RNA-Binding Protein MEX3A Interacting with DVL3 Stabilizes Wnt/β-Catenin Signaling in Endometrial Carcinoma
Abstract
:1. Introduction
2. Results
2.1. MEX3A, as a Representative RBP, Was Upregulated and Related to a Poor Prognosis in EC
2.2. MEX3A Promoted the Proliferation and Metastasis of EC Cells In Vitro
2.3. MEX3A Increased the Oncogenesis and Progression of EC Cells In Vivo
2.4. MEX3A Activated the EMT and Wnt/β-Catenin Signaling Pathway in EC Cells
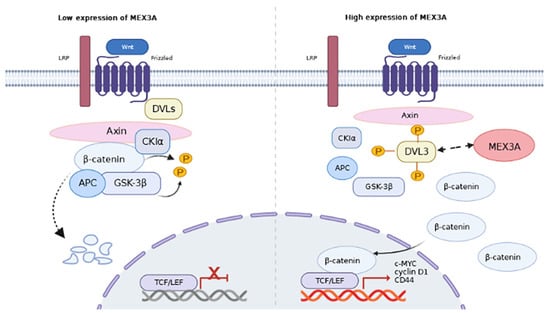
2.5. MEX3A Enhanced the Wnt/β-Catenin Signaling Pathway via DVL3
2.6. DVL3 Promoted the Progression of EC Cells In Vitro
3. Discussion
4. Materials and Methods
4.1. Bioinformatics Analysis
4.2. Immunohistochemistry (IHC) Assay
4.3. Cell Culture
4.4. RNA Interference, Lentiviral Infection, and Plasmid Transfection
4.5. Quantitative Real-Time PCR (qRT-PCR) and Western Blot Analysis
4.6. Cell Proliferation Assay
4.7. Migration and Invasion Assays
4.8. In Vivo Tumor Xenograft and Metastasis Assays
4.9. Co-Immunoprecipitation (Co-IP)
4.10. Immunofluorescence Assay (IF)
4.11. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cao, M.; Li, H.; Sun, D.; Chen, W. Cancer burden of major cancers in China: A need for sustainable actions. Cancer Commun. 2020, 40, 205–210. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics. CA A Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Connor, E.V.; Rose, P.G. Management Strategies for Recurrent Endometrial Cancer. Expert Rev. Anticancer. Ther. 2018, 18, 873–885. [Google Scholar] [CrossRef] [PubMed]
- Lortet-Tieulent, J.; Ferlay, J.; Bray, F.; Jemal, A. International Patterns and Trends in Endometrial Cancer Incidence, 1978–2013. Gynecol. Oncol. 2017, 110, 354–361. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davis, M.; Rauh-Hain, J.A.; Andrade, C.; Boruta, D.M., 2nd; Schorge, J.O.; Horowitz, N.S.; May, T.; del Carmen, M.G. Comparison of clinical outcomes of patients with clear cell and endometrioid ovarian cancer associated with endometriosis to papillary serous carcinoma of the ovary. Gynecol. Oncol. 2014, 132, 760–766. [Google Scholar] [CrossRef]
- Amant, F.; Moerman, P.; Neven, P.; Timmerman, D.; Van Limbergen, E.; Vergote, I. Endometrial cancer. Lancet 2005, 366, 491–505. [Google Scholar] [CrossRef]
- Qin, H.; Ni, H.; Liu, Y.; Yuan, Y.; Xi, T.; Li, X.; Zheng, L. RNA-binding proteins in tumor progression. J. Hematol. Oncol. 2020, 13, 90. [Google Scholar] [CrossRef]
- Li, K.; Guo, Z.-W.; Zhai, X.-M.; Yang, X.-X.; Wu, Y.-S.; Liu, T.-C. RBPTD: A database of cancer-related RNA-binding proteins in humans. Database 2020, 2020, baz156. [Google Scholar] [CrossRef] [Green Version]
- Mitobe, Y.; Iino, K.; Takayama, K.-I.; Ikeda, K.; Suzuki, T.; Aogi, K.; Kawabata, H.; Suzuki, Y.; Horie-Inoue, K.; Inoue, S. PSF Promotes ER-Positive Breast Cancer Progression via Posttranscriptional Regulation of ESR1 and SCFD2. Cancer Res. 2020, 80, 2230–2242. [Google Scholar] [CrossRef] [Green Version]
