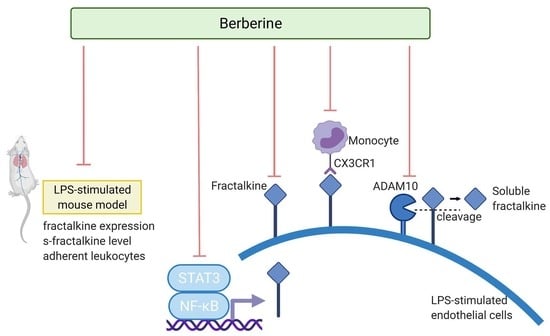
Berberine Suppresses Leukocyte Adherence by Downregulating CX3CL1 Expression and Shedding and ADAM10 in Lipopolysaccharide-Stimulated Vascular Endothelial Cells
Abstract
:1. Introduction
2. Results
2.1. Berberine Downregulated Cx3cl1 in the Endothelium of Lung Postcapillary Venules of LPS-Treated Rats
2.2. Berberine Mitigated the LPS-Induced Upregulation of CX3CL1 in Cultured Human Umbilical Cord Vein Endothelial Cells (HUVECs)
2.3. Berberine Mitigated the LPS-Induced Activation of NF-κB/STAT3 Pathways in HUVECs
2.4. Berberine Mitigated the LPS-Induced Upregulation of CX3CR1 in THP-1 Cells
2.5. Berberine Mitigated the LPS-Induced Upregulation of Soluble Fractalkine In Vivo and In Vitro
2.6. Berberine Mitigated the LPS-Induced Upregulation of ADAM10 in HUVECs
3. Discussion
4. Materials and Methods
4.1. Drugs and Reagent
4.2. Animals
4.3. Animal Experiments
4.4. Cell Culture
4.5. MTT (3-[4, 5-Dimethylthiazol-2-yl]-2, 5-Diphenyltetrazolium Bromide) Assay
4.6. RNA Isolation
4.7. Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
4.8. Western Blotting Analysis
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Parsons, P.E.; Eisner, M.D.; Thompson, B.T.; Matthay, M.A.; Ancukiewicz, M.; Bernard, G.R.; Wheeler, A.P. NHLBI Acute Respiratory Distress Syndrome Clinical Trials Network. Lower tidal volume ventilation and plasma cytokine markers of inflammation in patients with acute lung injury. Crit. Care Med. 2005, 33, 1–6. [Google Scholar] [CrossRef]
- Piantadosi, C.A.; Schwartz, D.A. The acute respiratory distress syndrome. Ann. Intern. Med. 2004, 141, 460–470. [Google Scholar] [CrossRef]
- Reiss, L.K.; Uhlig, U.; Uhlig, S. Models and mechanisms of acute lung injury caused by direct insults. Eur. J. Cell Biol. 2012, 91, 590–601. [Google Scholar] [CrossRef]
- Bazan, J.F.; Bacon, K.B.; Hardiman, G.; Wang, W.; Soo, K.; Rossi, D.; Greaves, D.R.; Zlotnik, A.; Schall, T.J. A new class of membrane-bound chemokine with a CX3C motif. Nature 1997, 385, 640–644. [Google Scholar] [CrossRef]
- Imai, T.; Nishimura, M.; Nanki, T.; Umehara, H. Fractalkine and inflammatory diseases. Nihon Rinsho Meneki Gakkai Kaishi 2005, 28, 131–139. [Google Scholar] [CrossRef] [Green Version]
- Imaizumi, T.; Yoshida, H.; Satoh, K. Regulation of CX3CL1/fractalkine expression in endothelial cells. J. Atheroscler. Thromb. 2004, 11, 15–21. [Google Scholar] [CrossRef] [Green Version]
- Garcia, G.E.; Xia, Y.; Chen, S.; Wang, Y.; Ye, R.D.; Harrison, J.K.; Bacon, K.B.; Zerwes, H.G.; Feng, L. NF-kappaB-dependent fractalkine induction in rat aortic endothelial cells stimulated by IL-1beta, TNF-alpha, and LPS. J. Leukoc. Biol. 2000, 67, 577–584. [Google Scholar] [CrossRef]
- Fong, A.M.; Robinson, L.A.; Steeber, D.A.; Tedder, T.F.; Yoshie, O.; Imai, T.; Patel, D.D. Fractalkine and CX3CR1 mediate a novel mechanism of leukocyte capture, firm adhesion, and activation under physiologic flow. J. Exp. Med. 1998, 188, 1413–1419. [Google Scholar] [CrossRef]
