Active nNOS Is Required for Grp94-Induced Antioxidant Cytoprotection: A Lesson from Myogenic to Cancer Cells
Abstract
:1. Introduction
2. Results
2.1. Effects of Grp94/gp96 Overexpression on Ca2+ Homeostasis and Antioxidant Protection in Myogenic and Epithelial Cell Lines
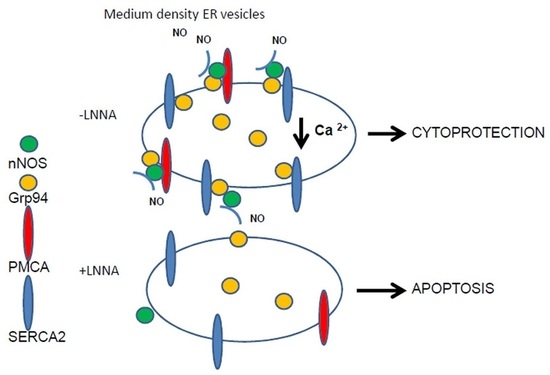
2.2. Grp94 Interacts with Ca2+-Handling Proteins and nNOS
2.3. Role of Grp94 Interactors in Antioxidant Cytoprotection
2.4. Grp94 Protein-Interaction and Cytoprotection in Breast Cancer Cell Lines
3. Discussion
4. Material and Methods
4.1. Constructs
4.2. Cell Culture and Transfection
4.3. Western Blotting and Immunoprecipitation
4.4. Aequorin Ca2+ Measurements
4.5. Cytoprotection Experiments and TUNEL Assays
4.6. Subcellular Fractionation
4.7. Statistical Analyses
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Hong, F.; Mohammad Rachidi, S.; Lundgren, D.; Han, D.; Huang, X.; Zhao, H.; Kimura, Y.; Hirano, H.; Ohara, O.; Udono, H.; et al. Mapping the Interactome of a Major Mammalian Endoplasmic Reticulum Heat Shock Protein 90. PLoS ONE 2017, 12, e0169260. [Google Scholar] [CrossRef] [PubMed]
- Wanderling, S.; Simen, B.B.; Ostrovsky, O.; Ahmed, N.T.; Vogen, S.M.; Gidalevitz, T.; Argon, Y. GRP94 is essential for mesoderm induction and muscle development because it regulates insulin-like growth factor secretion. Mol. Biol. Cell 2007, 18, 3764–3775. [Google Scholar] [CrossRef] [PubMed]
- Mao, C.; Wang, M.; Luo, B.; Wey, S.; Dong, D.; Wesselschmidt, R.; Rawlings, S.; Lee, A.S. Targeted mutation of the mouse Grp94 gene disrupts development and perturbs endoplasmic reticulum stress signaling. PLoS ONE 2010, 5, e10852. [Google Scholar] [CrossRef] [Green Version]
- Liu, B.; Li, Z. Endoplasmic reticulum HSP90b1 (gp96, grp94) optimizes B-cell function via chaperoning integrin and TLR but not immunoglobulin. Blood 2008, 112, 1223–1230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gorza, L.; Vitadello, M. Reduced amount of the glucose-regulated protein GRP94 in skeletal myoblasts results in loss of fusion competence. FASEB J. 2000, 14, 461–475. [Google Scholar] [CrossRef] [Green Version]
- Barton, E.R.; Park, S.; James, J.K.; Makarewich, C.A.; Philippou, A.; Eletto, D.; Lei, H.; Brisson, B.; Ostrovsky, O.; Li, Z.; et al. Deletion of muscle GRP94 impairs both muscle and body growth by inhibiting local IGF production. FASEB J. 2012, 26, 3691–3702. [Google Scholar] [CrossRef] [Green Version]
- Vitadello, M.; Gherardini, J.; Gorza, L. The stress protein/chaperone Grp94 counteracts muscle disuse atrophy by stabilizing subsarcolemmal neuronal nitric oxide synthase. Antioxid. Redox Signal. 2014, 20, 2479–2496. [Google Scholar] [CrossRef] [Green Version]
- Bando, Y.; Katayama, T.; Aleshin, A.N.; Manabe, T.; Tohyama, M. GRP94 reduces cell death in SH-SY5Y cells perturbated calcium homeostasis. Apoptosis 2004, 9, 501–508. [Google Scholar] [CrossRef]
- Vitadello, M.; Penzo, D.; Petronilli, V.; Michieli, G.; Gomirato, S.; Menabò, R.; Di Lisa, F.; Gorza, L. Overexpression of the stress protein Grp94 reduces cardiomyocyte necrosis due to calcium overload and simulated ischemia. FASEB J. 2003, 17, 923–925. [Google Scholar] [CrossRef] [Green Version]
- Pizzo, P.; Scapin, C.; Vitadello, M.; Florean, C.; Gorza, L. Grp94 acts as a mediator of curcumin induced antioxidant defence in myogenic cells. J. Cell. Mol. Med. 2010, 14, 970–981. [Google Scholar] [CrossRef] [Green Version]