- Takayama, K.-I.; Suzuki, T.; Fujimura, T.; Yamada, Y.; Takahashi, S.; Homma, Y.; Suzuki, Y.; Inoue, S. Dysregulation of spliceosome gene expression in advanced prostate cancer by RNA-binding protein PSF. Proc. Natl. Acad. Sci. USA 2017, 114, 10461–10466. [Google Scholar] [CrossRef]
- Gor, R.; Sampath, S.S.; Lazer, L.M.; Ramalingam, S. RNA binding protein PUM1 promotes colon cancer cell proliferation and migration. Int. J. Biol. Macromol. 2021, 174, 549–561. [Google Scholar] [CrossRef] [PubMed]
- Palomo-Irigoyen, M.; Pérez-Andrés, E.; Iruarrizaga-Lejarreta, M.; Barreira-Manrique, A.; Tamayo-Caro, M.; Vila-Vecilla, L.; Moreno-Cugnon, L.; Beitia, N.; Medrano, D.; Fernández-Ramos, D.; et al. HuR/ELAVL1 drives malignant peripheral nerve sheath tumor growth and metastasis. J. Clin. Investig. 2020, 130, 3848–3864. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buchet-Poyau, K.; Courchet, J.; Le Hir, H.; Séraphin, B.; Scoazec, J.-Y.; Duret, L.; Domon-Dell, C.; Freund, J.-N.; Billaud, M. Identification and characterization of human Mex-3 proteins, a novel family of evolutionarily conserved RNA-binding proteins differentially localized to processing bodies. Nucleic Acids Res. 2007, 35, 1289–1300. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liang, J.; Li, H.; Han, J.; Jiang, J.; Wang, J.; Li, Y.; Feng, Z.; Zhao, R.; Sun, Z.; Lv, B.; et al. Mex3a interacts with LAMA2 to promote lung adenocarcinoma metastasis via PI3K/AKT pathway. Cell Death Dis. 2020, 11, 614. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Zhan, H.; Zhao, Y.; Wu, Y.; Li, L.; Wang, H. MEX3A contributes to development and progression of glioma through regulating cell proliferation and cell migration and targeting CCL2. Cell Death Dis. 2021, 12, 14. [Google Scholar] [CrossRef]
- Li, F.; Zhao, C.; Diao, Y.; Wang, Z.; Peng, J.; Yang, N.; Qiu, C.; Kong, B.; Li, Y. MEX3A promotes the malignant progression of ovarian cancer by regulating intron retention in TIMELESS. Cell Death Dis. 2022, 13, 553. [Google Scholar] [CrossRef]
- Zhang, P.; Su, T.; Zhang, S. Comprehensive Analysis of Prognostic Value of MEX3A and Its Relationship with Immune Infiltrates in Ovarian Cancer. J. Immunol. Res. 2021, 2021, 5574176. [Google Scholar] [CrossRef]
- Wang, Y.; Liang, Q.; Lei, K.; Zhu, Q.; Zeng, D.; Liu, Y.; Lu, Y.; Kang, T.; Tang, N.; Huang, L.; et al. Targeting MEX3A attenuates metastasis of breast cancer via β-catenin signaling pathway inhibition. Cancer Lett. 2021, 521, 50–63. [Google Scholar] [CrossRef]
- Li, L.; Mao, B.; Yan, M.; Wu, S.; Ge, R.; Lian, Q.; Cheng, C.Y. Planar cell polarity protein Dishevelled 3 (Dvl3) regulates ectoplasmic specialization (ES) dynamics in the testis through changes in cytoskeletal organization. Cell Death Dis. 2019, 10, 194. [Google Scholar] [CrossRef] [Green Version]
- Harnoš, J.; Cañizal, M.C.A.; Jurásek, M.; Kumar, J.; Holler, C.; Schambony, A.; Hanakova, K.; Bernatik, O.; Zdrahal, Z.; Gömöryová, K.; et al. Dishevelled-3 conformation dynamics analyzed by FRET-based biosensors reveals a key role of casein kinase 1. Nat. Commun. 2019, 10, 1804. [Google Scholar] [CrossRef]
