–1290. [Google Scholar] [CrossRef]
- Nakamura, R.; Imanishi, A.; Murakoshi, K.; Nakato, Y. In Situ FTIR Studies of Primary Intermediates of Photocatalytic Reactions on Nanocrystalline TiO2 Films in Contact with Aqueous Solutions. J. Am. Chem. Soc. 2003, 125, 7443–7450. [Google Scholar] [CrossRef]
- Abdel-Latif, H.M.R.; Dawood, M.A.O.; Menanteau-Ledouble, S.; El-Matbouli, M. Environmental transformation of n-TiO2 in the aquatic systems and their ecotoxicity in bivalve mollusks: A systematic review. Ecotoxicol. Env. Saf. 2020, 200, 110776. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Mao, S.S. Titanium Dioxide Nanomaterials: Synthesis, Properties, Modifications, and Applications. Chem. Rev. 2007, 107, 2891–2959. [Google Scholar] [CrossRef] [PubMed]
- Bolibok, P.; Roszek, K.; Wiśniewski, M. Graphene Oxide-Mediated Protection from Photodamage. J. Phys. Chem. Lett. 2018, 9, 3241–3244. [Google Scholar] [CrossRef] [PubMed]






| Sample | Surface Area [m2/g] |
|---|---|
| A-n-TiO2 | 8 |
| SC-n-TiO2 | 248 |
| C-n-TiO2 | 109 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wiśniewski, M.; Roszek, K. Underestimated Properties of Nanosized Amorphous Titanium Dioxide. Int. J. Mol. Sci. 2022, 23, 2460. https://doi.org/10.3390/ijms23052460
Wiśniewski M, Roszek K. Underestimated Properties of Nanosized Amorphous Titanium Dioxide. International Journal of Molecular Sciences. 2022; 23(5):2460. https://doi.org/10.3390/ijms23052460
Chicago/Turabian StyleWiśniewski, Marek, and Katarzyna Roszek. 2022. "Underestimated Properties of Nanosized Amorphous Titanium Dioxide" International Journal of Molecular Sciences 23, no. 5: 2460. https://doi.org/10.3390/ijms23052460
APA StyleWiśniewski, M., & Roszek, K. (2022). Underestimated Properties of Nanosized Amorphous Titanium Dioxide. International Journal of Molecular Sciences, 23(5), 2460. https://doi.org/10.3390/ijms23052460