- Lee, A.S. The glucose-regulated proteins: Stress induction and clinical applications. Trends Biochem. Sci. 2001, 26, 504–510. [Google Scholar] [CrossRef]
- Randow, F.; Seed, B. Endoplasmic reticulum chaperone gp96 is required for innate immunity but not cell viability. Nat. Cell Biol. 2001, 3, 891–896. [Google Scholar] [CrossRef]
- Dejeans, N.; Glorieux, C.; Guenin, S.; Beck, R.; Sid, B.; Rousseau, R.; Bisig, B.; Delvenne, P.; Buc Calderon, P.; Verrax, J. Overexpression of GRP94 in breast cancer cells resistant to oxidative stress promotes high levels of cancer cell proliferation and migration: Implications for tumor recurrence. Free Radic. Biol. Med. 2012, 52, 993–1002. [Google Scholar] [CrossRef] [PubMed]
- Duan, X.; Iwanowycz, S.; Ngoi, S.; Hill, M.; Zhao, Q.; Liu, B. Molecular Chaperone GRP94/GP96 in Cancers: Oncogenesis and Therapeutic Target. Front. Oncol. 2021, 11, 629846. [Google Scholar] [CrossRef]
- Patel, P.D.; Yan, P.; Seidler, P.M.; Patel, H.J.; Sun, W.; Yang, C.; Que, N.S.; Taldone, T.; Finotti, P.; Stephani, R.A.; et al. Paralog-selective Hsp90 inhibitors define tumor-specific regulation of Her2. Nat. Chem. Biol. 2013, 9, 677–684. [Google Scholar] [CrossRef] [PubMed]
- Yaffe, D.; Saxel, O. Serial passaging and differentiation of myogenic cells isolated from dystrophic mouse muscle. Nature 1977, 270, 725–727. [Google Scholar] [CrossRef]
- Marzec, M.; Eletto, D.; Argon, Y. GRP94: An HSP90-like protein specialized for protein folding and quality control in the endoplasmic reticulum. Biochim. Biophys. Acta 2012, 1823, 774–787. [Google Scholar] [CrossRef] [Green Version]
- Strehler, E.E. Plasma membrane calcium ATPases: From generic Ca(2+) sump pumps to versatile systems for fine-tuning cellular Ca(2.). Biochem. Biophys. Res. Common. 2015, 460, 26–33. [Google Scholar] [CrossRef]
- Dremina, E.S.; Sharov, V.S.; Schöneich, C. Heat-shock proteins attenuate SERCA inactivation by the anti-apoptotic protein Bcl-2: Possible implications for the ER Ca2+-mediated apoptosis. Biochem. J. 2012, 444, 127–139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vogen, S.; Gidalevitz, T.; Biswas, C.; Simen, B.B.; Stein, E.; Gulmen, F.; Argon, Y. Radicicol-sensitive Peptide Binding to the N-terminal Portion of GRP94. J. Biol. Chem. 2002, 277, 40742–40750. [Google Scholar] [CrossRef] [Green Version]
- Peng, H.M.; Morishima, Y.; Clapp, K.M.; Lau, M.; Pratt, W.B.; Osawa, Y. Dynamic Cycling with Hsp90 Stabilizes Neuronal Nitric Oxide Synthase Through Calmodulin-dependent Inhibition of Ubiquitination. Biochemistry 2009, 48, 8483–8490. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Camacho, P. Ca2-dependent redox modulation of SERCA 2b by ERp57. J. Cell Biol. 2004, 164, 35–46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vandecaetsbeek, I.; Vangheluwe, P.; Raeymaekers, L.; Wuytack, F.; Vanoevelen, J. The Ca2+ Pumps of the Endoplasmic Reticulum and Golgi Apparatus. Cold Spring Harb. Perspect. Biol. 2011, 3, a004184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, S.; Hong, F.; Gewirth, D.; Guo, B.; Liu, B.; Li, Z. The molecular chaperone gp96/Grp94 interacts with toll-like receptors and integrins via its c-terminal hydrophobic domain. J. Biol. Chem. 2012, 287, 6735–6742. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Missiaen, L.; Van Acker, K.; Van Baelen, K.; Raeymaekers, L.; Wuytack, F.; Parys, J.B.; De Smedt, H.; Vanoevelen, J.; Dodea, L.; Rizzuto, R.; et al. Calcium release from the Golgi apparatus and the endoplasmic reticulum in HeLa cells stably expressing targeted aequorin to these compartments. Cell Calcium 2004, 36, 479–487. [Google Scholar] [CrossRef] [PubMed]