- Zhao, Q.; Zhuang, K.; Han, K.; Tang, H.; Wang, Y.; Si, W.; Yang, Z. Silencing DVL3 defeats MTX resistance and attenuates stemness via Notch Signaling Pathway in colorectal cancer. Pathol.—Res. Pract. 2020, 216, 152964. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.-Q.; Jiang, J.; Wang, X.-T.; Zhang, C.-L.; Ji, A.-Y.; Chen, X.-J. Role and mechanism of Dvl3 in the esophageal squamous cell carcinoma. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 7716–7725. [Google Scholar] [CrossRef] [PubMed]
- Kim, P.-J.; Park, J.Y.; Kim, H.-G.; Cho, Y.M.; Go, H. Dishevelled segment polarity protein 3 (DVL3): A novel and easily applicable recurrence predictor in localised prostate adenocarcinoma. Br. J. Urol. 2017, 120, 343–350. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gebauer, F.; Schwarzl, T.; Valcárcel, J.; Hentze, M.W. RNA-binding proteins in human genetic disease. Nat. Rev. Genet. 2020, 22, 185–198. [Google Scholar] [CrossRef]
- Neelamraju, Y.; Hashemikhabir, S.; Janga, S.C. The human RBPome: From genes and proteins to human disease. J. Proteom. 2015, 127, 61–70. [Google Scholar] [CrossRef] [Green Version]
- Makker, V.; MacKay, H.; Ray-Coquard, I.; Levine, D.A.; Westin, S.N.; Aoki, D.; Oaknin, A. Endometrial cancer. Nat. Rev. Dis. Prim. 2021, 7, 88. [Google Scholar] [CrossRef]
- Chen, Y.; Su, H.; Su, Y.; Zhang, Y.; Lin, Y.; Haglund, F. Identification of an RNA-Binding-Protein-Based Prognostic Model for Ewing Sarcoma. Cancers 2021, 13, 3736. [Google Scholar] [CrossRef]
- Wang, M.; Huang, S.; Chen, Z.; Han, Z.; Li, K.; Chen, C.; Wu, G.; Zhao, Y. Development and validation of an RNA binding protein-associated prognostic model for hepatocellular carcinoma. BMC Cancer 2020, 20, 1136. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, Y.; Li, C.; Liu, J.; Ren, H.; Li, L.; Zheng, X.; Wang, H.; Han, Z. RNA binding protein PUM2 promotes the stemness of breast cancer cells via competitively binding to neuropilin-1 (NRP-1) mRNA with miR-376a. Biomed. Pharmacother. 2019, 114, 108772. [Google Scholar] [CrossRef]
- Colorectal cancer cells expressing Mex3a drive recurrence after chemotherapy. Nat. Cancer 2022, 3, 1024–1025. [CrossRef]
- Mittal, V. Epithelial Mesenchymal Transition in Tumor Metastasis. Annu. Rev. Pathol. 2018, 13, 395–412. [Google Scholar] [CrossRef] [PubMed]
- Pastushenko, I.; Blanpain, C. EMT Transition States during Tumor Progression and Metastasis. Trends Cell Biol. 2019, 29, 212–226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, S.; Sun, Z.; Lei, Z.; Zhang, H.-T. RNA-binding proteins and cancer metastasis. Semin. Cancer Biol. 2022, 86, 748–768. [Google Scholar] [CrossRef] [PubMed]
- Bebee, T.W.; Cieply, B.W.; Carstens, R.P. Genome-Wide Activities of RNA Binding Proteins That Regulate Cellular Changes in the Epithelial to Mesenchymal Transition (EMT). Adv. Exp. Med. Biol. 2014, 825, 267–302. [Google Scholar] [CrossRef]
- Wu, C.-Y.; Tsai, Y.-P.; Wu, M.-Z.; Teng, S.-C.; Wu, K.-J. Epigenetic reprogramming and post-transcriptional regulation during the epithelial–mesenchymal transition. Trends Genet. 2012, 28, 454–463. [Google Scholar] [CrossRef]