- Gan, A.M.; Butoi, E.; Manea, A.; Pirvulescu, M.M.; Stan, D.; Simion, V.; Calin, M.; Simionescu, M.; Manduteanu, I. Functional analysis of the fractalkine gene promoter in human aortic smooth muscle cells exposed to proinflammatory conditions. FEBS J. 2014, 281, 3869–3881. [Google Scholar] [CrossRef]
- Zhang, J.; Patel, J.M. Role of the CX3CL1-CX3CR1 axis in chronic inflammatory lung diseases. Int. J. Clin. Exp. Med. 2010, 3, 233–244. [Google Scholar]
- Sukkar, M.B.; Issa, R.; Xie, S.; Oltmanns, U.; Newton, R.; Chung, K.F. Fractalkine/CX3CL1 production by human airway smooth muscle cells: Induction by IFN-gamma and TNF-alpha and regulation by TGF-beta and corticosteroids. Am. J. Physiol. Lung Cell. Mol. Physiol. 2004, 287, L1230–L1240. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Wei, Q.; Hu, Y.; Wang, C. Role of fractalkine in promoting inflammation in sepsis-induced multiple organ dysfunction. Infect. Genet. Evol. 2020, 85, 104569. [Google Scholar] [CrossRef]
- Schwarz, N.; Pruessmeyer, J.; Hess, F.M.; Dreymueller, D.; Pantaler, E.; Koelsch, A.; Windoffer, R.; Voss, M.; Sarabi, A.; Weber, C.; et al. Requirements for leukocyte transmigration via the transmembrane chemokine CX3CL1. Cell. Mol. Life Sci. 2010, 67, 4233–4248. [Google Scholar] [CrossRef]
- Zhang, H.Q.; Wang, H.D.; Lu, D.X.; Qi, R.B.; Wang, Y.P.; Yan, Y.X.; Fu, Y.M. Berberine inhibits cytosolic phospholipase A2 and protects against LPS-induced lung injury and lethality independent of the alpha2-adrenergic receptor in mice. Shock 2008, 29, 617–622. [Google Scholar] [CrossRef]
- Liu, S.J.; Yin, C.X.; Ding, M.C.; Wang, Y.Z.; Wang, H. Berberine inhibits tumor necrosis factor-α-induced expression of inflammatory molecules and activation of nuclear factor-κB via the activation of AMPK in vascular endothelial cells. Mol. Med. Rep. 2015, 12, 5580–5586. [Google Scholar] [CrossRef]
- Wu, Y.H.; Chuang, S.Y.; Hong, W.C.; Lai, Y.J.; Chang, G.J.; Pang, J.H. Berberine reduces leukocyte adhesion to LPS-stimulated endothelial cells and VCAM-1 expression both in vivo and in vitro. Int. J. Immunopathol. Pharmacol. 2012, 25, 741–750. [Google Scholar] [CrossRef] [Green Version]
- Dos Santos, C.; Heunks, L.; Wunsch, H. Update in critical Care 2016. Am. J. Respir. Crit. Care Med. 2017, 196, 11–17. [Google Scholar] [CrossRef]
- Duggal, A.; Ganapathy, A.; Ratnapalan, M.; Adhikari, N.K. Pharmacological treatments for acute respiratory distress syndrome: Systematic review. Minerva Anestesiol. 2015, 81, 567–588. [Google Scholar]
- Imenshahidi, M.; Hosseinzadeh, H. Berberis vulgaris and berberine: An update review. Phytother. Res. 2016, 30, 1745–1764. [Google Scholar] [CrossRef]
- Hu, Y.; Lou, J.; Mao, Y.Y.; Lai, T.W.; Liu, L.Y.; Zhu, C.; Zhang, C.; Liu, J.; Li, Y.Y.; Zhang, F.; et al. Activation of MTOR in pulmonary epithelium promotes LPS-induced acute lung injury. Autophagy 2016, 12, 2286–2299. [Google Scholar] [CrossRef] [Green Version]
- Liang, Y.; Fan, C.; Yan, X.; Lu, X.; Jiang, H.; Di, S.; Ma, Z.; Feng, Y.; Zhang, Z.; Feng, P.; et al. Berberine ameliorates lipopolysaccharide-induced acute lung injury via the PERK-mediated Nrf2/HO-1 signaling axis. Phytother. Res. 2019, 33, 130–148. [Google Scholar] [CrossRef] [Green Version]
