CPR Gene Contributes to Integument Function and Ovary Development in a Rice Planthopper
Abstract
:1. Introduction
2. Results
2.1. Development Stage and Tissue Expression Specificities of the CPR Gene
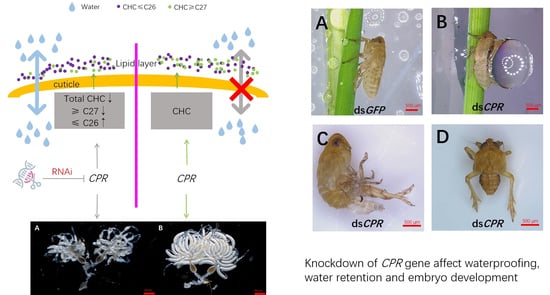
2.2. RNAi Result
2.3. Effect of CPR Knockdown on CHC Levels
3. Discussion
4. Materials and Methods
4.1. Insects
4.2. Cloning of a N. lugens CPR cDNA
4.3. Quantitative Real-Time qPCR (qRT-PCR) Analysis
4.4. RNAi
4.5. Measurement of Body Weight in dsRNA-Injected Insects
4.6. Oviposition Experiment
4.7. Waterproofing and Water Retention Assay
4.8. Scanning Electron Microscope (SEM) Analysis
4.9. Extraction and Quantification of CHCs
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Louërat-Oriou, B.; Perret, A.; Pompon, D. Differential redox and electron-transfer properties of purified yeast, plant and human NADPH-cytochrome P-450 reductases highly modulate cytochrome P-450 activities. Eur. J. Biochem. 1998, 258, 1040–1049. [Google Scholar] [CrossRef] [PubMed]
- Paine, M.J.I.; Scrutton, N.S.; Munro, A.W.; Gutierrez, A.; Roberts, G.C.K.; Wolf, C.R. Electron Transfer Partners of Cytochrome P450. In Cytochrome P450; Ortiz de Montellano, P.R., Ed.; Springer: Boston, MA, USA, 2005. [Google Scholar]
- Vermilion, J.L.; Coon, M.J. Highly purified detergent-solubilized NADPH-cytochrome P-450 reductase from phenobarbital-induced rat liver microsomes. Biochem. Biophys. Res. Commun. 1974, 60, 1315–1322. [Google Scholar] [CrossRef] [Green Version]
- Porter, T.D.; Wilson, T.E.; Kasper, C.B. Expression of a functional 78,000 dalton mammalian flavoprotein, NADPH-cytochrome P-450 oxidoreductase, in Escherichia coli. Arch. Biochem. Biophys. 1987, 254, 353–367. [Google Scholar] [CrossRef]
- Ming, W.; Roberts, D.L. Three-dimensional structure of NADPH-cytochrome P450 reductase: Prototype for FMN- and FAD-containing enzymes. Proc. Natl. Acad. Sci. USA 1997, 94, 8411. [Google Scholar] [CrossRef] [Green Version]
- Hovemann, B.T.; Sehlmeyer, F.; Malz, J. Drosophila melanogaster NADPH–cytochrome P450 oxidoreductase: Pronounced expression in antennae may be related toodorantclearance. Gene 1997, 189, 213–219. [Google Scholar] [CrossRef]
- Qiu, Y.; Tittiger, C.; Wicker-Thomas, C.; Le Goff, G.; Young, S.; Wajnberg, E.; Fricaux, T.; Taquet, N.; Blomquist, G.J.; Feyereisen, R. An insect-specific P450 oxidative decarbonylase for cuticular hydrocarbon biosynthesis. Proc. Natl. Acad. Sci. USA 2012, 109, 14858–14863. [Google Scholar] [CrossRef] [Green Version]
- Liu, D.; Zhou, X.; Li, M.; Zhu, S.; Qiu, X. Characterization of NADPH-cytochrome P450 reductase gene from the cotton bollworm, Helicoverpa armigera. Gene 2014, 545, 262–270. [Google Scholar] [CrossRef]
- Liu, S.; Liang, Q.M.; Zhou, W.W.; Jiang, Y.D.; Zhu, Q.Z.; Yu, H.; Zhang, C.X.; Gurr, G.M.; Zhu, Z.R. RNA interference of NADPH-cytochrome P450 reductase of the rice brown planthopper, Nilaparvata lugens, increases susceptibility to insecticides. Pest Manag. Sci. 2015, 71, 32–39. [Google Scholar] [CrossRef]