- Padányi, R.; Pászty, K.; Heged, H.; Varga, K.; Papp, B.; Penniston, J.T.; Enyedim, A. Multifaceted plasma membrane Ca2+ pumps: From structure to intracellular Ca2+ handling and cancer. Biochim. Biophys. Acta 2016, 1863, 1351–1363. [Google Scholar] [CrossRef] [Green Version]
- Fiscus, R.R.; Yuen, J.P.; Chan, S.L.; Kwong, J.H.; Chew, S.B. Nitric oxide and cyclic GMP as pro- and anti-apoptotic agents. J. Card. Surg. 2002, 17, 336–339. [Google Scholar] [CrossRef] [PubMed]
- Adachi, T.; Weisbrod, R.M.; Pimentel, D.R.; Ying, Y.; Sharov, V.S.; Schöneich, C.; Cohen, R.A. S-Glutathiolation by peroxynitrite activates SERCA during arterial relaxation by nitric oxide. Nat. Med. 2004, 10, 1200–1207. [Google Scholar] [CrossRef]
- Vangheluwe, P.; Raeymaekers, L.; Dode, L.; Wuytack, F. Modulating sarco(endo)plasmic reticulum Ca2+ ATPase 2 (SERCA2) activity: Cell biological implications. Cell Calcium 2005, 38, 291–302. [Google Scholar] [CrossRef]
- Ansa-Addo, E.A.; Thaxton, J.; Hong, F.; Wu, B.X.; Zhang, Y.; Fugle, C.W.; Metelli, A.; Riesenberg, B.; Williams, K.; Gewirth, D.T.; et al. Clients and Oncogenic Roles of Molecular Chaperone gp96/grp94. Curr. Top. Med. Chem. 2016, 16, 2765–2778. [Google Scholar] [CrossRef] [Green Version]
- Brini, M.; Calì, T.; Ottolini, D.; Carafoli, E. The plasma membrane calcium pump in health and disease. FEBS J. 2013, 280, 5385–5397. [Google Scholar] [CrossRef] [PubMed]
- Monteith, G.; Davis, F.M.; Roberts-Thomson, S.J. Calcium Channels and Pumps in Cancer: Changes and Consequences. J. Biol. Chem. 2012, 287, 31666–31673. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muntanè, J.; Mata, D.L.M. Nitric oxide and cancer. World J. Hepatol. 2010, 2, 337–344. [Google Scholar] [CrossRef]
- Cheng, R.Y.S.; Basudhar, D.; Ridnour, L.A.; Heinecke, J.L.; Kesarwala, A.H.; Glynn, S.; Switzer, C.H.; Ambs, S.; Miranda, K.M.; Wink, D.A. Gene expression profiles of NO- and HNO-donor treated breast cancer cells: Insights into tumor response and resistance pathways. Nitric Oxide 2014, 43, 17–28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, L.H.; Boivin, G.P.; Prasad, V.; Periasamy, M.; Shull, G.E. Squamous cell tumors in mice heterozygous for the null allele of Atp2a2, encoding the sarco(endo)plasmic reticulum Ca2+- ATPase isoform 2 Ca2+ pump. J. Biol. Chem. 2001, 276, 26737–26740. [Google Scholar] [CrossRef] [Green Version]
- Baggott, R.R.; Mohamed, T.M.A.; Oceandy, D.; Holton, M.; Blanc, M.C.; Roux-Soro, S.C.; Brown, S.; Brown, J.E.; Cartwright, E.J.; Wang, W.; et al. Disruption of the interaction between PMCA2 and calcineurin triggers apoptosis and enhances paclitaxel-induced cytotoxicity in breast cancer cells. Carcinogenesis 2012, 33, 2362–2368. [Google Scholar] [CrossRef] [Green Version]