- Hoogendijk, A.J.; Wiewel, M.A.; van Vught, L.A.; Scicluna, B.P.; Belkasim-Bohoudi, H.; Horn, J.; Zwinderman, A.H.; Klouwenberg, P.M.C.K.; Cremer, O.L.; Bonten, M.J.; et al. Plasma fractalkine is a sustained marker of disease severity and outcome in sepsis patients. Crit. Care 2015, 19, 412. [Google Scholar] [CrossRef] [Green Version]
- Dijkstra, A.; Postma, D.S.; Noordhoek, J.A.; Lodewijk, M.E.; Kauffman, H.F.; ten Hacken, N.H.T.; Timens, W. Expression of ADAMs (“a disintegrin and metalloprotease”) in the human lung. Virchows Arch. 2009, 454, 441–449. [Google Scholar] [CrossRef] [Green Version]
- Hundhausen, C.; Misztela, D.; Berkhout, T.A.; Broadway, N.; Saftig, P.; Reiss, K.; Hartmann, D.; Fahrenholz, F.; Postina, R.; Matthews, V.; et al. The disintegrin-like metalloproteinase ADAM10 is involved in constitutive cleavage of CX3CL1 (fractalkine) and regulates CX3CL1-mediated cell-cell adhesion. Blood 2003, 102, 1186–1195. [Google Scholar] [CrossRef] [Green Version]
- Dreymueller, D.; Uhlig, S.; Ludwig, A. ADAM-family metalloproteinases in lung inflammation: Potential therapeutic targets. Am. J. Physiol. Lung Cell. Mol. Physiol. 2015, 308, L325–L343. [Google Scholar] [CrossRef] [Green Version]
- Wetzel, S.; Seipold, L.; Saftig, P. The metalloproteinase ADAM10: A useful therapeutic target? Biochim. Biophys. Acta Mol. Cell Res. 2017, 1864, 2071–2081. [Google Scholar] [CrossRef]
- Antonelli, A.; Di Maggio, S.; Rejman, J.; Sanvito, F.; Rossi, A.; Catucci, A.; Gorzanelli, A.; Bragonzi, A.; Bianchi, M.E.; Raucci, A. The shedding-derived soluble receptor for advanced glycation endproducts sustains inflammation during acute Pseudomonas aeruginosa lung infection. Biochim. Biophys. Acta Gen. Subj. 2017, 1861, 354–364. [Google Scholar] [CrossRef]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Y.-H.; Wei, C.-Y.; Hong, W.-C.; Pang, J.-H.S. Berberine Suppresses Leukocyte Adherence by Downregulating CX3CL1 Expression and Shedding and ADAM10 in Lipopolysaccharide-Stimulated Vascular Endothelial Cells. Int. J. Mol. Sci. 2022, 23, 4801. https://doi.org/10.3390/ijms23094801
Wu Y-H, Wei C-Y, Hong W-C, Pang J-HS. Berberine Suppresses Leukocyte Adherence by Downregulating CX3CL1 Expression and Shedding and ADAM10 in Lipopolysaccharide-Stimulated Vascular Endothelial Cells. International Journal of Molecular Sciences. 2022; 23(9):4801. https://doi.org/10.3390/ijms23094801
Chicago/Turabian StyleWu, Yi-Hong, Chen-Ying Wei, Wei-Chin Hong, and Jong-Hwei Su Pang. 2022. "Berberine Suppresses Leukocyte Adherence by Downregulating CX3CL1 Expression and Shedding and ADAM10 in Lipopolysaccharide-Stimulated Vascular Endothelial Cells" International Journal of Molecular Sciences 23, no. 9: 4801. https://doi.org/10.3390/ijms23094801
APA StyleWu, Y.-H., Wei, C.-Y., Hong, W.-C., & Pang, J.-H. S. (2022). Berberine Suppresses Leukocyte Adherence by Downregulating CX3CL1 Expression and Shedding and ADAM10 in Lipopolysaccharide-Stimulated Vascular Endothelial Cells. International Journal of Molecular Sciences, 23(9), 4801. https://doi.org/10.3390/ijms23094801