- Wang, K.; Peng, X.; Zuo, Y.; Li, Y.; Chen, M. Molecular Cloning, Expression Pattern and Polymorphisms of NADPH-Cytochrome P450 Reductase in the Bird Cherry-Oat Aphid Rhopalosiphum padi (L.). PLoS ONE 2016, 11, e0154633. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Wang, Y.; Wang, L.; Yao, J.; Guo, H.; Fang, J. Knockdown of NADPH-cytochrome P450 reductase results in reduced resistance to buprofezin in the small brown planthopper, Laodelphax striatellus (fallen). Pestic. Biochem. Physiol. 2016, 127, 21–27. [Google Scholar] [CrossRef]
- Zhang, Y.-X.; Li, S.-G.; Rao, X.-J.; Liu, S. Molecular characterization of a NADPH–cytochrome P450 reductase gene from the rice leaffolder, Cnaphalocrocis medinalis (Lepidoptera: Pyralidae). Appl. Entomol. Zool. 2017, 53, 19–27. [Google Scholar] [CrossRef]
- Ji, H.Y.; Staehelin, C.; Jiang, Y.P.; Liu, S.W.; Ma, Z.H.; Su, Y.J.; Zhang, J.E.; Wang, R.L. Tobacco Cutworm (Spodoptera Litura) Larvae Silenced in the NADPH-Cytochrome P450 Reductase Gene Show Increased Susceptibility to Phoxim. Int. J. Mol. Sci. 2019, 20, 14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, C.; Liang, J.J.; Liu, S.N.; Zeng, Y.; Wang, S.L.; Wu, Q.J.; Xie, W.; Zhang, Y.J. Molecular characterization of an NADPH cytochrome P450 reductase from Bemisia tabaci Q: Potential involvement in susceptibility to imidacloprid. Pestic. Biochem. Physiol. 2020, 162, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Qiao, J.-W.; Fan, Y.-L.; Wu, B.-J.; Bai, T.-T.; Wang, Y.-H.; Zhang, Z.-F.; Wang, D.; Liu, T.-X. Downregulation of NADPH-cytochrome P450 reductase via RNA interference increases the susceptibility of Acyrthosiphon pisum to desiccation and insecticides. Insect Sci. 2021. [Google Scholar] [CrossRef]
- Yuan, C.Y.; Jing, T.X.; Li, W.; Liu, X.Q.; Liu, T.Y.; Liu, Y.; Chen, M.L.; Jiang, R.X.; Yuan, G.R.; Dou, W.; et al. NADPH-cytochrome P450 reductase mediates the susceptibility of Asian citrus psyllid Diaphorina citri to imidacloprid and thiamethoxam. Pest Manag. Sci. 2021, 77, 677–685. [Google Scholar] [CrossRef]
- Ginzel, M.D.; Blomquist, G.J. Insect Hydrocarbons: Biochemistry and Chemical Ecology. In Extracellular Composite Matrices in Arthropods; Cohen, E., Moussian, B., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 221–252. [Google Scholar]
- Gibbs, A.G. Thermodynamics of cuticular transpiration. J. Insect Physiol. 2011, 57, 1066–1069. [Google Scholar] [CrossRef]
- Quinlan, M.C.; Gibbs, A.G. Discontinuous gas exchange in insects. Respir. Physiol. Neurobiol. 2006, 154, 18–29. [Google Scholar] [CrossRef]
- Li, D.-T.; Chen, X.; Wang, X.-Q.; Moussian, B.; Zhang, C.-X. The fatty acid elongase gene family in the brown planthopper, Nilaparvata lugens. Insect Biochem. Mol. Biol. 2019, 108, 32–43. [Google Scholar] [CrossRef]