- Xu, Y.; Pan, S.; Chen, H.; Qian, H.; Wang, Z.; Zhu, X. MEX3A suppresses proliferation and EMT via inhibiting Akt signaling pathway in cervical cancer. Am. J. Cancer Res. 2021, 11, 1446–1462. [Google Scholar]
- Wang, X.; Shan, Y.-Q.; Tan, Q.-Q.; Tan, C.-L.; Zhang, H.; Liu, J.-H.; Ke, N.-W.; Chen, Y.-H.; Liu, X.-B. MEX3A knockdown inhibits the development of pancreatic ductal adenocarcinoma. Cancer Cell Int. 2020, 20, 63. [Google Scholar] [CrossRef] [Green Version]
- Parsons, M.J.; Tammela, T.; Dow, L.E. WNT as a Driver and Dependency in Cancer. Cancer Discov. 2021, 11, 2413–2429. [Google Scholar] [CrossRef]
- Tang, Q.; Chen, J.; Di, Z.; Yuan, W.; Zhou, Z.; Liu, Z.; Han, S.; Liu, Y.; Ying, G.; Shu, X.; et al. TM4SF1 promotes EMT and cancer stemness via the Wnt/β-catenin/SOX2 pathway in colorectal cancer. J. Exp. Clin. Cancer Res. 2020, 39, 232. [Google Scholar] [CrossRef]
- Yang, S.; Liu, Y.; Li, M.-Y.; Ng, C.S.H.; Yang, S.-L.; Wang, S.; Zou, C.; Dong, Y.; Du, J.; Long, X.; et al. FOXP3 promotes tumor growth and metastasis by activating Wnt/β-catenin signaling pathway and EMT in non-small cell lung cancer. Mol. Cancer 2017, 16, 124. [Google Scholar] [CrossRef]
- Zhao, H.; Zhao, Y.; Jiang, G.; Zhang, X.; Zhang, Y.; Dong, Q.; Luan, L.; Papavassiliou, P.; Wang, E.; Wang, E. Dishevelled-3 activates p65 to upregulate p120-catenin transcription via a p38-dependent pathway in non-small cell lung cancer. Mol. Carcinog. 2014, 54 (Suppl. 1), E112–E121. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Bajrami, I.; Verrill, C.; Kigozi, A.; Ouaret, D.; Aleksic, T.; Asher, R.; Han, C.; Allen, P.; Bailey, D.; et al. Dsh Homolog DVL3 Mediates Resistance to IGFIR Inhibition by Regulating IGF-RAS Signaling. Cancer Res. 2014, 74, 5866–5877. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shen, W.; Song, Z.; Zhong, X.; Huang, M.; Shen, D.; Gao, P.; Qian, X.; Wang, M.; He, X.; Wang, T.; et al. Sangerbox: A comprehensive, interaction-friendly clinical bioinformatics analysis platform. Imeta 2022, 1, e36. [Google Scholar] [CrossRef]







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, P.; Zhang, P.; Zhang, S. RNA-Binding Protein MEX3A Interacting with DVL3 Stabilizes Wnt/β-Catenin Signaling in Endometrial Carcinoma. Int. J. Mol. Sci. 2023, 24, 592. https://doi.org/10.3390/ijms24010592
Yang P, Zhang P, Zhang S. RNA-Binding Protein MEX3A Interacting with DVL3 Stabilizes Wnt/β-Catenin Signaling in Endometrial Carcinoma. International Journal of Molecular Sciences. 2023; 24(1):592. https://doi.org/10.3390/ijms24010592
Chicago/Turabian StyleYang, Pusheng, Panpan Zhang, and Shu Zhang. 2023. "RNA-Binding Protein MEX3A Interacting with DVL3 Stabilizes Wnt/β-Catenin Signaling in Endometrial Carcinoma" International Journal of Molecular Sciences 24, no. 1: 592. https://doi.org/10.3390/ijms24010592
APA StyleYang, P., Zhang, P., & Zhang, S. (2023). RNA-Binding Protein MEX3A Interacting with DVL3 Stabilizes Wnt/β-Catenin Signaling in Endometrial Carcinoma. International Journal of Molecular Sciences, 24(1), 592. https://doi.org/10.3390/ijms24010592