- Jeong, J.; VanHouten, J.N.; Dann, P.; Kim, W.; Sullivan, C.; Yu, H.; Liotta, L.; Espina, V.; Stern, D.F.; Friedman, P.A.; et al. PMCA2 regulates HER2 protein kinase localization and signaling and promotes HER2-mediated breast cancer. Proc. Natl. Acad. Sci. USA 2016, 113, E282–E290. [Google Scholar] [CrossRef] [Green Version]
- Curry, C.M.; Luk, N.A.; Kenny, P.A.; Roberts-Thomson, S.J.; Monteith, G.R. Distinct Regulation of Cytoplasmic Calcium Signals and Cell Death Pathways by Different Plasma Membrane Calcium ATPase Isoforms in MDA-MB-231 Breast Cancer Cells. J. Biol. Chem. 2012, 287, 28598–28608. [Google Scholar] [CrossRef] [Green Version]
- Karihtala, P.; Kinnula, V.L.; Soini, Y. Antioxidative response for nitric oxide production in breast carcinoma. Oncol. Rep. 2004, 12, 755–759. [Google Scholar] [CrossRef]
- Yang, Z.; Misner, B.; Ji, H.; Poulos, T.L.; Silverman, R.B.; Meyskens, F.L.; Yang, S. Targeting nitric oxide signaling with nNOS inhibitors as a novel strategy for the therapy and prevention of human melanoma. Antioxid. Redox Signal. 2013, 19, 433–447. [Google Scholar] [CrossRef] [Green Version]
- Leung, E.L.; Fraser, M.; Fiscus, R.R.; Tsang, B.K. Cisplatin alters nitric oxide synthase levels in human ovarian cancer cells: Involvement in p53 regulation and cisplatin resistance. Br. J. Cancer 2008, 98, 1803–1809. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bryk, R.; Wolff, D.J. Pharmacological modulation of nitric oxide synthesis by mechanism-based inactivators and related inhibitors. Pharmacol. Ther. 1999, 84, 157–178. [Google Scholar] [CrossRef]
- Montero, M.; Brini, M.; Marsault, R.; Alvarez, J.; Sitia, R.; Pozzan, T.; Rizzuto, R. Monitoring dynamic changes in free Ca2+ concentration in the endoplasmic reticulum of intact cells. EMBO J. 1995, 14, 5467–5475. [Google Scholar] [CrossRef] [PubMed]
- Pinton, P.; Rimessi, A.; Romagnoli, A.; Prandini, A.; Rizzuto, R. Biosensors for the detection of calcium and pH. Methods Cell Biol. 2007, 80, 297–325. [Google Scholar] [PubMed]
- Sucrose Gradient Separation Protocol—MitoSciences VIII: Optimization Steps and General Tips. 2007, Page 11. Available online: https://pdf4pro.com/view/sucrose-gradient-separation-protocol-mitosciences-1598bd.html (accessed on 8 January 2022).




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fornasiero, F.; Scapin, C.; Vitadello, M.; Pizzo, P.; Gorza, L. Active nNOS Is Required for Grp94-Induced Antioxidant Cytoprotection: A Lesson from Myogenic to Cancer Cells. Int. J. Mol. Sci. 2022, 23, 2915. https://doi.org/10.3390/ijms23062915
Fornasiero F, Scapin C, Vitadello M, Pizzo P, Gorza L. Active nNOS Is Required for Grp94-Induced Antioxidant Cytoprotection: A Lesson from Myogenic to Cancer Cells. International Journal of Molecular Sciences. 2022; 23(6):2915. https://doi.org/10.3390/ijms23062915
Chicago/Turabian StyleFornasiero, Filippo, Cristina Scapin, Maurizio Vitadello, Paola Pizzo, and Luisa Gorza. 2022. "Active nNOS Is Required for Grp94-Induced Antioxidant Cytoprotection: A Lesson from Myogenic to Cancer Cells" International Journal of Molecular Sciences 23, no. 6: 2915. https://doi.org/10.3390/ijms23062915
APA StyleFornasiero, F., Scapin, C., Vitadello, M., Pizzo, P., & Gorza, L. (2022). Active nNOS Is Required for Grp94-Induced Antioxidant Cytoprotection: A Lesson from Myogenic to Cancer Cells. International Journal of Molecular Sciences, 23(6), 2915. https://doi.org/10.3390/ijms23062915