- Li, D.-T.; Chen, X.; Wang, X.-Q.; Zhang, C.-X. FAR gene enables the brown planthopper to walk and jump on water in paddy field. Sci. China Life Sci. 2019, 62, 1521–1531. [Google Scholar] [CrossRef]
- Chung, H.; Carroll, S.B. Wax, sex and the origin of species: Dual roles of insect cuticular hydrocarbons in adaptation and mating. BioEssays 2015, 37, 822–830. [Google Scholar] [CrossRef]
- Gibbs, A.G. Water-proofing properties of cuticular lipids. Am. Zool. 1998, 38, 471–482. [Google Scholar] [CrossRef]
- Xu, H.J.; Xue, J.; Lu, B.; Zhang, X.C.; Zhuo, J.C.; He, S.F.; Ma, X.F.; Jiang, Y.Q.; Fan, H.W.; Xu, J.Y.; et al. Two insulin receptors determine alternative wing morphs in planthoppers. Nature 2015, 519, 464–467. [Google Scholar] [CrossRef] [PubMed]
- Xue, J.; Zhou, X.; Zhang, C.X.; Yu, L.L.; Fan, H.W.; Wang, Z.; Xu, H.J.; Xi, Y.; Zhu, Z.R.; Zhou, W.W.; et al. Genomes of the rice pest brown planthopper and its endosymbionts reveal complex complementary contributions for host adaptation. Genome Biol. 2014, 15, 521. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, S.Y.; Price, J.H.; Zhang, D. Hydrocarbons catalysed by TmCYP4G122 and TmCYP4G123 in Tenebrio molitor modulate the olfactory response of the parasitoid Scleroderma guani. Insect Mol. Biol. 2019, 28, 637–648. [Google Scholar] [CrossRef]
- Wang, W.; Yang, R.R.; Peng, L.Y.; Zhang, L.; Yao, Y.L.; Bao, Y.Y. Proteolytic activity of the proteasome is required for female insect reproduction. Open Biol. 2021, 11, 200251. [Google Scholar] [CrossRef] [PubMed]
- Xue, J.A.; Bao, Y.Y.; Li, B.L.; Cheng, Y.B.; Peng, Z.Y.; Liu, H.; Xu, H.J.; Zhu, Z.R.; Lou, Y.G.; Cheng, J.A.; et al. Transcriptome Analysis of the Brown Planthopper Nilaparvata lugens. PLoS ONE 2010, 5, 11. [Google Scholar] [CrossRef] [Green Version]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2− ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Huang, H.J.; Liu, C.W.; Xu, H.J.; Bao, Y.Y.; Zhang, C.X. Mucin-like protein, a saliva component involved in brown planthopper virulence and host adaptation. J. Insect Physiol. 2017, 98, 223–230. [Google Scholar] [CrossRef]
- Zhou, X.; Peng, L.Y.; Wang, Z.C.; Wang, W.; Zhu, Z.; Huang, X.H.; Chen, L.B.; Song, Q.S.; Bao, Y.Y. Identification of novel antimicrobial peptides from rice planthopper, Nilaparvata lugens. Insect Biochem. Mol. Biol. 2019, 113, 103215. [Google Scholar] [CrossRef]
- Peng, L.Y.; Dai, Z.W.; Yang, R.R.; Zhu, Z.; Wang, W.; Zhou, X.; Bao, Y.Y. NADPH Oxidase 5 Is Essential for Molting and Oviposition in a Rice Planthopper Nilaparvata lugens. Insects 2020, 11, 642. [Google Scholar] [CrossRef]
- Li, D.T.; Pei, X.J.; Ye, Y.X.; Wang, X.Q.; Wang, Z.C.; Chen, N.; Liu, T.X.; Fan, Y.L.; Zhang, C.X. Cuticular Hydrocarbon Plasticity in Three Rice Planthopper Species. Int. J. Mol. Sci. 2021, 22, 7733. [Google Scholar] [CrossRef] [PubMed]






| CHC | dsGFP | dsCPR | Content Change Rate | dsGFP Percentage of the Total | dsCPR Percentage of the Total | Proportion Change Rate |
|---|---|---|---|---|---|---|
| C15 | 0.005789 ± 0.005148 | 0.003344 ± 0.001431 | −42.25% | 0.74% | 0.57% | −23.65% |
| C16 | 0.01252 ± 0.002376 | 0.017922 ± 0.006255 | 43.14% | 1.60% | 3.04% | 89.24% |
| C17 | 0.018235 ± 0.004875 | 0.016934 ± 0.002075 | −7.14% | 2.34% | 2.87% | 22.77% |
| C18 | 0.093074 ± 0.006788 | 0.103419 ± 0.006303 | 11.11% | 11.93% | 17.52% | 46.90% |
| C19 | 0.011544 ± 0.001355 | 0.013049 ± 0.000938 | 13.04% | 1.48% | 2.21% | 49.44% |
| C20 | 0.089822 ± 0.003363 | 0.101096 ± 0.00619 | 12.55% | 11.51% | 17.13% | 48.80% |
| C22 | 0.06181 ± 0.002839 | 0.064111 ± 0.004132 | 3.72% | 7.92% | 10.86% | 37.13% |
| C23 | 0.041042 ± 0.00212 | 0.042432 ± 0.004224 | 3.39% | 5.26% | 7.19% | 36.68% |
| C24 | 0.036321 ± 0.002465 | 0.036459 ± 0.004968 | 0.38% | 4.65% | 6.18% | 32.71% |
| C25 | 0.026527 ± 0.004492 | 0.024414 ± 0.002927 | −7.96% | 3.40% | 4.14% | 21.68% |
| C26 | 0.02426 ± 0.004057 | 0.022278 ± 0.002021 | −8.17% | 3.11% | 3.77% | 21.40% |
| C27 | 0.057761 ± 0.010241 | 0.033636 ± 0.003238 | −41.77% | 7.40% | 5.70% | −23.01% |
| C28 | 0.019489 ± 0.002171 | 0.013462 ± 0.001666 | −30.93% | 2.50% | 2.28% | −8.68% |
| C29 | 0.254038 ± 0.030532 | 0.080512 ± 0.011656 | −68.31% | 32.55% | 13.64% | −58.10% |
| C30 | 0.008287 ± 0.001302 | 0.005653 ± 0.000758 | −31.78% | 1.06% | 0.96% | −9.81% |
| C31 | 0.014764 ± 0.002829 | 0.007224 ± 0.000978 | −51.07% | 1.89% | 1.22% | −35.31% |
| C32 | 0.002795 ± 0.000268 | 0.002523 ± 0.0004 | −9.73% | 0.36% | 0.43% | 19.34% |
| C33 | 0.002263 ± 0.000237 | 0.001783 ± 0.000497 | −21.20% | 0.29% | 0.30% | 4.17% |
| Total | 0.780341 ± 0.052279 | 0.590251 ± 0.032017 | −24.36% | 100.00% | 100.00% | 0.00% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.-C.; Tao, S.; Cheng, X.; Li, D.-T.; Zhang, C.-X.; Bao, Y.-Y. CPR Gene Contributes to Integument Function and Ovary Development in a Rice Planthopper. Int. J. Mol. Sci. 2022, 23, 2875. https://doi.org/10.3390/ijms23052875
Wang Z-C, Tao S, Cheng X, Li D-T, Zhang C-X, Bao Y-Y. CPR Gene Contributes to Integument Function and Ovary Development in a Rice Planthopper. International Journal of Molecular Sciences. 2022; 23(5):2875. https://doi.org/10.3390/ijms23052875
Chicago/Turabian StyleWang, Zhe-Chao, Shuai Tao, Xu Cheng, Dan-Ting Li, Chuan-Xi Zhang, and Yan-Yuan Bao. 2022. "CPR Gene Contributes to Integument Function and Ovary Development in a Rice Planthopper" International Journal of Molecular Sciences 23, no. 5: 2875. https://doi.org/10.3390/ijms23052875
APA StyleWang, Z.-C., Tao, S., Cheng, X., Li, D.-T., Zhang, C.-X., & Bao, Y.-Y. (2022). CPR Gene Contributes to Integument Function and Ovary Development in a Rice Planthopper. International Journal of Molecular Sciences, 23(5), 2875. https://doi.org/10.3390/ijms23052875